Structural Characterization, Magnetic and Luminescent Properties of Praseodymium(III)-4,4,4-Trifluoro-1-(2-Naphthyl)Butane-1,3-Dionato(1-) Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis of the Complexes
2.3. Single Crystal X-ray Diffraction Analysis
2.4. Powder X-ray Diffraction
2.5. Magnetic Measurements
2.6. Luminescence Measurements
3. Results and Discussion
3.1. Synthesis and Spectra
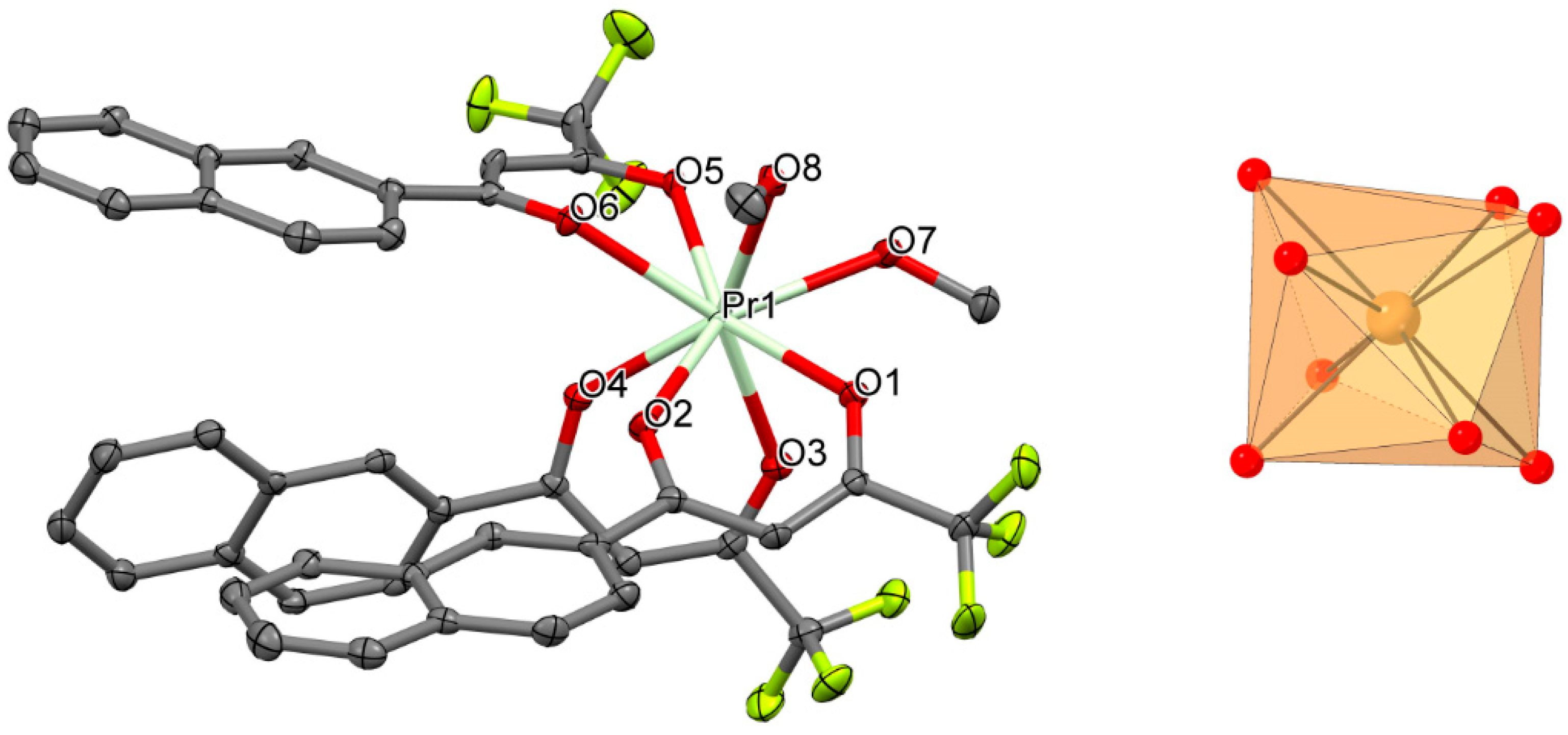
3.2. Description of the Crystal Structures (1–4)
3.3. Magnetic Properties
3.4. Luminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single-molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Moore, E.G.; Xu, J.; Jocher, C.J.; Werner, E.J.; Raymond, K.N. Cymothoe sangaris: An extremely stable and highly luminescent 1,2-hydroxy-pyridinonate chelate of Eu(III). J. Am. Chem. Soc. 2006, 128, 10648–10649. [Google Scholar] [CrossRef]
- Quici, S.; Cavazzini, M.; Marzanni, G.; Accorsi, G.; Armaroli, N.; Ventura, B.; Barigelletti, F. Visible and near-infrared intense luminescence from water-soluble lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] complexes. Inorg. Chem. 2005, 44, 529–537. [Google Scholar] [CrossRef]
- Petoud, S.; Cohen, S.M.; Bünzli, J.-C.G.; Raymond, K.N. Stable lanthanide luminescence agents highly emissive in aqueous solution: Multidentate 2-hydroxyisophthalamide complexes of Sm3+, Eu3+, Tb3+, Dy3+. J. Am. Chem. Soc. 2003, 126, 13324–13325. [Google Scholar] [CrossRef]
- Bakker, B.H.; Goes, M.; Hoebe, N.; Van Ramesdonk, H.J.; Verhoeven, J.W.; Werts, M.H.V.; Hofstraat, J.W. Luminescent materials and devices: Lanthanide azatriphenylene complexes and electroluminescent charge transfer systems. Coord. Chem. Rev. 2000, 208, 3–16. [Google Scholar] [CrossRef]
- Mara, D.; Artizzu, F.; Laforce, B.; Vincze, L.; Van Hecke, K.; Van Deun, R.; Kaczmarek, A.M. Novel tetrakis lanthanide β-diketonate complexes: Structural study, luminescence properties and temperature sensing. J. Lumin. 2019, 213, 343–355. [Google Scholar] [CrossRef]
- Dai, P.-P.; Li, C.; Zhang, X.-T.; Xu, J.; Chen, X.; Wang, X.-L.; Jia, Y.; Wang, X.; Liu, Y.-C. A single Eu2+-activated high-color-rendering oxychloride white-light phosphor for white-light-emitting diodes. Light Sci. Appl. 2016, 5, e16024. [Google Scholar] [CrossRef]
- Dai, P.; Lee, S.-P.; Chan, T.-S.; Huang, C.-H.; Chiang, Y.-W.; Chen, T.-M. Sr3Ce(PO4)3: Eu2+: A broadband yellow-emitting phosphor for near ultraviolet-pumped white light-emitting devices. J. Mater. Chem. 2016, C4, 1170–1177. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, D.; Zhou, Y.; Wan, Z.; Ding, M.; Bai, W.; Ji, Z. New Eu3+-activated perovskite La0.5Na0.5TiO3 phosphors in glass for warm white light emitting diodes. Dalton Trans. 2016, 45, 4762–4770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Fang, M.; Huang, Z.; Liu, Y.G.; Chen, K.; Min, X.; Mao, Y.; Wang, M. Photoluminescence properties of Li2Mg2 (WO4)3: Eu3+ red phosphor with high color purity for white LEDs applications. J. Lumin. 2016, 172, 180–184. [Google Scholar] [CrossRef]
- Li, C.; Dai, J.; Huang, J.; Deng, D.; Yu, H.; Wang, L.; Ma, Y.; Hua, Y.; Xu, S. Crystal structure, luminescent properties and white light emitting diode application of Ba3GdNa(PO4)3F: Eu2+ single-phase white light-emitting phosphor. Ceram. Int. 2016, 42, 6891–6898. [Google Scholar] [CrossRef]
- Pavitra, E.; Raju, G.S.R.; Ko, Y.H.; Yu, J.S. A novel strategy for controllable emissions from Eu3+ or Sm3+ ions co-doped SrY2O4: Tb3+ phosphors. Phys. Chem. Chem. Phys. 2012, 14, 11296–11307. [Google Scholar] [CrossRef]
- Lin, C.C.; Meijerink, A.; Liu, R.-S. Critical red components for next-generation white LEDs. J. Phys. Chem. Lett. 2016, 7, 495–503. [Google Scholar] [CrossRef]
- Dar, W.A.; Ahmed, Z.; Iftikhar, K. Cool white light emission from the yellow and blue emission bands of the Dy(III) complex under UV excitation. J. Photoch. Photobio. A 2018, 356, 502–511. [Google Scholar] [CrossRef]
- de Bettencourt-Dias, A. Lanthanide-based emitting materials in light emitting diodes. Dalton Trans. 2007, 22, 2229–2241. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millan, A.; Carlos, L.D.; Bünzli, J.C.G.; Pecharsky, V.K. (Eds.) Handbook of the Physics and Chemistry of Rare Earths, Lanthanides in Luminescent Thermometry; Elsevier B.V.: Amsterdan, The Netherlands, 2016; Volume 49, Chapter 281; pp. 339–427. [Google Scholar]
- An, R.; Zhao, H.; Hu, H.-M.; Wang, X.; Yang, M.-L.; Xue, G. Synthesis, structure, white light emission, and temperature recognition properties of Eu/Tb mixed coordination polymer. Inorg. Chem. 2016, 55, 871–876. [Google Scholar] [CrossRef]
- Miyata, K.; Konno, Y.; Nakanishi, T.; Kobayashi, A.; Kato, M.; Fushimi, K.; Hasegawa, Y. Chameleon luminophore for sensing temperatures: Control of metal-to-metal and energy back transfer in lanthanide coordination polymers. Angew. Chem. Int. Ed. 2013, 52, 6413–6416. [Google Scholar] [CrossRef]
- Oshishi, Y.; Kanamori, T.; Kitagawa, T.; Takashashi, S.; Snitzer, E.; Sigel, G.H. Pr3+-doped fluoride fiber amplifier operating at 1.31 μm. Opt. Lett. 1991, 16, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Slooff, L.H.; Polman, A.; Wolbers, M.P.O.; van Veggel, F.; Reinhoudt, D.N.; Hofstraat, J.W. Optical properties of erbium-doped organic polydentate cage complexes. J. Appl. Phys. 1998, 83, 497–503. [Google Scholar] [CrossRef]
- Surender, E.M.; Comby, S.; Martyn, S.; Cavanagh, B.; Lee, C.T.; Brougham, D.F.; Gunnlaugsson, T. Cyclen lanthanide-based micellar structures for application as luminescent [Eu(III)] and magnetic [Gd(III)] resonance imaging (MRI) contrast agents. Chem. Commun. 2016, 52, 1085–10861. [Google Scholar] [CrossRef] [Green Version]
- Nchimi-Nono, K.; Wenger, K.D.; Linden, S.; Lecointre, A.; Ehret-Sabatier, L.; Shakir, S.; Hildebrandt, N.; Charbonniere, L.J. Activated phosphonated trifunctional chelates for highly sensitive lanthanide-based FRET immunoassays applied to total prostate specific antigen detection. Org. Biomol. Chem. 2013, 11, 6493–6501. [Google Scholar] [CrossRef]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. In vitro cytotoxicity activity of novel Schiff base ligand–lanthanide complexes. Sci. Rep. 2018, 8, 3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrza, R.; Nowak, M.; Skroban’ska, M.; Tolin’ska, A.; Zabiszak, M.; Gabryel, M.; Marciniak, Ł.; Kaczmarek, M.T. DNA as a target for lanthanide(III) complexes influence. Coord. Chem. Rev. 2019, 382, 145–159. [Google Scholar] [CrossRef]
- Singh, S.K.; Gupta, T.; Rajaraman, G. Magnetic anisotropy and mechanism of magnetic relaxation in Er(III) single-ion magnets. Inorg. Chem. 2014, 53, 10835–10845. [Google Scholar] [CrossRef]
- Takamatsu, S.; Ishikawa, T.; Koshihara, S.; Ishikawa, N. Significant increase of the barrier energy for magnetization reversal of a single-4f-ionic single-molecule magnet by a longitudinal contraction of the coordination space. Inorg. Chem. 2007, 46, 7250–7252. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Wernsdorfer, W.G. Nuclear spin driven quantum tunneling of magnetization in a new lanthanide single-molecule magnet: Bis(phthalocyaninato)holmium anion. J. Am. Chem. Soc. 2005, 127, 3650–3651. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Benelli, C.; Caneschi, A.; Guillou, O.; Pardi, L. Synthesis, crystal structure, and magnetic properties of tetranuclear complexes containing exchange-coupled dilanthanide-dicopper (lanthanide = gadolinium, dysprosium) species. Inorg. Chem. 1990, 29, 1750–1755. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, X.-L.; Liuac, S.; Tang, J. External stimuli modulate the magnetic relaxation of lanthanide single-molecule magnets. Inorg. Chem. Front. 2020, 7, 3315–3326. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining new light on multifunctional lanthanide single-molecule magnets. Angew. Chem. 2019, 60, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, B.; Speed, S.; Maury, O.; Font-Bardía, M.; Vicente, R. Homodinuclear lanthanide 9-anthracenecarboxylate complexes: Field induced SMM and NIR-luminescence. Polyhedron 2019, 169, 187–194. [Google Scholar] [CrossRef]
- Dey, A.; Kalita, P.; Chandrasekhar, V. Lanthanide(III)-based single-ion magnets. ACS Omega 2018, 3, 9462–9475. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, M.; Tang, J. Recent Developments in lanthanide single-molecule magnets. Chem. Asian J. 2017, 12, 2772–2779. [Google Scholar] [CrossRef]
- Layfield, R.A.; Murugesu, M. (Eds.) Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2015. [Google Scholar]
- Yao, X.; An, G.; Li, Y.; Yan, P.; Li, W.; Li, G. Effect of nuclearity and symmetry on the single-molecule magnets behavior of seven-coordinated β-diketonate Dy(III) complexes. J. Solid State Chem. 2019, 274, 295–302. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Tian, L.; Liu, Z.Y.; Wang, X.G. Experimental and theoretical interpretation of the magnetic behavior of two Dy(III) single-ion magnets constructed through β-diketonate ligands with different substituent groups (–Cl/ OCH3). RSC Adv. 2015, 5, 74864–74873. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Wang, C.; Xue, S.; Lin, S.-Y.; Tang, J. Equatorially coordinated lanthanide single ion magnets. J. Am. Chem. Soc. 2014, 136, 4484–4487. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.Q.; Zhu, D.-B. Field-induced single-ion magnets based on enantiopure chiral β-diketonate ligands. Inorg. Chem. 2013, 52, 8933–8940. [Google Scholar] [CrossRef]
- Rizkalla, E.N. Systematics of lanthanide coordination. Radiochim. Acta 1993, 61, 181–188. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ganaie, A.B.; Iftikhar, K. Synthesis and 4f-4f absorption studies of tris(acetylacetonato) praseodymium(III) and holmium(III) complexes with imidazole and pyrazole in non-aqueous solvents. Structure elucidation by sparkle/PM7. J. Mol. Struct. 2019, 1198, 126826. [Google Scholar] [CrossRef]
- Wang, W.-M.; Liu, S.-Y.; Xu, M.; Bai, L.; Wang, H.-Q.; Wen, X.; Zhao, X.-Y.; Qiao, H.; Wu, Z.-L. Structures and magnetic properties of phenoxo-O-bridged dinuclear lanthanide(III) compounds: Single-molecule magnet behaviour and magnetic refrigeration. Polyhedron 2018, 145, 114–119. [Google Scholar] [CrossRef]
- Chen, G.-J.; Zhou, Y.; Jin, G.-X.; Dong, Y.-B. [Dy(acac)3(dppn)]·C2H5OH: Construction of a single-ion magnet based on the square-antiprism dysprosium(III) ion. Dalton Trans. 2014, 43, 16659–16665. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Ilmi, R.; Iftikhar, K. Hypersensitivity in the 4f–4f absorption spectra of tris(acetylacetonato)neodymium(III) complexes with imidazole and pyrazole in non-aqueous solutions. Effect of environment on hypersensitive transitions. J. Lumin. 2012, 132, 51–60. [Google Scholar] [CrossRef]
- Mautner, F.A.; Bierbaumer, F.; Gyurkac, M.; Fischer, R.C.; Torvisco, A.; Massoud, S.S.; Vicente, R. Synthesis and characterization of lanthanum(III) complexes containing 4,4,4-trifluoro-1-(2-naphthalen-yl)-butane-1,3-dionate. Polyhedron 2020, 179, 114384. [Google Scholar] [CrossRef]
- Ansari, A.A.; Hussain, H.A.; Iftikhar, K. Optical absorption spectroscopic studies on holmium(III) complexes with β-diketone and heterocyclic amines. The environment effect on 4f–4f hypersensitive transitions. Spectrochim. Acta Part A 2007, 68, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Ahmed, Z.; Iftikhar, K. Nuclear magnetic resonance and optical absorption spectroscopic studies on paramagnetic praseodymium(III) complexes with β-diketone and heterocyclic amines. Spectrochim. Acta Part A 2007, 68, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bruker APEX, SAINT v. 8.37A; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A Short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J.J. Mercury: Visualization and analysis of crystal structures. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Chem. Soc. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Cirera, J.; Alvarez, S. Stereospinomers of pentacoordinate iron porphyrin complexes: The case of the [Fe(porphyrinato)(CN)]− anions. Dalton. Trans. 2013, 42, 7002–7008. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Fu, L.; Deng, R.; Zhou, L.; Li, H.; Liu, F.; Fu, H. Synthesis, structure and luminescent properties of a new praseodymium(ш) complex with β-diketone. Inorg. Chem. Commun. 2003, 6, 852–854. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Li, P.; Ma, J.; Zheng, W. 1,2,4-Diazaphospholide complexes of lanthanum(III), cerium(III), neodymium(III), praseodymium(III), and samarium(III): Synthesis, X-ray structural characterization, and magnetic susceptibility studies. Dalton Trans. 2016, 45, 11172–11181. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.C. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Voloshin, A.I.; Shavaleev, N.M.; Kazakov, V.P. Luminescence of praseodymium(III) chelates from two excited states (3P0 and 1D2) and its dependence on ligand triplet state energy. J. Lumin. 2001, 93, 199–204. [Google Scholar] [CrossRef]
- Sveshnikova, E.B.; Timofeev, N.T. Disruption of cascading of nonradiative transitions in the Pr3+ ion. Opt. Spektrosk. 1980, 48, 503–509. [Google Scholar]
- Kazakov, V.P.; Voloshin, A.I.; Shavaleev, N.M. Chemiluminescence in visible and infrared spectral regions and quantum chain reactions upon thermal and photochemical decomposition of adamantylideneadamantane-1,2-dioxetane in the presence of chelates Pr(dpm)3 and Pr(fod)3. J. Photo Chem. Photobiol. A Chem. 1998, 119, 177–186. [Google Scholar] [CrossRef]
- Voloshin, A.I.; Shavaleev, N.M.; Kazakov, V.P. Chemiluminescence of praseodymium(III), neodymium (III) and ytterbium(III) β-diketonates in solution excited from 1,2-dioxetane decomposition and singlet–singlet energy transfer from ketone to rare-earth β-diketonates. J. Lumin. 2000, 91, 49–58. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels of the trivalent Lanthanide aquo ions. III. Tb3+. J. Chem. Phys. 1968, 49, 4424. [Google Scholar] [CrossRef]
- Chrysochoos, J.; Qusti, A.H. Electronic relaxation of 3P0 and 1D2 states of Pr(3+) in POCl3:SnCl4. J. Less Common Met. 1986, 126, 169–174. [Google Scholar] [CrossRef]
- Qusti, A.H.; Chrysochoos, J. Concentration and temperature dependence of the luminescence arising from 3P0 and 1D2-states of Pr3+ in POCl3:SnCl4. J. Less Common Met. 1985, 112, 291–295. [Google Scholar] [CrossRef]
- Davies, G.M.; Aarons, R.J.; Motson, G.R.; Jeffery, J.C.; Adams, H.; Faulkner, S.; Ward, M.D. Structural and near-IR photophysical studies on ternary lanthanide complexes containing poly(pyrazolyl)borate and 1,3-diketonate ligands. Dalton Trans. 2004, 8, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.M.; Costa, A.L.; Feldl, J.; Maria, T.M.R.; de Melo, J.S.; Martín-Ramos, P.; Martín-Gile, J.; Silva, R.M. Synthesis, structure and physical properties of luminescent Pr(III) β-diketonate complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Fatila, E.M.; Maahs, A.C.; Hetherington, E.H.; Cooper, B.J.; Cooper, R.E.; Daanen, N.N.; Jennings, M.; Skrabalak, S.E.; Preuss, K.E. Stoichiometric control: 8- and 10-coordinate Ln(hfac)3(bpy) and Ln(hfac)3(bpy)2 complexes of the early lanthanides La–Sm†. Dalton Trans. 2018, 47, 16232–16241. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Ohba, M.; Ōkawa, H. A Series of Trinuclear CuIILnIIICuII complexes derived from 2,6-di(acetoacetyl)pyridine: synthesis, structure, and magnetism. Inorg. Chem. 2004, 43, 4435–4446. [Google Scholar] [CrossRef]
- Joan Gónzalez-Fabra, J.; Bandeira, N.A.G.; Velasco, V.; Barrios, L.A.; David Aguilà, D.; Teat, S.J.; Roubeau, O.R.; Bo, C.; Aromí, G. Thermodynamic stability of heterodimetallic [LnLn] complexes: Synthesis and DFT Studies. Chem A Eur. J. 2017, 23, 5117–5125. [Google Scholar] [CrossRef] [Green Version]
- Aguilà, D.; Barrios, L.A.; Velasco, V.; Arnedo, L.; Aliaga-Alcalde, N.; Menelaou, M.; Teat, S.J.; Roubeau, O.; Luis, F.; Aromí, G. Lanthanide contraction within a series of asymmetric dinuclear [Ln2] complexes. Chem. A Eur. J. 2013, 19, 5881–5891. [Google Scholar] [CrossRef]








| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C44H32F9O8Pr | C62H40F9N4O6Pr |
| Formula mass | 1000.61 | 1248.89 |
| System | Monoclinic | Triclinic |
| Space group | P21/c | P-1 |
| a (Å) | 8.9307(4) | 11.9059(4) |
| b (Å) | 28.9448(12) | 15.3694(5) |
| c (Å) | 16.2421(7) | 16.5675(6) |
| α (°) | 90 | 79.097(2) |
| β (°) | 105.674(2) | 70.308(2) |
| γ (°) | 90 | 67.575(2) |
| V (Å3) | 4042.4(3) | 2632.08(16) |
| Z | 4 | 2 |
| T (K) | 100(2) | 100(2) |
| μ (mm−1) | 1.301 | 1.016 |
| Dcalc (Mg/m3) | 1.644 | 1.576 |
| θ max (°) | 30.110 | 27.000 |
| Data collected | 185055 | 95375 |
| Unique refl./Rint | 11862/0.0797 | 11469/0.0642 |
| Parameters/Restraints | 567/0 | 767/66 |
| Goodness-of-Fit on F2 | 1.234 | 0.991 |
| R1/wR2 (all data) | 0.0437/0.0853 | 0.0276/0.0728 |
| Compound | 3 | 4 |
| Empirical formula | C54H36F9N2O8Pr | C54H36F9N2O6Pr |
| Formula mass | 1152.76 | 1120.76 |
| System | Monoclinic | Orthorhombic |
| Space group | P21/c | Pca21 |
| a (Å) | 13.2021(5) | 20.1848(6) |
| b (Å) | 14.6509(6) | 11.9034(3) |
| c (Å) | 24.8108(10) | 19.6102(6) |
| α (°) | 90 | 90 |
| β (°) | 98.938(2) | 90 |
| γ (°) | 90 | 90 |
| V (Å3) | 4740.7(3) | 4711.7(2) |
| Z | 4 | 4 |
| T (K) | 100(2) | 100(2) |
| μ (mm−1) | 1.123 | 1.124 |
| Dcalc (Mg/m3) | 1.615 | 1.580 |
| θ max (°) | 30.641 | 29.995 |
| Data collected | 405492 | 163636 |
| Unique refl./Rint | 14586/0.0514 | 13727/0.0648 |
| Parameters/Restraints | 669/0 | 651/7 |
| Goodness-of-Fit on F2 | 1.146 | 1.029 |
| R1/wR2 (all data) | 0.0260/0.0622 | 0.0266/0.0534 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Vicente, R.; Tubau, À.; Ferran, A.; Massoud, S.S. Structural Characterization, Magnetic and Luminescent Properties of Praseodymium(III)-4,4,4-Trifluoro-1-(2-Naphthyl)Butane-1,3-Dionato(1-) Complexes. Crystals 2021, 11, 179. https://doi.org/10.3390/cryst11020179
Mautner FA, Bierbaumer F, Fischer RC, Vicente R, Tubau À, Ferran A, Massoud SS. Structural Characterization, Magnetic and Luminescent Properties of Praseodymium(III)-4,4,4-Trifluoro-1-(2-Naphthyl)Butane-1,3-Dionato(1-) Complexes. Crystals. 2021; 11(2):179. https://doi.org/10.3390/cryst11020179
Chicago/Turabian StyleMautner, Franz A., Florian Bierbaumer, Roland C. Fischer, Ramon Vicente, Ànnia Tubau, Arnau Ferran, and Salah S. Massoud. 2021. "Structural Characterization, Magnetic and Luminescent Properties of Praseodymium(III)-4,4,4-Trifluoro-1-(2-Naphthyl)Butane-1,3-Dionato(1-) Complexes" Crystals 11, no. 2: 179. https://doi.org/10.3390/cryst11020179
APA StyleMautner, F. A., Bierbaumer, F., Fischer, R. C., Vicente, R., Tubau, À., Ferran, A., & Massoud, S. S. (2021). Structural Characterization, Magnetic and Luminescent Properties of Praseodymium(III)-4,4,4-Trifluoro-1-(2-Naphthyl)Butane-1,3-Dionato(1-) Complexes. Crystals, 11(2), 179. https://doi.org/10.3390/cryst11020179








