Low Temperature and High-Pressure Study of Bending L-Leucinium Hydrogen Maleate Crystals
Abstract
:1. Introduction
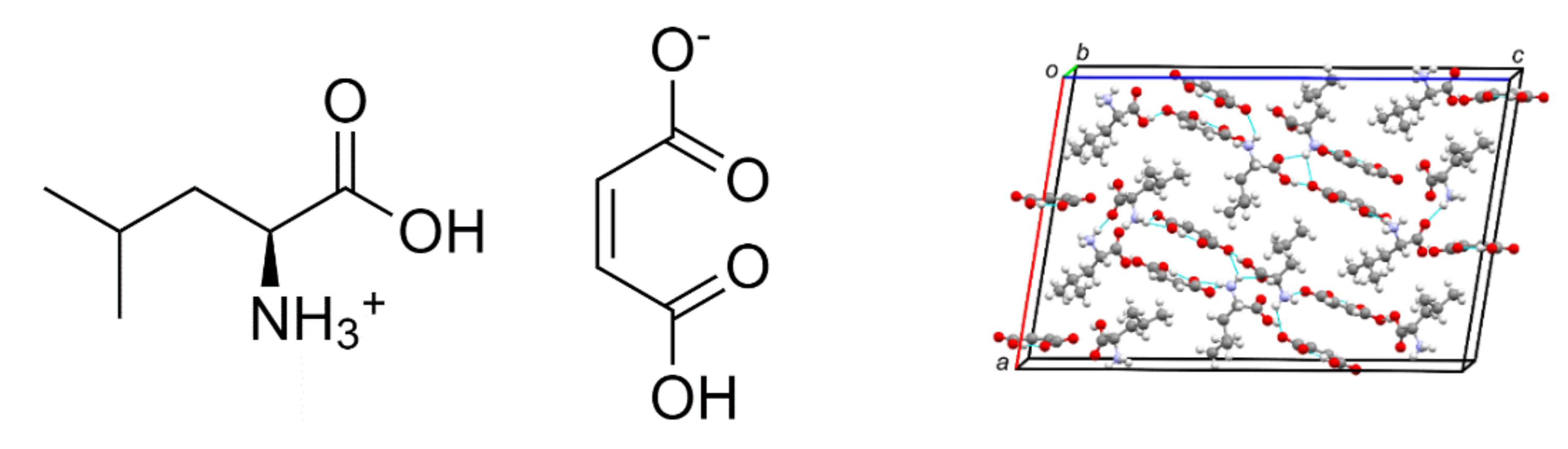
2. Materials and Methods
2.1. Crystal Growth
2.2. High-Pressure Generation and Measurement
2.3. Optical Microscopy
2.4. Single-Crystal X-ray Diffraction (SCXRD)
2.5. Raman Spectroscopy
2.6. Computational Methods
3. Results
3.1. Room Temperature Raman Spectra and Vibrational Mode Assignment
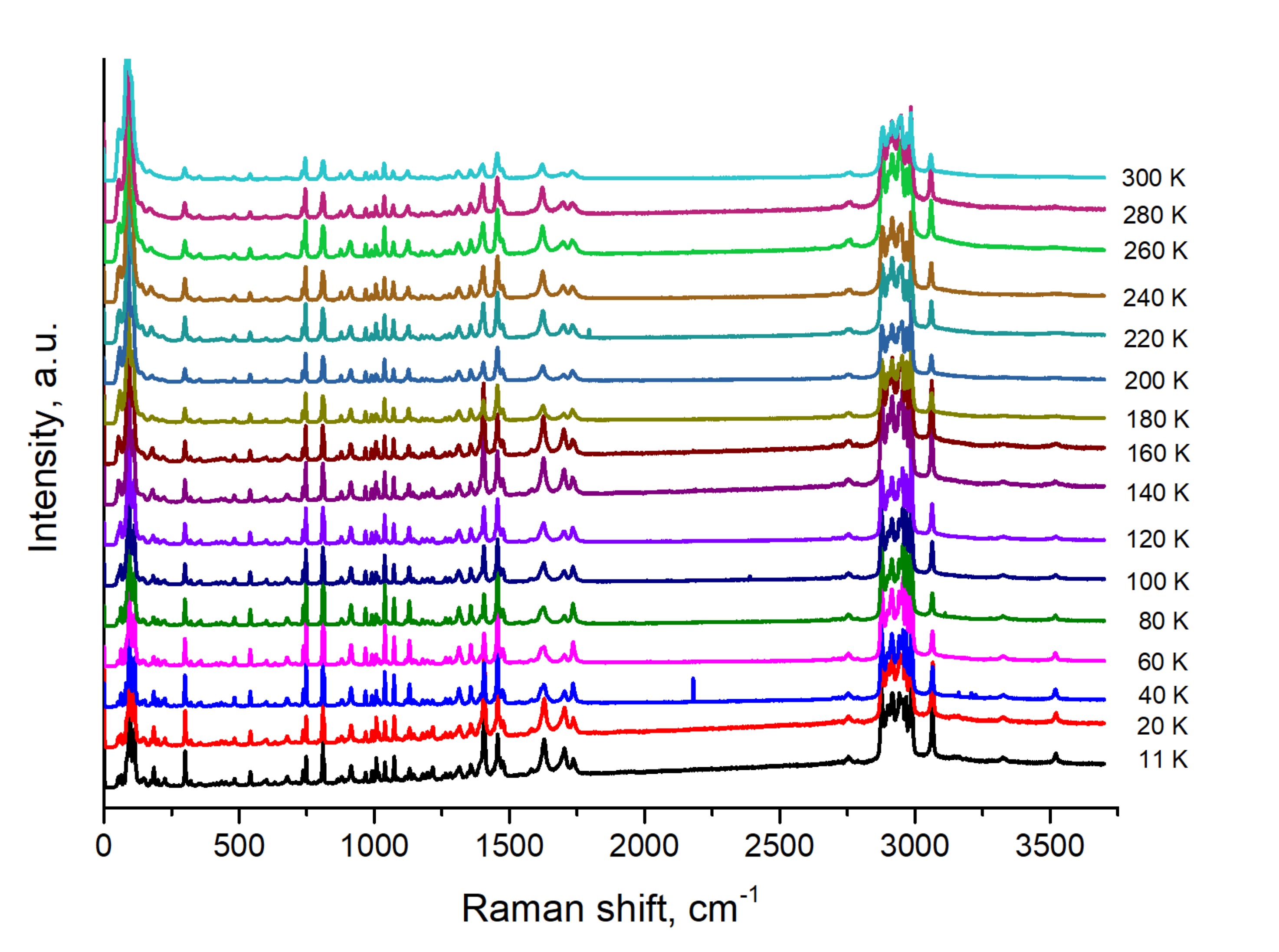
3.2. Low Temperature Study
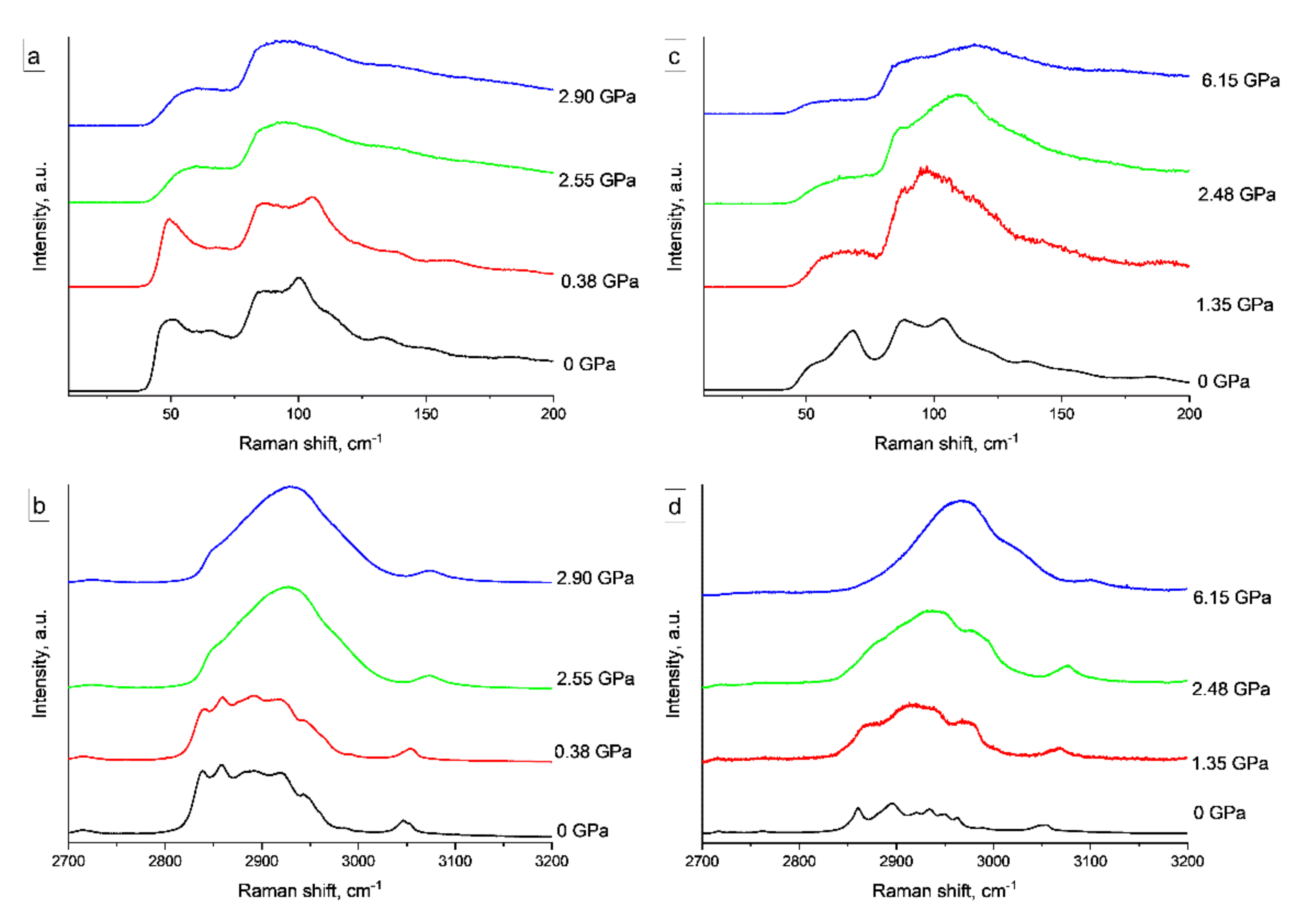
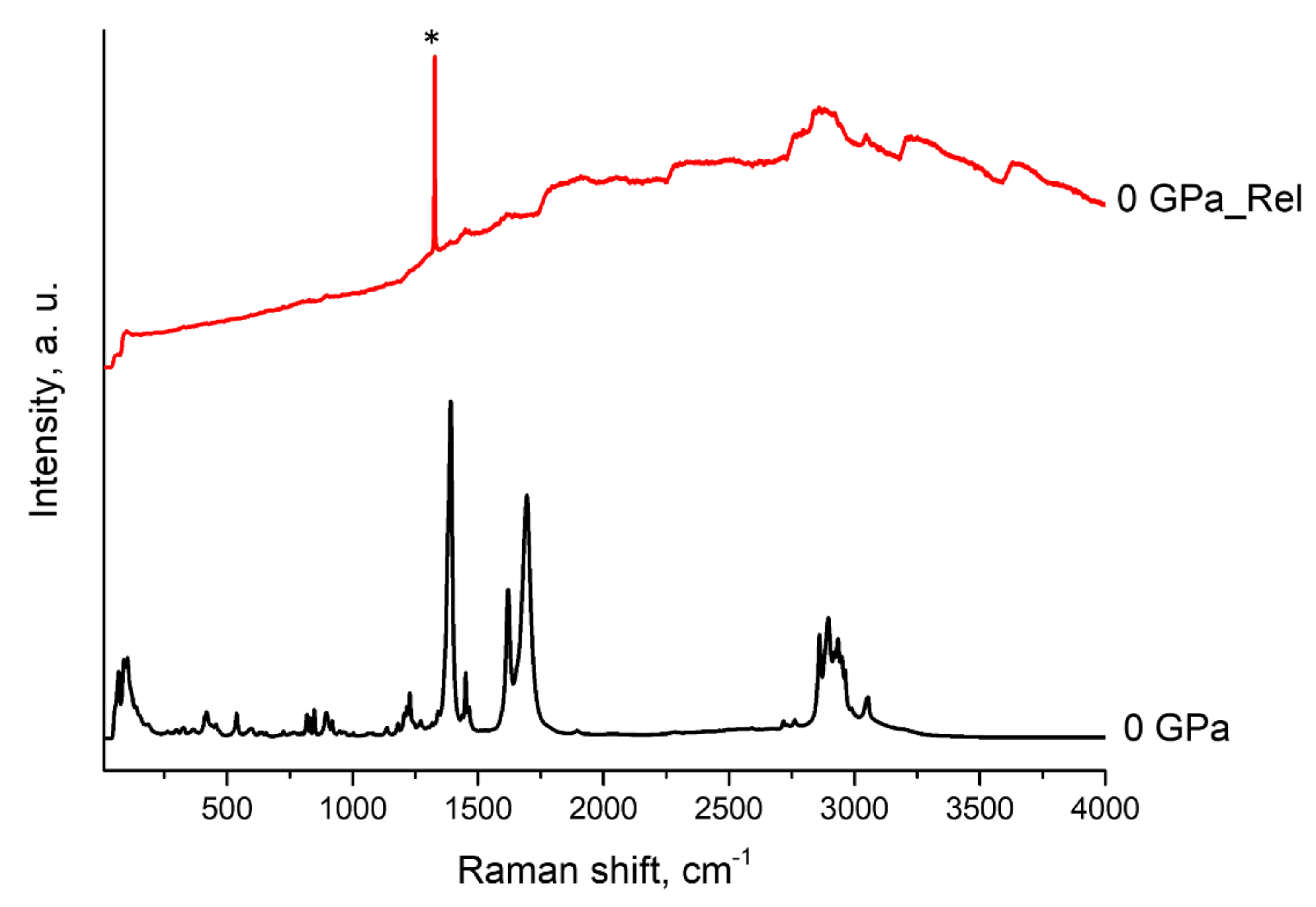
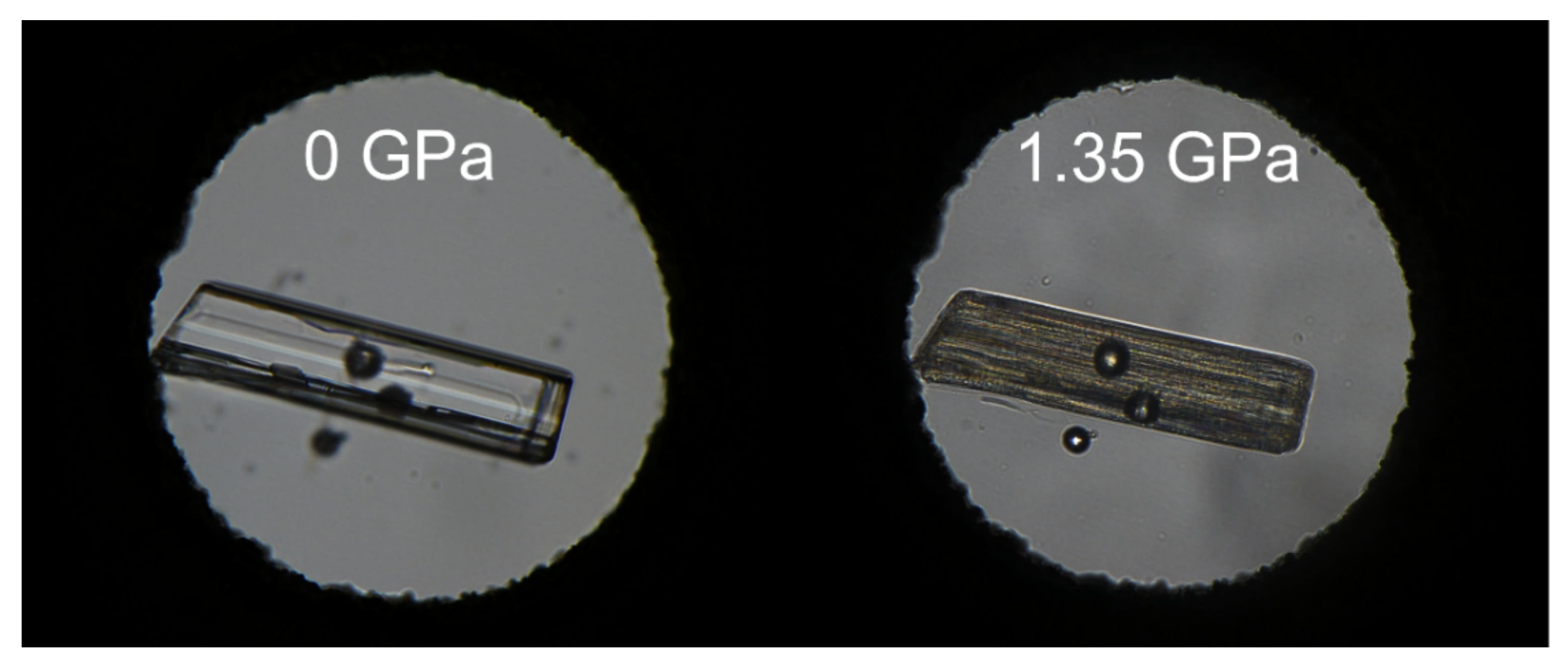
3.3. High Pressure Study
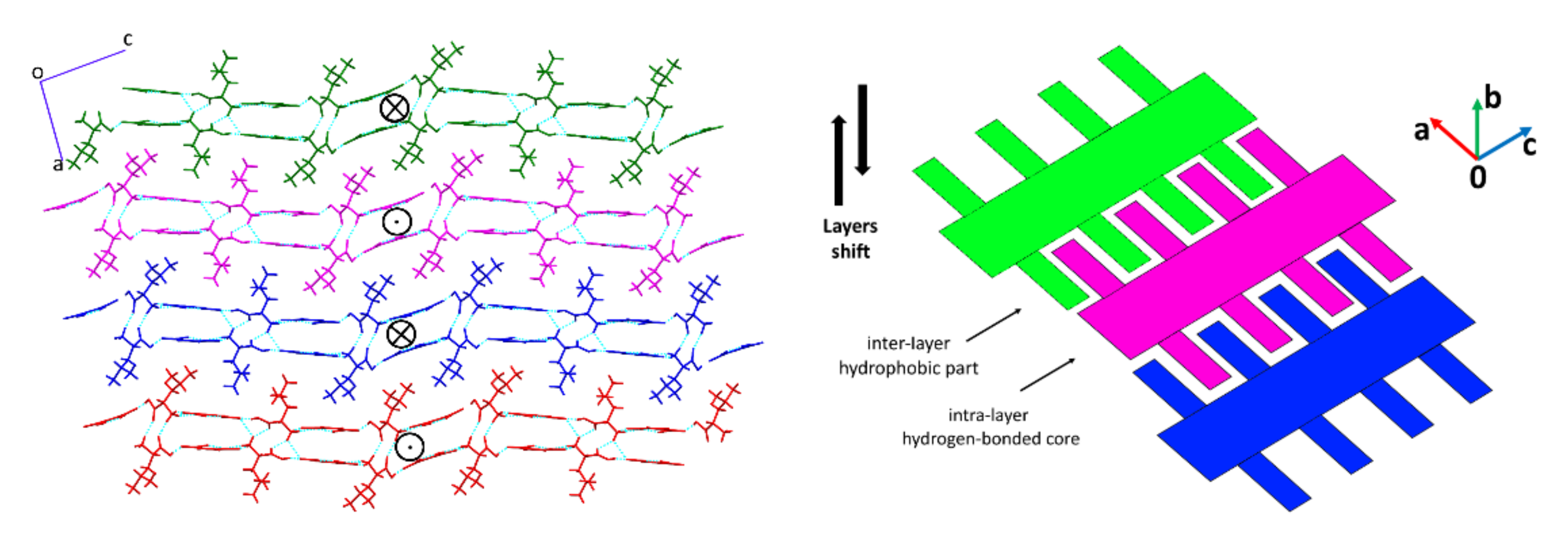
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montis, R.; Fusaro, L.; Falqui, A.; Hursthouse, M.B.; Tumanov, N.; Coles, S.J.; Threlfall, T.L.; Horton, P.N.; Sougrat, R.; Lafontaine, A.; et al. Complex structures arising from the self-assembly of a simple organic salt. Nature 2021, 590, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, S.; Ratajczyk, P.; Katrusiak, A. High-pressure Nucleation of Low-Density Polymorphs. Chemistry 2021, 27, 7069–7073. [Google Scholar] [CrossRef] [PubMed]
- Rychkov, D.A.; Stare, J.; Boldyreva, E.V. Pressure-driven phase transition mechanisms revealed by quantum chemistry: L-serine polymorphs. Phys. Chem. Chem. Phys. 2017, 19, 6671–6676. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mondal, A.; Kiran, M.S.R.N.; Ramamurty, U.; Reddy, C.M. The Role of Weak Interactions in the Phase Transition and Distinct Mechanical Behavior of Two Structurally Similar Caffeine Co-Crystal Polymorphs Studied by Nanoindentation. Cryst. Growth Des. 2013, 13, 4435–4441. [Google Scholar] [CrossRef]
- Korabel’nikov, D.V.; Zhuravlev, Y.N. Semi-empirical and ab initio calculations for crystals under pressure at fixed temperatures: The case of guanidinium perchlorate. RSC Adv. 2020, 10, 42204–42211. [Google Scholar] [CrossRef]
- Fedorov, A.Y.; Rychkov, D.A. Comparison of Different Computational Approaches for Unveiling the High-Pressure Behavior of Organic Crystals at a Molecular Level. Case Study of Tolazamide Polymorphs. J. Struct. Chem. 2020, 61, 1356–1366. [Google Scholar] [CrossRef]
- Rychkov, D.A. A Short Review of Current Computational Concepts for High-Pressure Phase Transition Studies in Molecular Crystals. Crystals 2020, 10, 81. [Google Scholar] [CrossRef]
- Errandonea, D. Pressure-Induced Phase Transformations. Crystals 2020, 10, 595. [Google Scholar] [CrossRef]
- Casati, N.; Macchi, P.; Sironi, A. Molecular Crystals Under High Pressure: Theoretical and Experimental Investigations of the Solid-Solid Phase Transitions in [Co2(CO)6(XPh3)2] (X=P, As). Chemistry 2009, 15, 4446–4457. [Google Scholar] [CrossRef]
- Zakharov, B.A.; Boldyreva, E.V. High pressure: A complementary tool for probing solid-state processes. CrystEngComm 2019, 21, 10–22. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M. Periodic DFT Calculations—Review of Applications in the Pharmaceutical Sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef]
- McGregor, L.; Rychkov, D.A.; Coster, P.L.; Day, S.; Drebushchak, V.A.; Achkasov, A.F.; Nichol, G.S.; Pulham, C.R.; Boldyreva, E.V. A new polymorph of metacetamol. CrystEngComm 2015, 17, 6183–6192. [Google Scholar] [CrossRef]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An extraordinary example of conformational polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Zhang, G. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv. Drug Deliv. Rev. 2004, 56, 371–390. [Google Scholar] [CrossRef]
- Llinàs, A.; Goodman, J.M. Polymorph control: Past, present and future. Drug Discov. Today 2008, 13, 198–210. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Rehman, H.U.; Zheng, X.; Li, H.; Liu, H.; Hedenqvist, M.S. Shape-Memory Polymeric Artificial Muscles: Mechanisms, Applications and Challenges. Molecules 2020, 25, 4246. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Z.; Tang, B.; Qu, C.; Zhang, Z.; Zhang, H. A Flexible Organic Single Crystal with Plastic-Twisting and Elastic-Bending Capabilities and Polarization-Rotation Function. Angew. Chem. Int. Ed. 2020, 59, 12944–12950. [Google Scholar] [CrossRef]
- Liu, H.; Bian, Z.; Cheng, Q.; Lan, L.; Wang, Y.; Zhang, H. Controllably realizing elastic/plastic bending based on a room-temperature phosphorescent waveguiding organic crystal. Chem. Sci. 2019, 10, 227–232. [Google Scholar] [CrossRef]
- Ghosh, S.; Reddy, C.M. Elastic and Bendable Caffeine Cocrystals: Implications for the Design of Flexible Organic Materials. Angew. Chem. Int. Ed. 2012, 51, 10319–10323. [Google Scholar] [CrossRef]
- Gupta, P.; Karothu, D.P.; Ahmed, E.; Naumov, P.; Nath, N.K. Thermally Twistable, Photobendable, Elastically Deformable, and Self-Healable Soft Crystals. Angew. Chem. Int. Ed. 2018, 57, 8498–8502. [Google Scholar] [CrossRef]
- Commins, P.; Desta, I.T.; Karothu, D.P.; Panda, M.K.; Naumov, P. Crystals on the move: Mechanical effects in dynamic solids. Chem. Commun. 2016, 52, 13941–13954. [Google Scholar] [CrossRef]
- Kakkar, S.; Bhattacharya, B.; Reddy, C.M.; Ghosh, S. Tuning mechanical behaviour by controlling the structure of a series of theophylline co-crystals. CrystEngComm 2018, 20, 1101–1109. [Google Scholar] [CrossRef]
- Colmenero, F. Mechanical properties of anhydrous oxalic acid and oxalic acid dihydrate. Phys. Chem. Chem. Phys. 2019, 21, 2673–2690. [Google Scholar] [CrossRef]
- Mishra, M.K.; Mishra, K.; Narayan, A.; Reddy, C.M.; Vangala, V.R. Structural Basis for Mechanical Anisotropy in Polymorphs of a Caffeine–Glutaric Acid Cocrystal. Cryst. Growth Des. 2020, 20, 6306–6315. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Roy, D.; Dey, S.; Puthuvakkal, A.; Bhunia, S.; Mondal, S.; Chowdhury, R.; Bhattacharya, M.; Mandal, M.; Manoj, K.; et al. Mechanical-Bending-Induced Fluorescence Enhancement in Plastically Flexible Crystals of a GFP Chromophore Analogue. Angew. Chem. 2020, 132, 20050–20055. [Google Scholar] [CrossRef]
- Colmenero, F. Negative linear compressibility in nanoporous metal–organic frameworks rationalized by the empty channel structural mechanism. Phys. Chem. Chem. Phys. 2021, 23, 8508–8524. [Google Scholar] [CrossRef]
- Masunov, A.E.; Wiratmo, M.; Dyakov, A.A.; Matveychuk, Y.V.; Bartashevich, E.V. Virtual Tensile Test for Brittle, Plastic, and Elastic Polymorphs of 4-Bromophenyl 4-Bromobenzoate. Cryst. Growth Des. 2020, 20, 6093–6100. [Google Scholar] [CrossRef]
- Thomas, S.P.; Shi, M.W.; Koutsantonis, G.A.; Jayatilaka, D.; Edwards, A.J.; Spackman, M.A. The Elusive Structural Origin of Plastic Bending in Dimethyl Sulfone Crystals with Quasi-Isotropic Crystal Packing. Angew. Chem. Int. Ed. 2017, 56, 8468–8472. [Google Scholar] [CrossRef]
- Reddy, C.M.; Gundakaram, R.C.; Basavoju, S.; Kirchner, M.T.; Padmanabhan, K.A.; Desiraju, G.R. Structural basis for bending of organic crystals. Chem. Commun. 2005, 1, 3945. [Google Scholar] [CrossRef]
- Reddy, C.M.; Padmanabhan, K.A.; Desiraju, G.R. Structure−Property Correlations in Bending and Brittle Organic Crystals. Cryst. Growth Des. 2006, 6, 2720–2731. [Google Scholar] [CrossRef]
- Worthy, A.; Grosjean, A.; Pfrunder, M.C.; Xu, Y.; Yan, C.; Edwards, G.; Clegg, J.K.; McMurtrie, J.C. Atomic resolution of structural changes in elastic crystals of copper(II) acetylacetonate. Nat. Chem. 2017, 10, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.P.; Chen, M.; Sun, C.C.; Reddy, C.M. Direct correlation among crystal structure, mechanical behaviour and tabletability in a trimorphic molecular compound. CrystEngComm 2012, 14, 3865. [Google Scholar] [CrossRef]
- Colmenero, F. Organic acids under pressure: Elastic properties, negative mechanical phenomena and pressure induced phase transitions in the lactic, maleic, succinic and citric acids. Mater. Adv. 2020, 1, 1399–1426. [Google Scholar] [CrossRef]
- Arkhipov, S.G.; Losev, E.A.; Nguyen, T.T.; Rychkov, D.A.; Boldyreva, E.V. A large anisotropic plasticity of L-leucinium hydrogen maleate preserved at cryogenic temperatures. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 143–151. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Arkhipov, S.G.; Rychkov, D.A. Simple crystallographic model for anomalous plasticity of L-Leucinium hydrogen maleate crystals. Mater. Today Proc. 2020, 25, 412–415. [Google Scholar] [CrossRef]
- Barrio, M.; Maccaroni, E.; Rietveld, I.B.; Malpezzi, L.; Masciocchi, N.; Céolin, R.; Tamarit, J.-L. Pressure-temperature state diagram for the phase relationships between benfluorex hydrochloride forms I and II: A case of enantiotropic behavior. J. Pharm. Sci. 2012, 101, 1073–1078. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Allan, D.R.; David, W.I.F.; Moggach, S.A.; Parsons, S.; Pulham, C.R. High-pressure recrystallisation—A route to new polymorphs and solvates. CrystEngComm 2004, 6, 504–511. [Google Scholar] [CrossRef]
- Drebushchak, V.A.; McGregor, L.; Rychkov, D.A. Cooling rate “window” in the crystallization of metacetamol form II. J. Therm. Anal. Calorim. 2017, 127, 1807–1814. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Pulham, C.R. High-pressure studies of pharmaceutical compounds and energetic materials. Chem. Soc. Rev. 2006, 35, 932. [Google Scholar] [CrossRef]
- Moggach, S.A.; Marshall, W.G.; Parsons, S. High-pressure neutron diffraction study of L-serine-I and L-serine-II, and the structure of L-serine-III at 8.1 GPa. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 815–825. [Google Scholar] [CrossRef]
- Wood, P.A.; Francis, D.; Marshall, W.G.; Moggach, S.A.; Parsons, S.; Pidcock, E.; Rohl, A.L. A study of the high-pressure polymorphs of L-serine using ab initio structures and PIXEL calculations. CrystEngComm 2008, 10, 1154. [Google Scholar] [CrossRef]
- Zakharov, B.A.; Kolesov, B.A.; Boldyreva, E.V. Effect of pressure on crystalline L- and DL-serine: Revisited by a combined single-crystal X-ray diffraction at a laboratory source and polarized Raman spectroscopy study. Acta Crystallogr. Sect. B Struct. Sci. 2012, 68, 275–286. [Google Scholar] [CrossRef]
- Fisch, M.; Lanza, A.; Boldyreva, E.; Macchi, P.; Casati, N. Kinetic Control of High-Pressure Solid-State Phase Transitions: A Case Study on l-Serine. J. Phys. Chem. C 2015, 119, 18611–18617. [Google Scholar] [CrossRef]
- Millar, D.I.A.; Oswald, I.D.H.; Francis, D.J.; Marshall, W.G.; Pulham, C.R.; Cumming, A.S. The crystal structure of β-RDX-an elusive form of an explosive revealed. Chem. Commun. 2009, 5, 562–564. [Google Scholar] [CrossRef]
- Munday, L.B.; Chung, P.W.; Rice, B.M.; Solares, S.D. Simulations of High-Pressure Phases in RDX. J. Phys. Chem. B 2011, 115, 4378–4386. [Google Scholar] [CrossRef]
- Hunter, S.; Sutinen, T.; Parker, S.F.; Morrison, C.A.; Williamson, D.M.; Thompson, S.; Gould, P.J.; Pulham, C.R. Experimental and DFT-D Studies of the Molecular Organic Energetic Material RDX. J. Phys. Chem. C 2013, 117, 8062–8071. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, A.; Xue, X.; Jiang, D.; Zhu, Y.; Zhang, C. Crystal packing of impact-sensitive high-energy explosives. Cryst. Growth Des. 2014, 14, 6101–6114. [Google Scholar] [CrossRef]
- Millar, D.I.A. Energetic Materials at Extreme Conditions; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642231315. [Google Scholar]
- Gude, V.; Choubey, P.S.; Das, S.; Bhaktha, B.N.S.; Reddy, C.M.; Biradha, K. Elastic orange emissive single crystals of 1,3-diamino-2,4,5,6-tetrabromobenzene as flexible optical waveguides. J. Mater. Chem. C 2021, 9, 9465–9472. [Google Scholar] [CrossRef]
- Das, S.; Mondal, A.; Reddy, C.M. Harnessing molecular rotations in plastic crystals: A holistic view for crystal engineering of adaptive soft materials. Chem. Soc. Rev. 2020, 49, 8878–8896. [Google Scholar] [CrossRef]
- Matveychuk, Y.V.; Bartashevich, E.V.; Tsirelson, V.G. How the H-Bond Layout Determines Mechanical Properties of Crystalline Amino Acid Hydrogen Maleates. Cryst. Growth Des. 2018, 18, 3366–3375. [Google Scholar] [CrossRef]
- Arkhipov, S.G.; Rychkov, D.A.; Pugachev, A.M.; Boldyreva, E.V. New hydrophobic L-amino acid salts: Maleates of L-leucine, L-isoleucine and L-norvaline. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Rychkov, D.A.; Arkhipov, S.G.; Boldyreva, E.V. Simple and efficient modifications of well known techniques for reliable growth of high-quality crystals of small bioorganic molecules. J. Appl. Crystallogr. 2014, 47, 1435–1442. [Google Scholar] [CrossRef]
- Boehler, R. New diamond cell for single-crystal x-ray diffraction. Rev. Sci. Instrum. 2006, 77, 115103. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J.D. Hydrostatic limits in liquids and solids to 100 kbar. J. Appl. Phys. 1973, 44, 5377–5382. [Google Scholar] [CrossRef]
- Zakharov, B.A.; Achkasov, A.F. A compact device for loading diamond anvil cells with low-boiling pressure-transmitting media. J. Appl. Crystallogr. 2013, 46, 267–269. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J.D.; Forman, R.A. Calibration of the pressure dependence of the R1 ruby fluorescence line to 195 kbar. J. Appl. Phys. 1975, 46, 2774–2780. [Google Scholar] [CrossRef]
- Forman, R.A.; Piermarini, G.J.; Barnett, J.D.; Block, S. Pressure Measurement Made by the Utilization of Ruby Sharp-Line Luminescence. Science 1972, 176, 284–285. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09. Revision D.01. Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Mirone, P.; Chiorboli, P. Infrared and Raman spectra and vibrational assignment of maleic anhydride. Spectrochim. Acta 1962, 18, 1425–1432. [Google Scholar]
- Shakhse Emampour, J.; Suh, J.S.; Moskovits, M. Raman Study of the Photochemistry of Maleic Acid Adsorbed on the Surface of Colloidal Silver. Iran. J. Chem. Chem. Eng. 1994, 13, 30–36. [Google Scholar]
- Façanha Filho, P.F.; Freire, P.T.C.; Lima, K.C.V.; Mendes Filho, J.; Melo, F.E.A.; Pizani, P.S. Raman spectra of L-leucine crystals. Brazilian J. Phys. 2007, 38, 12. [Google Scholar]
- Façanha Filho, P.F.; Freire, P.T.C.; Lima, K.C.V.; Mendes Filho, J.; Melo, F.E.A.; Pizani, P.S. High temperature raman spectra of L-leucine crystals. Brazilian J. Phys. 2008, 38, 131–137. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 2nd ed.; Springer: Dordrecht, The Netherlands, 1980; Volume 2, ISBN 9789401165228. [Google Scholar]
- Socrates, G. Infrared Characteristic Group Frequencies. Tables and Charts. Anal. Chim. Acta 1994, 296, 221–222. [Google Scholar]
- Hemalatha, A.; Arulmani, S.; Sanjay, P.; Deepa, K.; Madhavan, J.; Senthil, S. Growth and Characterization of L-Leucenium Hydrogen Maleate Single Crystals for Nonlinear Optical Applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 360, 012044. [Google Scholar] [CrossRef]
- Galkina, Y.A.; Vershinin, M.A.; Kolesov, B.A. Raman Spectra of Molecular Crystals with Strong Hydrogen Bonds N-H⋯N in the Temperature Range of 5–300 K. J. Struct. Chem. 2019, 60, 398–404. [Google Scholar] [CrossRef]
- Kolesov, B.A. IR and Raman spectra of strong OHO hydrogen bonds. J. Mol. Struct. 2021, 1233, 130093. [Google Scholar] [CrossRef]
- Kolesov, B. Hydrogen Bonds: Raman Spectroscopic Study. Int. J. Mol. Sci. 2021, 22, 5380. [Google Scholar] [CrossRef]
- Zakharov, B.A.; Tumanov, N.A.; Boldyreva, E.V. β-Alanine under pressure: Towards understanding the nature of phase transitions. CrystEngComm 2015, 17, 2074–2079. [Google Scholar] [CrossRef]
- Anis, B.; Börrnert, F.; Rümmeli, M.H.; Kuntscher, C.A. Role of the pressure transmitting medium on the pressure effects in DWCNTs. Phys. Status Solidi 2013, 250, 2616–2621. [Google Scholar] [CrossRef]
- Gaydamaka, A.A.; Arkhipov, S.G.; Zakharov, B.A.; Seryotkin, Y.V.; Boldyreva, E.V. Effect of pressure on slit channels in guanine sodium salt hydrate: A link to nucleobase intermolecular interactions. CrystEngComm 2019, 21, 4484–4492. [Google Scholar] [CrossRef]
- Yang, S.Y.; Butler, I.S. Pressure-tuning infrared and Raman microscopy study of the DNA bases: Adenine, guanine, cytosine, and thymine. J. Biomol. Struct. Dyn. 2013, 31, 1490–1496. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skakunova, K.D.; Rychkov, D.A. Low Temperature and High-Pressure Study of Bending L-Leucinium Hydrogen Maleate Crystals. Crystals 2021, 11, 1575. https://doi.org/10.3390/cryst11121575
Skakunova KD, Rychkov DA. Low Temperature and High-Pressure Study of Bending L-Leucinium Hydrogen Maleate Crystals. Crystals. 2021; 11(12):1575. https://doi.org/10.3390/cryst11121575
Chicago/Turabian StyleSkakunova, Kseniya D., and Denis A. Rychkov. 2021. "Low Temperature and High-Pressure Study of Bending L-Leucinium Hydrogen Maleate Crystals" Crystals 11, no. 12: 1575. https://doi.org/10.3390/cryst11121575
APA StyleSkakunova, K. D., & Rychkov, D. A. (2021). Low Temperature and High-Pressure Study of Bending L-Leucinium Hydrogen Maleate Crystals. Crystals, 11(12), 1575. https://doi.org/10.3390/cryst11121575