Transformation of SnS Nanocompisites to Sn and S Nanoparticles during Lithiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Electrochemical Cycling
2.3. Microstructure Analysis
3. Results and Discussion
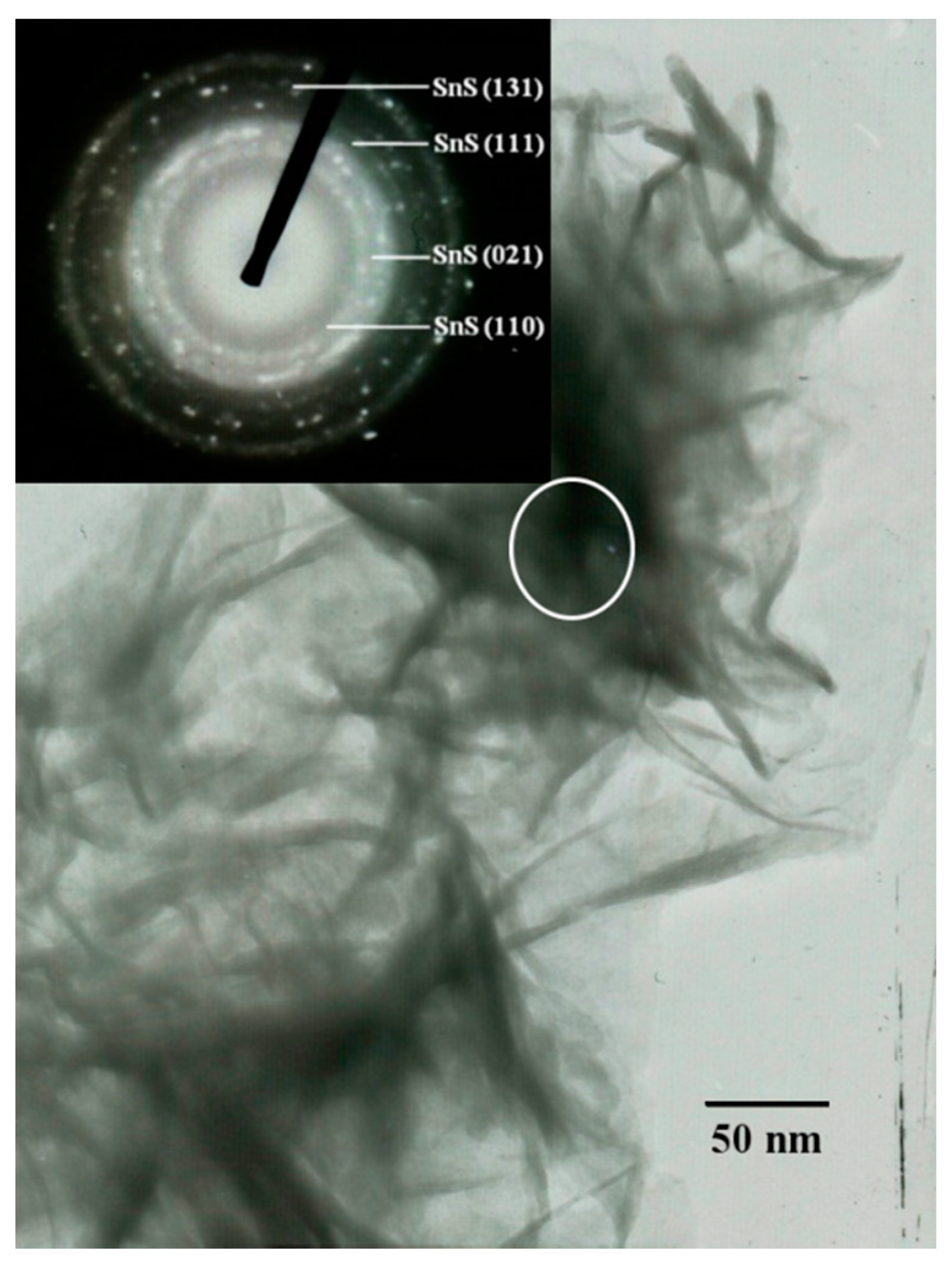
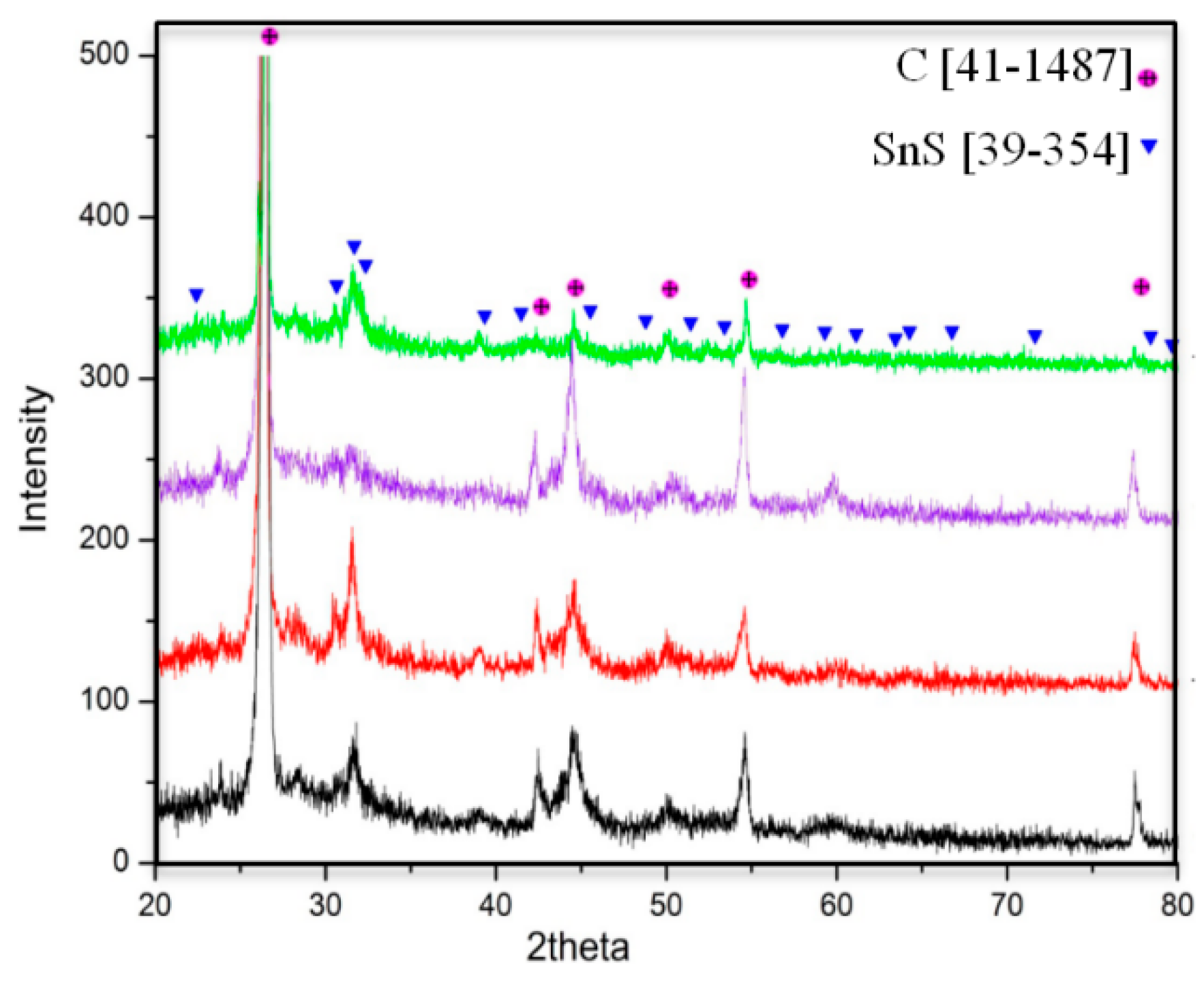
3.1. Microstructure Prior Electrochemical Cycling
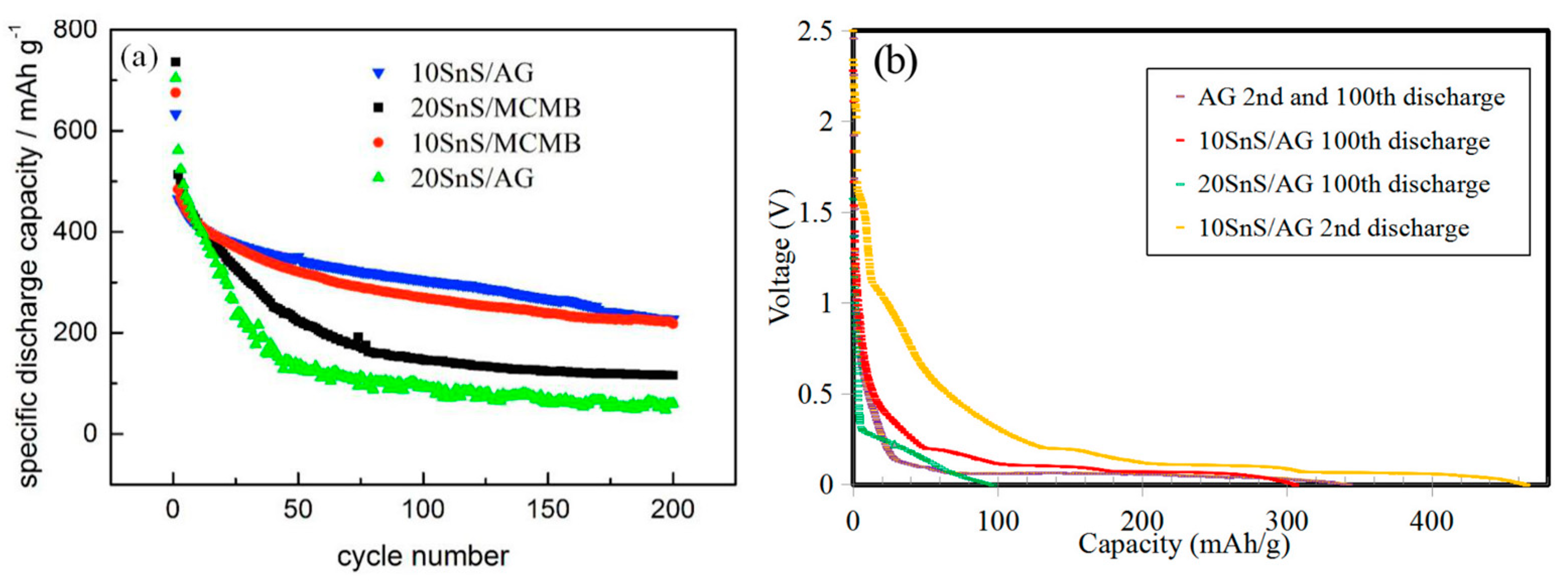
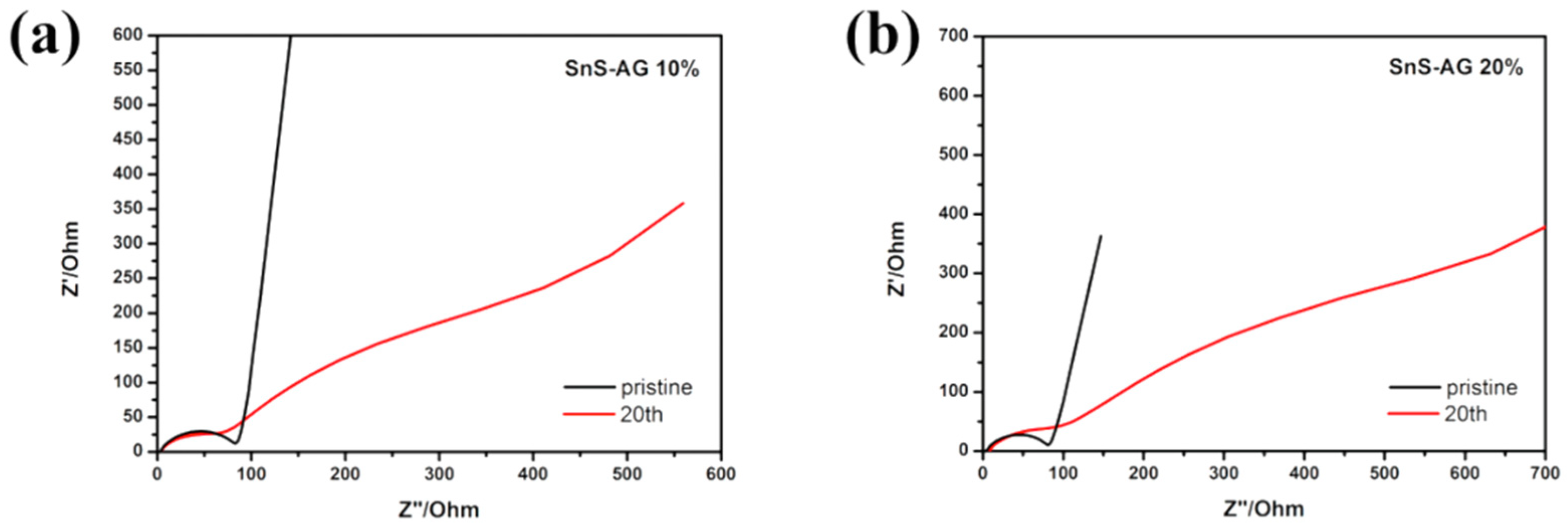
3.2. Electrochemical Performance
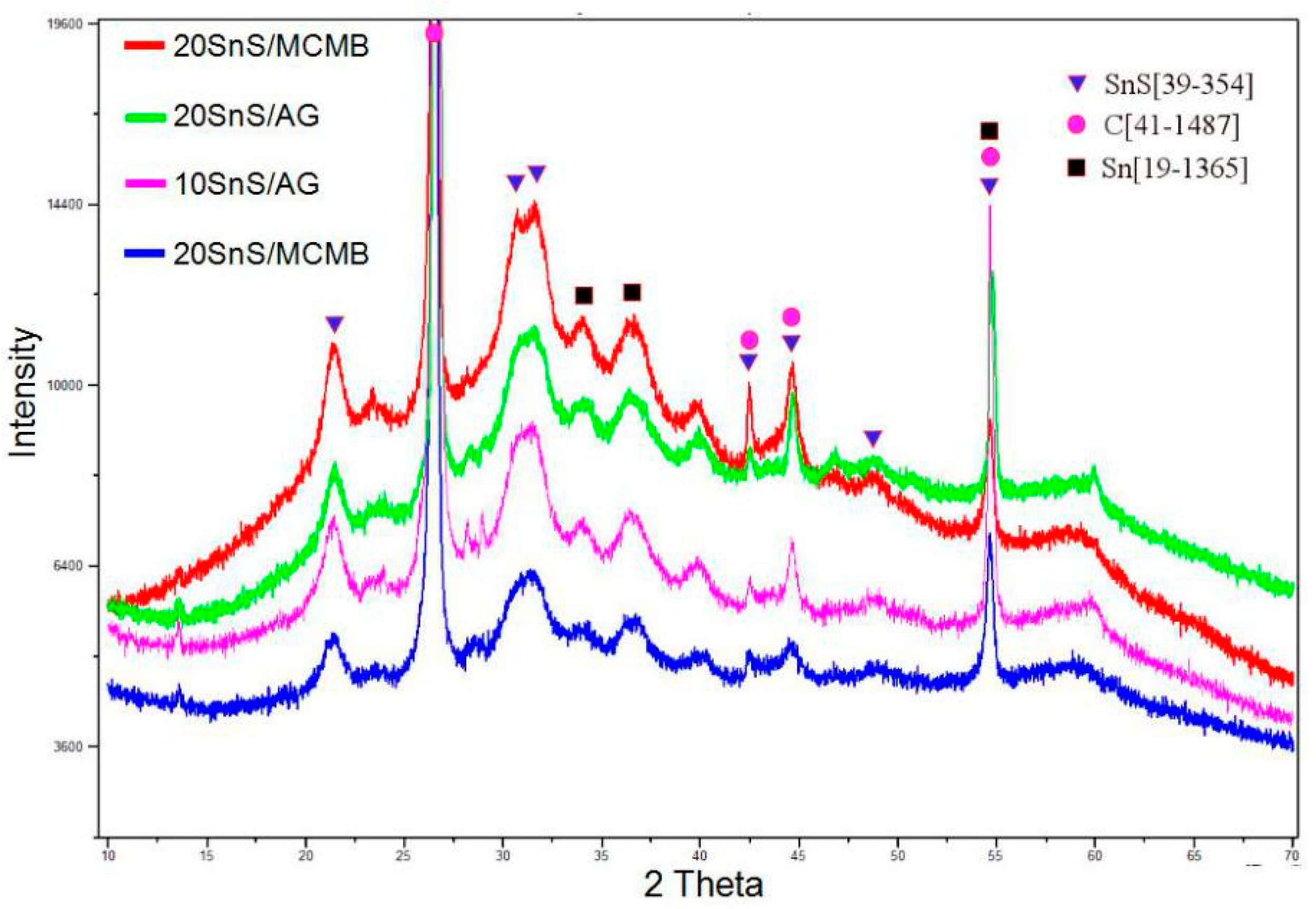
3.3. Microstructure after Cycling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Tian, W.; Li, X. Synthesis and Electrochemistry Properties of Sn-Sb Ultrafine Particles as Anode of Lithium-ion Batteries. J. Alloys Compd. 2007, 439, 350–354. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009–2013. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Ren, J.; Wang, L.; Jiang, C.; Wan, C. Preparation of Sn2Sb alloy encapsulated carbon microsphere anode materials for Li-ion batteries by carbothermal reduction of the oxides. Electrochim. Acta 2006, 52, 1221–1225. [Google Scholar] [CrossRef]
- Li, Y.; Tu, J.P.; Huang, X.H.; Wu, H.M.; Yuan, Y.F. Nanoscale SnS with and without carbon-coating as an anode material for lithium ion batteries. Electrochim. Acta 2006, 52, 1383–1389. [Google Scholar] [CrossRef]
- Li, Y.; Tu, J.P.; Huang, X.H.; Wu, H.M.; Yuan, Y.F. Net-like SnS/carbon nanocomposite film anode material for lithium ion batteries. Electrochem. Commun. 2007, 9, 49–53. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Yang, J.; Winter, M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries? J. Power Sources 1997, 68, 87–90. [Google Scholar] [CrossRef]
- Armstrong, R.W. Crystal Engineering for Mechanical Strength at Nano-Scale Dimensions. Crystals 2017, 7, 315. [Google Scholar] [CrossRef]
- Kumar, G.G.; Reddy, K.; Nahm, K.S.; Angulakshmi, N.; Stephan, A.M. Manuel Stephan, Synthesis and electrochemical properties of SnS as possible anode material for lithium batteries. J. Phys. Chem. Solids 2012, 73, 1187–1190. [Google Scholar] [CrossRef]
- Gou, X.; Chen, J.; Shen, P.W. Synthesis, characterization and application of SnSx (x = 1, 2) nanoparticles. Mat. Chem. Phys. 2005, 93, 557–566. [Google Scholar] [CrossRef]
- Aifantis, K.E.; Haycock, M.; Sanders, P.; Hackney, S.A. Fracture of nanostructured Sn/C anodes during Li-insertion. Mat. Sci. Eng. A 2011, 529, 55–61. [Google Scholar] [CrossRef]
- Cai, W.; Hu, J.; Zhao, T.; Yang, H.; Wang, J.; Xiang, W. Synthesis and characterization of nanoplate-based SnS microflowers via a simple solvothermal process with biomolecule assistance. Adv. Powder Technol. 2012, 23, 850–854. [Google Scholar] [CrossRef]
- Aifantis, K.E.; Huang, T.; Hackney, S.A.; Sarakonsri, T.; Yu, A. Capacity fade in Sn-C nanopowder anodes due to fracture. J. Power Sources 2012, 197, 246–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adpakpang, K.; Sarakonsri, T.; Aifantis, K.E.; Hackney, S.A. Morphological study of SnSb/graphitecomposites influenced by different ratio of Sn:Sb. Rev. Adv. Mater. Sci. 2012, 32, 12–18. [Google Scholar]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Brousse, T.; Lee, S.M.; Pasquereau, L.; Defives, D.; Schleich, D.M. Composite negative electrodes for lithium ion cells. Solid State Ionics 1998, 115, 51–56. [Google Scholar] [CrossRef]
- Denis, Y.W.Y.; Hoster, H.E.; Batabyal, S.K. Bulk antimony sulfide with excellent cycle stability as next-generation anode for lithium-ion batteries. Sci. Rep. 2014, 4, 4562–4567. [Google Scholar]


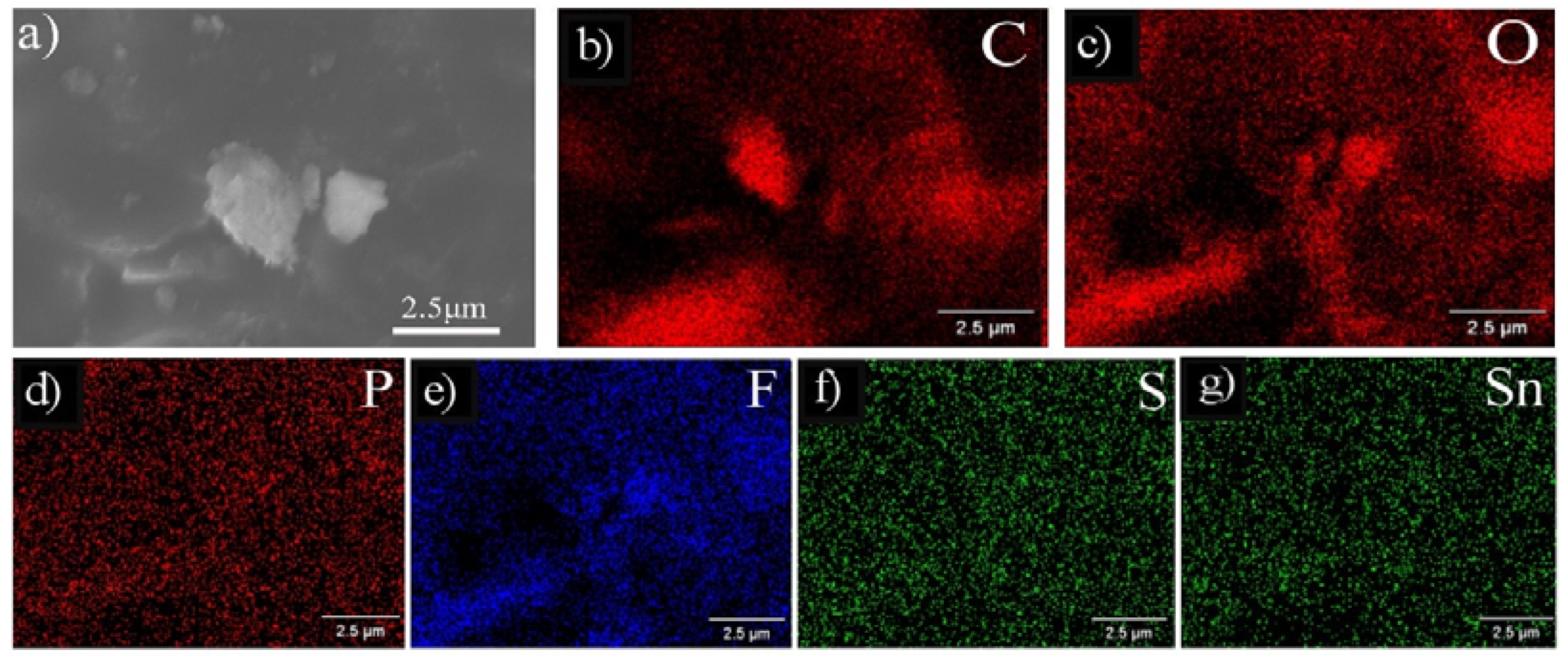
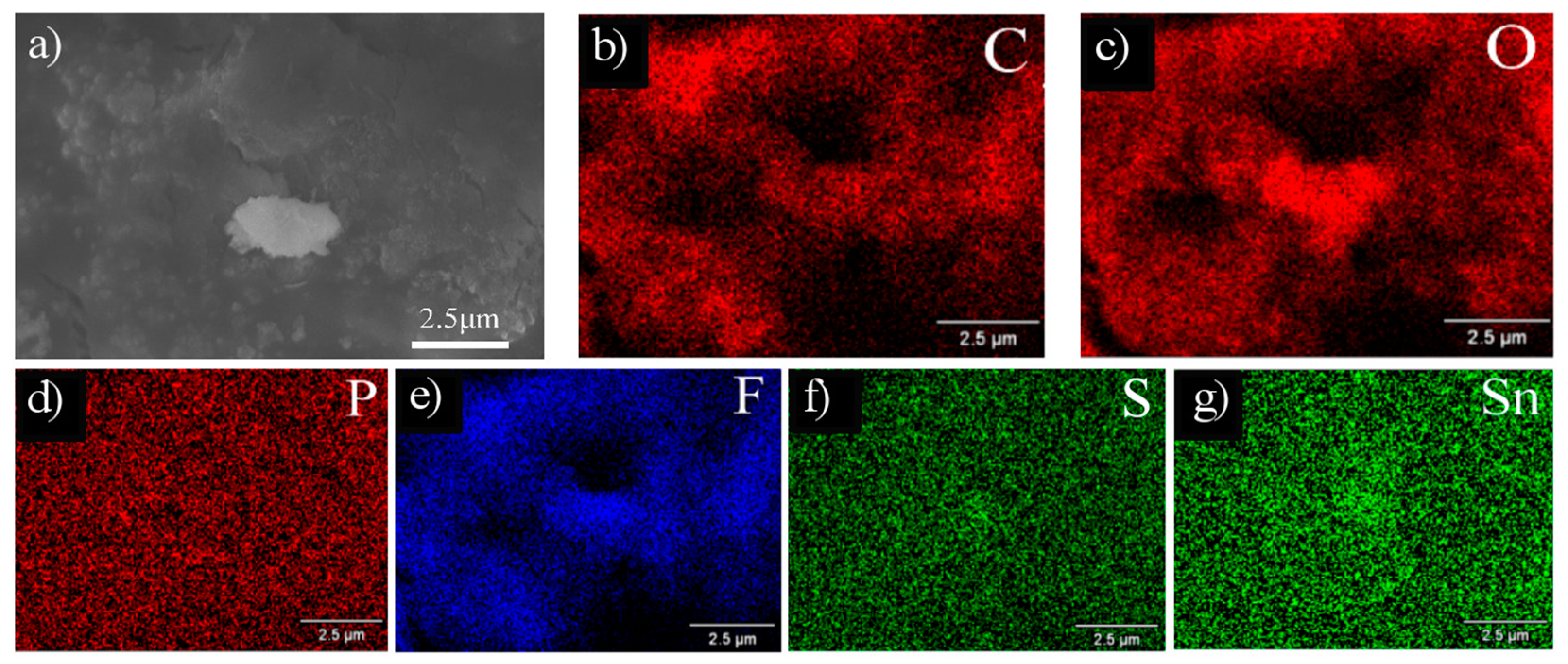
| Sample | Particle Size (nm) | Elements (% atom) | ||
|---|---|---|---|---|
| Sn | C | S | ||
| 10SnS/AG | 254.33 | 3.04 | 95.48 | 1.48 |
| 10SnS/MCMB | 494.35 | 3.5 | 94.17 | 2.34 |
| 20SnS/AG | 411.34 | 3.71 | 92.16 | 4.13 |
| 20SnS/MCMB | 559.91 | 5.34 | 89.09 | 5.57 |
| Sample | Area | % Elements (% atom) | |||||
|---|---|---|---|---|---|---|---|
| Sn | S | C | O | F | P | ||
| 10SnS/AG | Inside particle | 1.25 | 0.66 | 37.79 | 37.73 | 22.07 | 0.50 |
| Adjacent matrix | 1.75 | 1.52 | 45.45 | 29.60 | 19.40 | 2.28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Sarakonsri, T.; Huang, T.; Yu, A.; Aifantis, K. Transformation of SnS Nanocompisites to Sn and S Nanoparticles during Lithiation. Crystals 2021, 11, 145. https://doi.org/10.3390/cryst11020145
Deng H, Sarakonsri T, Huang T, Yu A, Aifantis K. Transformation of SnS Nanocompisites to Sn and S Nanoparticles during Lithiation. Crystals. 2021; 11(2):145. https://doi.org/10.3390/cryst11020145
Chicago/Turabian StyleDeng, Haokun, Thapanee Sarakonsri, Tao Huang, Aishui Yu, and Katerina Aifantis. 2021. "Transformation of SnS Nanocompisites to Sn and S Nanoparticles during Lithiation" Crystals 11, no. 2: 145. https://doi.org/10.3390/cryst11020145
APA StyleDeng, H., Sarakonsri, T., Huang, T., Yu, A., & Aifantis, K. (2021). Transformation of SnS Nanocompisites to Sn and S Nanoparticles during Lithiation. Crystals, 11(2), 145. https://doi.org/10.3390/cryst11020145





