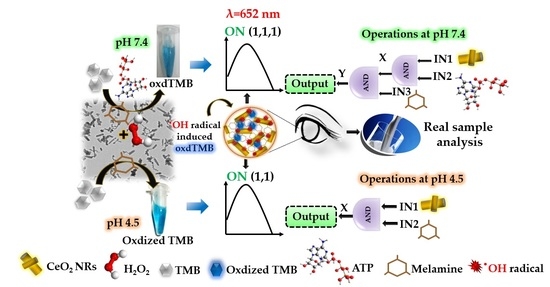
Picomolar-Level Melamine Detection via ATP Regulated CeO2 Nanorods Tunable Peroxidase-Like Nanozyme-Activity-Based Colorimetric Sensor: Logic Gate Implementation and Real Sample Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
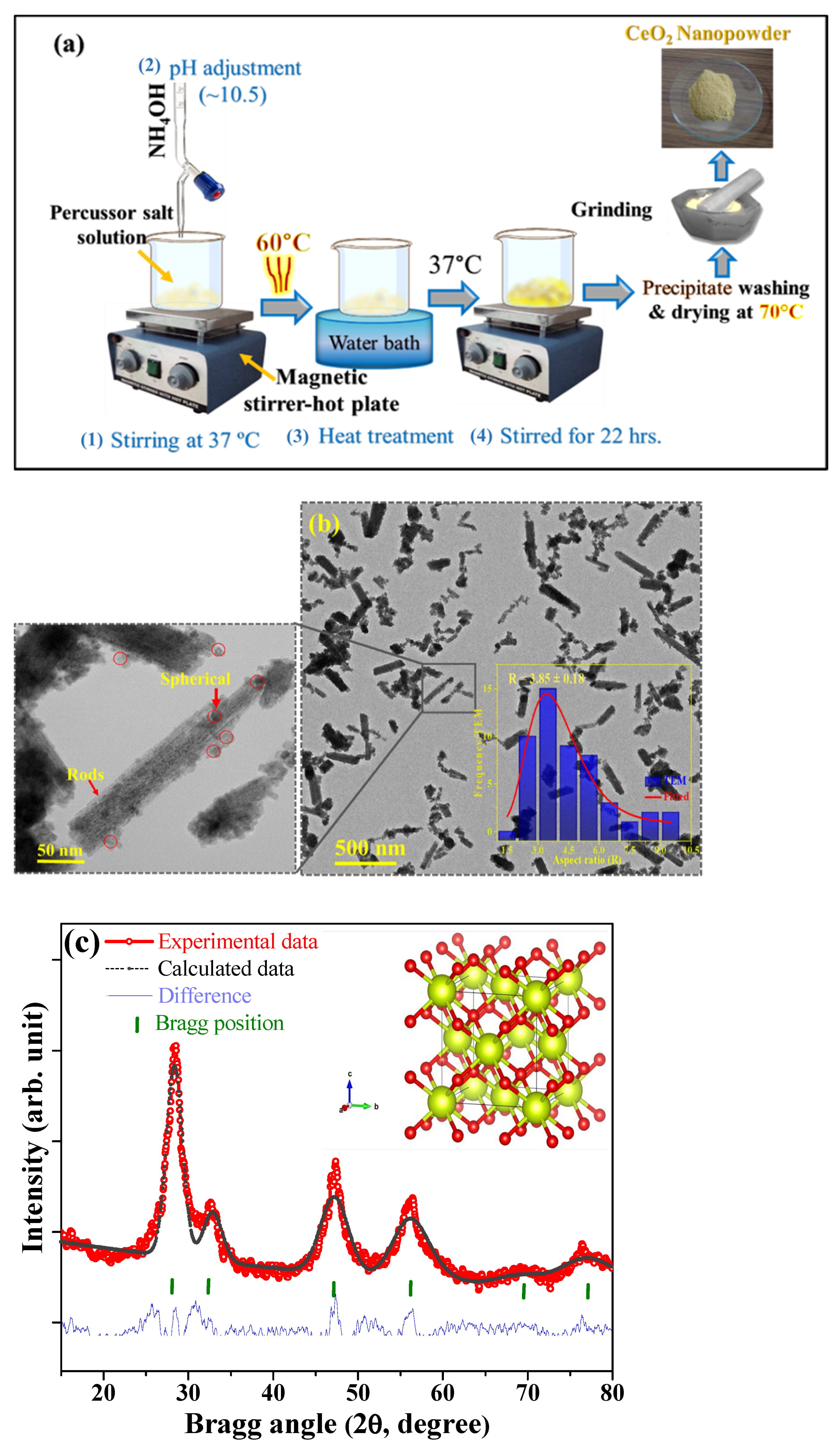
2.2. Preparation of CeO2 Nanozyme
2.3. Instrumentation and Characterization
2.4. Preparation of Buffer
2.5. POD-like Activity and Kinetic Measurement of CeO2 NRs
2.6. Construction of CeO2 NRs POD-like Nanozyme-Based Logic Operation
2.7. Experimental Procedure for Visual Colorimetric Detection of MEL
2.8. Feasibility Study in Real Samples
3. Results and Discussion
3.1. Characterization of CeO2 Nanozyme
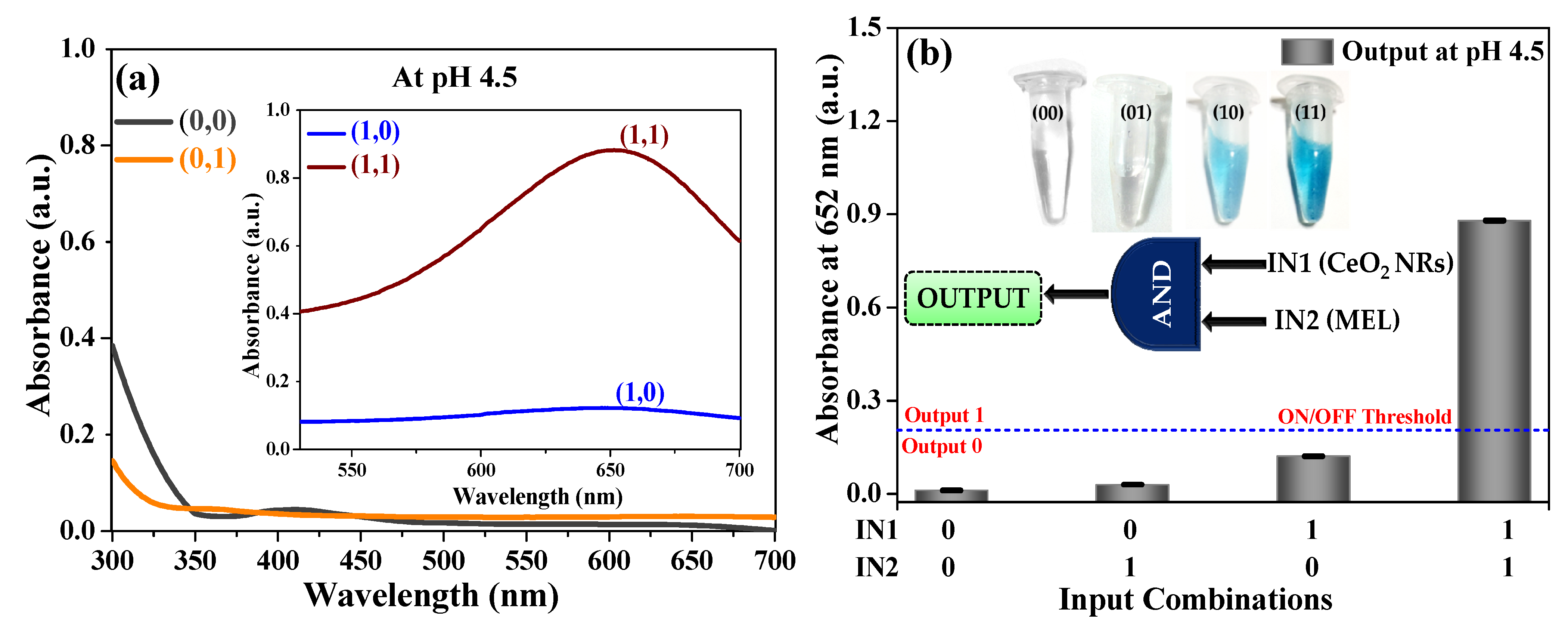
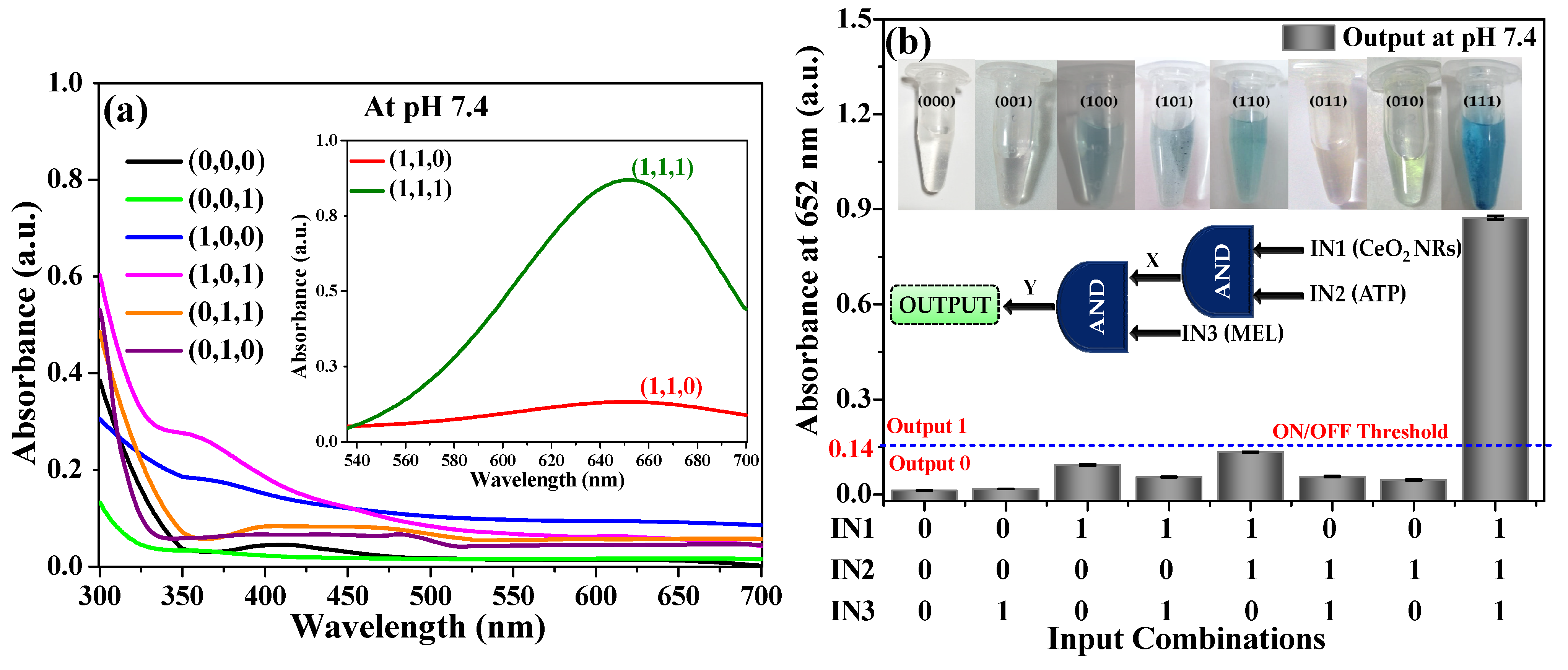
3.2. AND Logic Operation for MEL Sensing
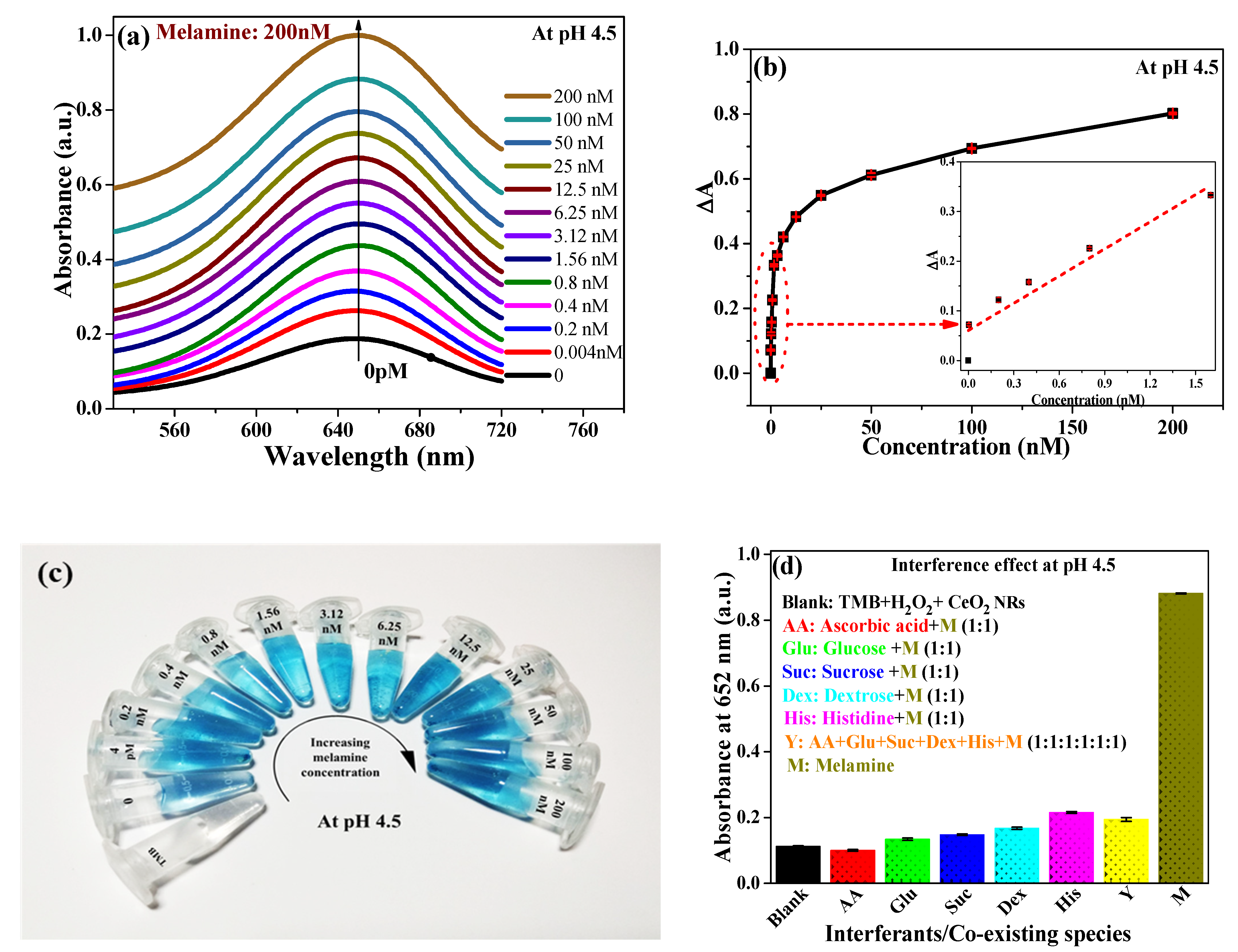
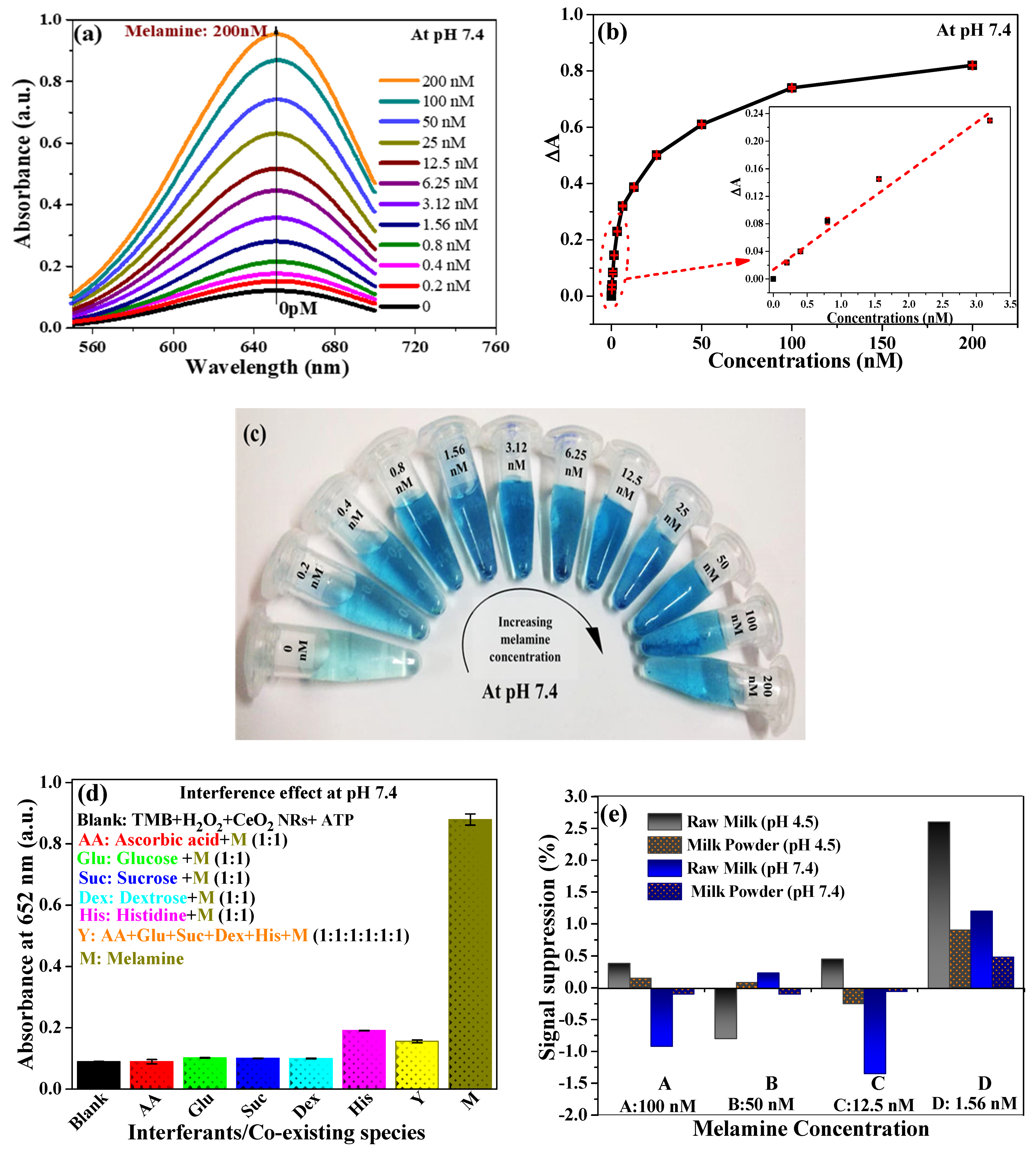
3.3. Analytical Performance of AND Logic Gate-Based Colorimetric Detection of MEL
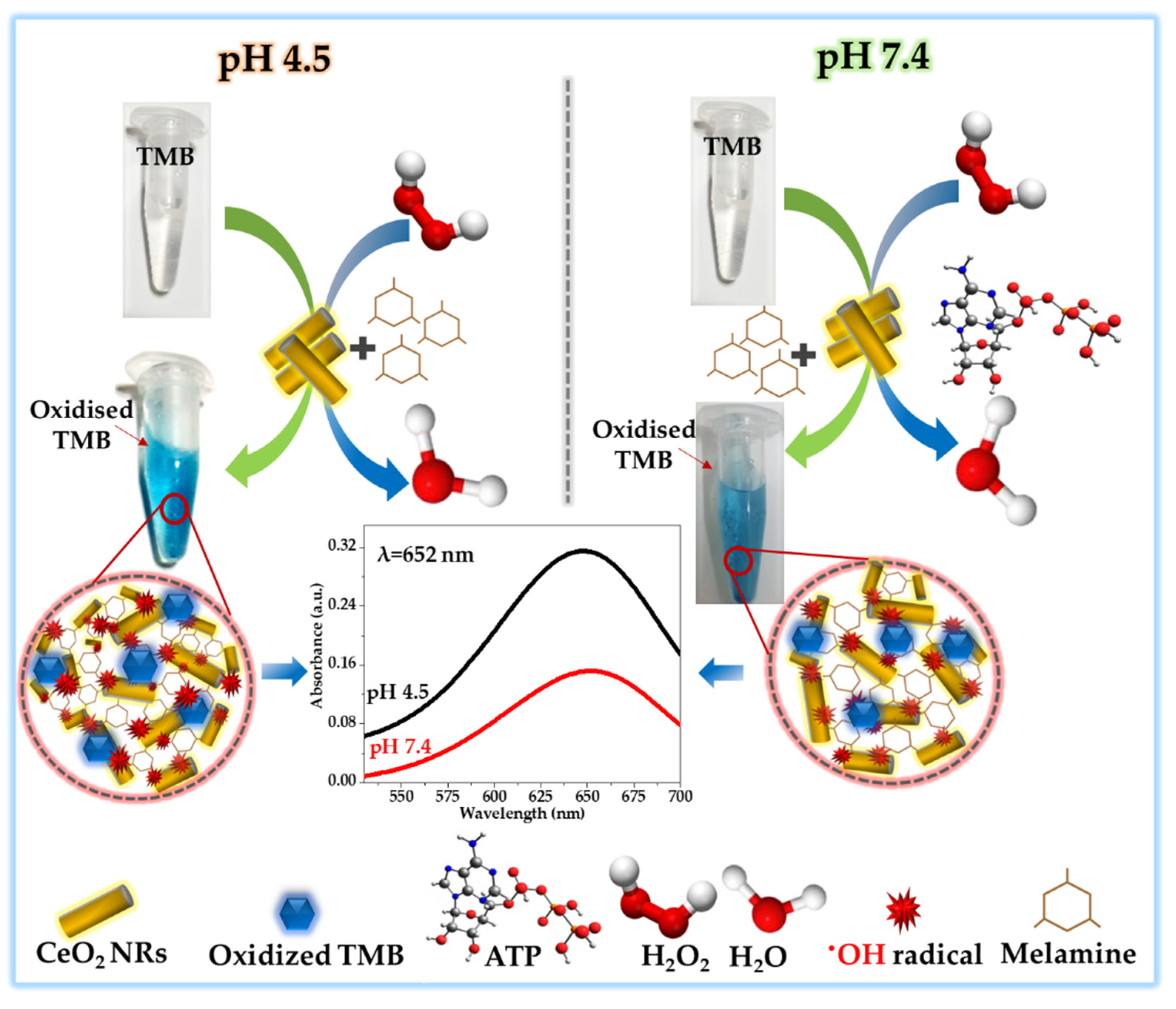
3.4. Implication of CeO2 NRs POD-Like Activity in MEL Sensing
3.5. Real Sample Analysis and Validation of the Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S. Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Z.; Li, T.-T.; Chen, W.; Song, Y.-Y. Co4N Nanowires: Noble-Metal-Free Peroxidase Mimetic with Excellent Salt- and Temperature-Resistant Abilities. ACS Appl. Mater. Interfaces 2017, 9, 29881–29888. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Feng, Y.-B.; Hong, L.; Chen, Q.-Y.; Wu, L.; Lin, X.; Xia, X.-H. Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 2012, 137, 1706–1712. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Catalytically Active Nanomaterials: A Promising Candidate for Artificial Enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Frasco, T.; Andreescu, D.; Andreescu, S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Analyst 2012, 138, 249–262. [Google Scholar] [CrossRef]
- Bülbül, G.; Hayat, A.; Andreescu, S. ssDNA-Functionalized Nanoceria: A Redox-Active Aptaswitch for Biomolecular Recognition. Adv. Healthc. Mater. 2016, 5, 822–828. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Xue, S.-F.; Deng, J.; Zhang, M.; Shi, G.; Zhou, T. Polyacrylic acid-coated cerium oxide nanoparticles: An oxidase mimic applied for colorimetric assay to organophosphorus pesticides. Biosens. Bioelectron. 2016, 85, 457–463. [Google Scholar] [CrossRef]
- Hayat, A.; Andreescu, D.; Bülbül, G.; Andreescu, S. Redox reactivity of cerium oxide nanoparticles against dopamine. J. Colloid Interface Sci. 2014, 418, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yin, W.; Ni, G.; Peng, J. Hydrogen-bonding-induced colorimetric detection of melamine based on the peroxidase activity of gelatin-coated cerium oxide nanospheres. Anal. Methods 2018, 10, 841–847. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Wang, W.; Gunasekaran, S. Nanozymes-based biosensors for food quality and safety. TrAC Trends Anal. Chem. 2020, 126, 115841. [Google Scholar] [CrossRef]
- Xin, H.; Stone, R. TAINTED MILK SCANDAL: Chinese Probe Unmasks High-Tech Adulteration With Melamine. Science 2008, 322, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhao, H.; He, Y.; Li, X.; Zeng, L.; Ding, N.; Wang, J.; Yang, J.; Wang, G. Hydrogen-bonding-induced colorimetric detection of melamine by nonaggregation-based Au-NPs as a probe. Biosens. Bioelectron. 2010, 25, 2680–2685. [Google Scholar] [CrossRef]
- Brown, C.A.; Jeong, K.-S.; Poppenga, R.H.; Puschner, B.; Miller, D.M.; Ellis, A.E.; Kang, K.-I.; Sum, S.; Cistola, A.M.; Brown, S.A. Outbreaks of Renal Failure Associated with Melamine and Cyanuric Acid in Dogs and Cats in 2004 and 2007. J. Veter. Diagn. Investig. 2007, 19, 525–531. [Google Scholar] [CrossRef]
- Xu, X.-M.; Ren, Y.-P.; Zhu, Y.; Cai, Z.-X.; Han, J.-L.; Huang, B.-F.; Zhu, Y. Direct determination of melamine in dairy products by gas chromatography/mass spectrometry with coupled column separation. Anal. Chim. Acta 2009, 650, 39–43. [Google Scholar] [CrossRef]
- Hau, A.K.-C.; Kwan, T.H.; Li, P.K.-T. Melamine Toxicity and the Kidney. J. Am. Soc. Nephrol. 2009, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Goscinny, S.; Hanot, V.; Halbardier, J.-F.; Michelet, J.-Y.; Van Loco, J. Rapid analysis of melamine residue in milk, milk products, bakery goods and flour by ultra-performance liquid chromatography/tandem mass spectrometry: From food crisis to accreditation. Food Control. 2011, 22, 226–230. [Google Scholar] [CrossRef]
- Lei, H.; Shen, Y.; Song, L.; Yang, J.; Chevallier, O.P.; Haughey, S.A.; Wang, H.; Sun, Y.; Elliott, C.T. Hapten synthesis and antibody production for the development of a melamine immunoassay. Anal. Chim. Acta 2010, 665, 84–90. [Google Scholar] [CrossRef]
- Zhao, N.; Li, H.; Tian, C.; Xie, Y.; Feng, Z.; Wang, Z.; Yan, X.; Wang, W.; Yu, H. Bioscaffold arrays decorated with Ag nanoparticles as a SERS substrate for direct detection of melamine in infant formula. RSC Adv. 2019, 9, 21771–21776. [Google Scholar] [CrossRef]
- Nicoli, F.; Paltrinieri, E.; Bakić, M.T.; Baroncini, M.; Silvi, S.; Credi, A. Binary logic operations with artificial molecular machines. Coord. Chem. Rev. 2021, 428, 213589. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, Y.; Wen, X.; Li, Y.; Cao, C.; Xiong, Q. A coordination and ligand replacement based three-input colorimetric logic gate sensing platform for melamine, mercury ions, and cysteine. RSC Adv. 2015, 5, 59106–59113. [Google Scholar] [CrossRef]
- Rajput, J.K. Bio-polyphenols promoted green synthesis of silver nanoparticles for facile and ultra-sensitive colorimetric detection of melamine in milk. Biosens. Bioelectron. 2018, 120, 153–159. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Ha, W.; Shi, Y.-P. Sensitive colorimetric detection of melamine in processed raw milk using asymmetrically PEGylated gold nanoparticles. Talanta 2019, 194, 475–484. [Google Scholar] [CrossRef]
- Han, X.; Qin, Z.; Zhao, M.; Song, J.; Qu, F.; Qu, F.; Kong, R.-M. Convenient and sensitive colorimetric detection of melamine in dairy products based on Cu(ii)-H2O2-3,3′,5,5′-tetramethylbenzidine system. RSC Adv. 2018, 8, 34877–34882. [Google Scholar] [CrossRef]
- Skinner, C.G.; Thomas, J.D.; Osterloh, J.D. Melamine Toxicity. J. Med. Toxicol. 2010, 6, 50–55. [Google Scholar] [CrossRef]
- Dorne, J.L.; Doerge, D.R.; Vandenbroeck, M.; Fink-Gremmels, J.; Mennes, W.; Knutsen, H.K.; Vernazza, F.; Castle, L.; Edler, L.; Benford, D. Recent advances in the risk assessment of melamine and cyanuric acid in animal feed. Toxicol. Appl. Pharmacol. 2013, 270, 218–229. [Google Scholar] [CrossRef]
- Chishti, B.; Fouad, H.; Seo, H.K.; Alothman, O.Y.; Ansari, Z.A.; Ansari, S.A. ATP fosters the tuning of nanostructured CeO2 peroxidase-like activity for promising antibacterial performance. New J. Chem. 2020, 44, 11291–11303. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L.; Scardi, P.; Maistrelli, P. LSI- a computer program for simultaneous refinement of material structure and microstructure. J. Appl. Crystallogr. 1992, 25, 459–462. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Huebner, W.; Anderson, H.U. Processing of Nanometer-Scale CeO2 Particles. Chem. Mater. 2003, 15, 378–382. [Google Scholar] [CrossRef]
- Kosack, C.S.; Page, A.-L.; Klatser, P.R. A guide to aid the selection of diagnostic tests. Bull. World Health Organ. 2017, 95, 639–645. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Concentrations of Melamine (nM) | Recovery b (%) | |||
|---|---|---|---|---|---|
| Original Amount | Concentration Spiked | Amount Found a | |||
| At pH 4.5 | Raw Milk | 0 | 1.56 | 1.4 ± 0.24 | 90 |
| 0 | 12.5 | 12.76 ± 0.24 | 102 | ||
| 0 | 50 | 50.12 ± 0.87 | 100 | ||
| 0 | 100 | 105.67 ± 2.68 | 105 | ||
| Milk Powder | 0 | 1.56 | 1.51 ± 0.41 | 97 | |
| 0 | 12.5 | 12.92 ± 0.41 | 103 | ||
| 0 | 50 | 50.05 ± 0.47 | 100 | ||
| 0 | 100 | 103.43 ± 3.51 | 103 | ||
| At pH 7.4 | Raw Milk | 0 | 1.56 | 1.61 ± 0.14 | 103 |
| 0 | 12.5 | 12.15 ± 0.14 | 97 | ||
| 0 | 50 | 50.29 ± 2.21 | 101 | ||
| 0 | 100 | 99.63 ± 0.14 | 97 | ||
| Milk Powder | 0 | 1.56 | 1.58 ± 0.12 | 101 | |
| 0 | 12.5 | 12.48 ± 0.12 | 100 | ||
| 0 | 50 | 50.13 ± 0.21 | 101 | ||
| 0 | 100 | 100.1 ± 0.12 | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chishti, B.; Ansari, Z.A.; Fouad, H.; Alothman, O.Y.; Hashem, M.; Ansari, S.G. Picomolar-Level Melamine Detection via ATP Regulated CeO2 Nanorods Tunable Peroxidase-Like Nanozyme-Activity-Based Colorimetric Sensor: Logic Gate Implementation and Real Sample Analysis. Crystals 2021, 11, 178. https://doi.org/10.3390/cryst11020178
Chishti B, Ansari ZA, Fouad H, Alothman OY, Hashem M, Ansari SG. Picomolar-Level Melamine Detection via ATP Regulated CeO2 Nanorods Tunable Peroxidase-Like Nanozyme-Activity-Based Colorimetric Sensor: Logic Gate Implementation and Real Sample Analysis. Crystals. 2021; 11(2):178. https://doi.org/10.3390/cryst11020178
Chicago/Turabian StyleChishti, Benazir, Zubaida A. Ansari, Hassan Fouad, Othman Y. Alothman, Mohamed Hashem, and Shafeeque G. Ansari. 2021. "Picomolar-Level Melamine Detection via ATP Regulated CeO2 Nanorods Tunable Peroxidase-Like Nanozyme-Activity-Based Colorimetric Sensor: Logic Gate Implementation and Real Sample Analysis" Crystals 11, no. 2: 178. https://doi.org/10.3390/cryst11020178
APA StyleChishti, B., Ansari, Z. A., Fouad, H., Alothman, O. Y., Hashem, M., & Ansari, S. G. (2021). Picomolar-Level Melamine Detection via ATP Regulated CeO2 Nanorods Tunable Peroxidase-Like Nanozyme-Activity-Based Colorimetric Sensor: Logic Gate Implementation and Real Sample Analysis. Crystals, 11(2), 178. https://doi.org/10.3390/cryst11020178