A Photomicroscopic Study on the Growth Rates of Calcium Oxalate Crystals in a New Synthetic Urine without Inhibitors and with Various Inhibitors
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Notation
| A | area |
| AG | growth kinetic parameter |
| Ci | impurity concentration |
| EG | growth activation energy |
| G | growth rate |
| g | growth rate order |
| L | size |
| R | ideal gas constant |
| S | supersaturation |
| T | temperature |
References
- Scales, C.D., Jr.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Abram, V.; Coe, F.L. Isolation of calcium oxalate crystal growth inhibitor from rat kidney and urine. Am. J. Physiol. 1984, 247, 765–772. [Google Scholar] [CrossRef]
- Ogbuji, L.U.; Batich, C.D. Ultrastructure of whewellite kidney stones: Electron-analytical investigation. J. Ultrastruct Res. 1985, 90, 1–8. [Google Scholar] [CrossRef]
- Kaloustian, J.; El-Moselhy, T.F.; Portugal, T.F. Determination of calcium oxalate (mono-and dihydrate) in mixtures with magnesium ammonium phosphate or uric acid: The use of simultaneous thermal analysis in urinary calculi. Clin. Chim. Acta 2003, 334, 117–129. [Google Scholar] [CrossRef]
- Opalko, F.J.; Adair, J.H.; Khan, S.R. Heterogeneous nucleation of calcium oxalate trihydrate in artificial urine by constant composition. J. Cryst. Growth 1997, 181, 410–417. [Google Scholar] [CrossRef]
- Rabinovich, Y.I.; Esayanur, M.; Daosukho, S.; Byer, K.J.; El-Shall, H.E.; Khan, S.R. Adhesion force between calcium oxalate monohydrate crystal and kidney epithelial cells and possible relevance for kidney stone formation. J. Colloid Interface Sci. 2006, 300, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Coe, F.L. Acidic peptide and polyribonucleotide crystal growth inhibitors in human urine. Am. J. Physiol. 1977, 233, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Drach, G.W.; Randolph, A.D.; Miller, J.D. Inhibition of calcium oxalate dihydrate crystallization by chemical modifiers. I. Pyrophosphate and methylene blue. J. Urol. 1978, 119, 99–103. [Google Scholar] [CrossRef]
- Hallson, P.C.; Rose, G.A. Uromucoids and urinary stone formation. Lancet 1979, 1, 1000–1002. [Google Scholar] [CrossRef]
- Randolph, A.D.; Drach, G.W. Some measurements of calcium oxalate nucleation and growth rates in urine-like liquors. J. Cryst. Growth 1981, 53, 195–201. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S. Factors influencing the crystallization of calcium oxalate-a critique. J. Cryst. Growth 1981, 53, 182–194. [Google Scholar] [CrossRef]
- Ryall, R.L.; Harnett, R.M.; Marshall, V.R. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin. Chim. Acta 1981, 112, 349–356. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Abram, V.; Kezdy, F.J.; Kaiser, E.T.; Coe, F.L. Purification and characterization of the principal inhibitor of calcium oxalate crystal growth in human urine. J. Biol. Chem. 1983, 258, 12594–12600. [Google Scholar] [CrossRef]
- Li, M.K.; Blacklock, N.J.; Garside, J. Effects of magnesium on calcium oxalate crystallization. J. Urol. 1985, 133, 123–125. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S.; Sergeant, V.J. Ionic and macromolecular modifiers of crystallization of calcium salts in urine. Fortschr. Urol. Nephrol. 1985, 23, 1–11. [Google Scholar]
- Robertson, W.G.; Scurr, D.S. Modifiers of calcium oxalate crystallization found in urine. I. Studies with a continuous crystallizer using an artificial urine. J. Urol. 1986, 86, 1322–1326. [Google Scholar] [CrossRef]
- Pak, C.Y. Citrate and renal calculi: New insights and future directions. Am. J. Kidney Dis. 1991, 17, 420–425. [Google Scholar] [CrossRef]
- Ryall, R.L. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J. Urol. 1997, 15, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Marangella, M.; Bagnis, C.; Bruno, M.; Vitale, C.; Petrarulo, M.; Ramello, A. Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol. Int. 2004, 72, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Isern, B.; Sanchis, P.; Perello, J.; Torres, J.J.; Costa-Bauza, A. Phytate acts as an inhibitor in formation of renal calculi. Front. Biosci. 2007, 12, 2580–2587. [Google Scholar] [CrossRef]
- Farmanesh, S.; Ramamoorthy, S.; Chung, J.; Asplin, J.R.; Karande, P.; Rimer, J.D. Specificity of Growth Inhibitors and their Cooperative Effects in Calcium Oxalate Monohydrate Crystallization. J. Am. Chem. Soc. 2013, 136, 367–376. [Google Scholar] [CrossRef]
- Grases, F.; Rodriguez, A.; Costa-Bauza, A. Efficacy of mixtures of magnesium, citrate and phytate as calcium oxalate crystallization inhibitors in urine. J. Urol. 2015, 194, 812–819. [Google Scholar] [CrossRef]
- Chung, J.; Granja, I.; Taylor, M.G.; Mpourmpakis, G.; Asplin, J.R.; Rimer, J.D. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature 2016, 536, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.L.; Jackson, G.E. Determination of thermodynamic parameters for complexation of calcium and magnesium with chondroitin sulfate isomers using isothermal titration calorimetry: Implications for calcium kidney-stone research. J. Cryst. Growth 2017, 463, 14–18. [Google Scholar] [CrossRef]
- Kim, D.; Rimer, J.D.; Asplin, J.R. Hydroxycitrate: A potential new therapy for calcium urolithiasis. Urolithiasis 2019, 47, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Costa-Bauza, A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef] [PubMed]
- Izatulina, A.R.; Golovanova, O.A.; Punin, Y.O. Effect of Amino Acids, Magnesium Ions and Hydroxyapatite on the Formation of Oxalate Nephroliths. Chem. Sustain. Dev. 2008, 2, 163–167. [Google Scholar]
- Golovanova, O.A.; Punin, Y.O.; Izatulina, A.R.; Korol’kov, V.V. Crystallization of calcium oxalate monohydrate in the presence of amino acids: Features and regularities. J. Struct. Chem. 2014, 55, 1356–1370. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Lin, Y.H.; Shiau, L.D. Effects of various inhibitors on the nucleation of calcium oxalate in synthetic urine. Crystals 2020, 10, 333. [Google Scholar] [CrossRef]
- Shiau, L.D. The distribution of dislocation activities among crystals in sucrose crystallization. Chem. Eng. Sci. 2003, 58, 5299–53042. [Google Scholar] [CrossRef]
- Coe, F.; Parks, J.H. Defenses of an unstable compromise: Crystallization inhibitors and the kidney’s role in mineral regulation. Kidney Int. 1990, 38, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, B. Calcium stones: Some physical and clinical aspects, Chapter 10. In Calcium Metabolism in Renal Failure and Nephrolithiasis; David, D.S., Ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Shiau, L.D.; Berglund, K.A. Growth kinetic of fructose crystals formed by contact nucleation. AIChE J. 1987, 33, 1028–1033. [Google Scholar] [CrossRef]
- Shiau, L.D.; Berglund, K.A. Growth rate dispersion in batch crystallization. AIChE J. 1990, 36, 1669–1678. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Yu, H.; Sheikholeslami, R.; Doherty, W.O.S. The effects of silica and sugar on the crystallographic and morphological properties of calcium oxalate. J. Cryst. Growth 2004, 265, 592–603. [Google Scholar] [CrossRef]
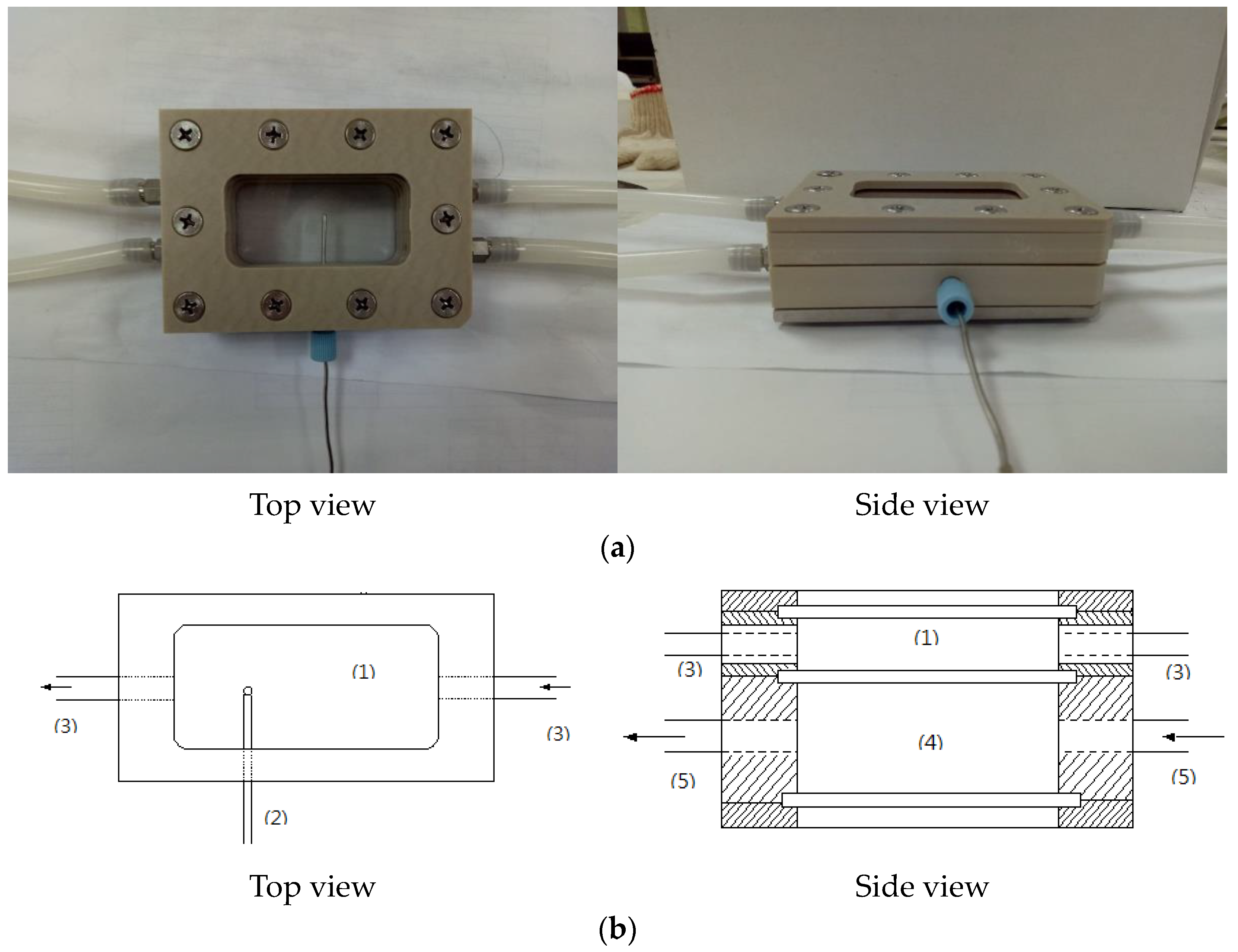
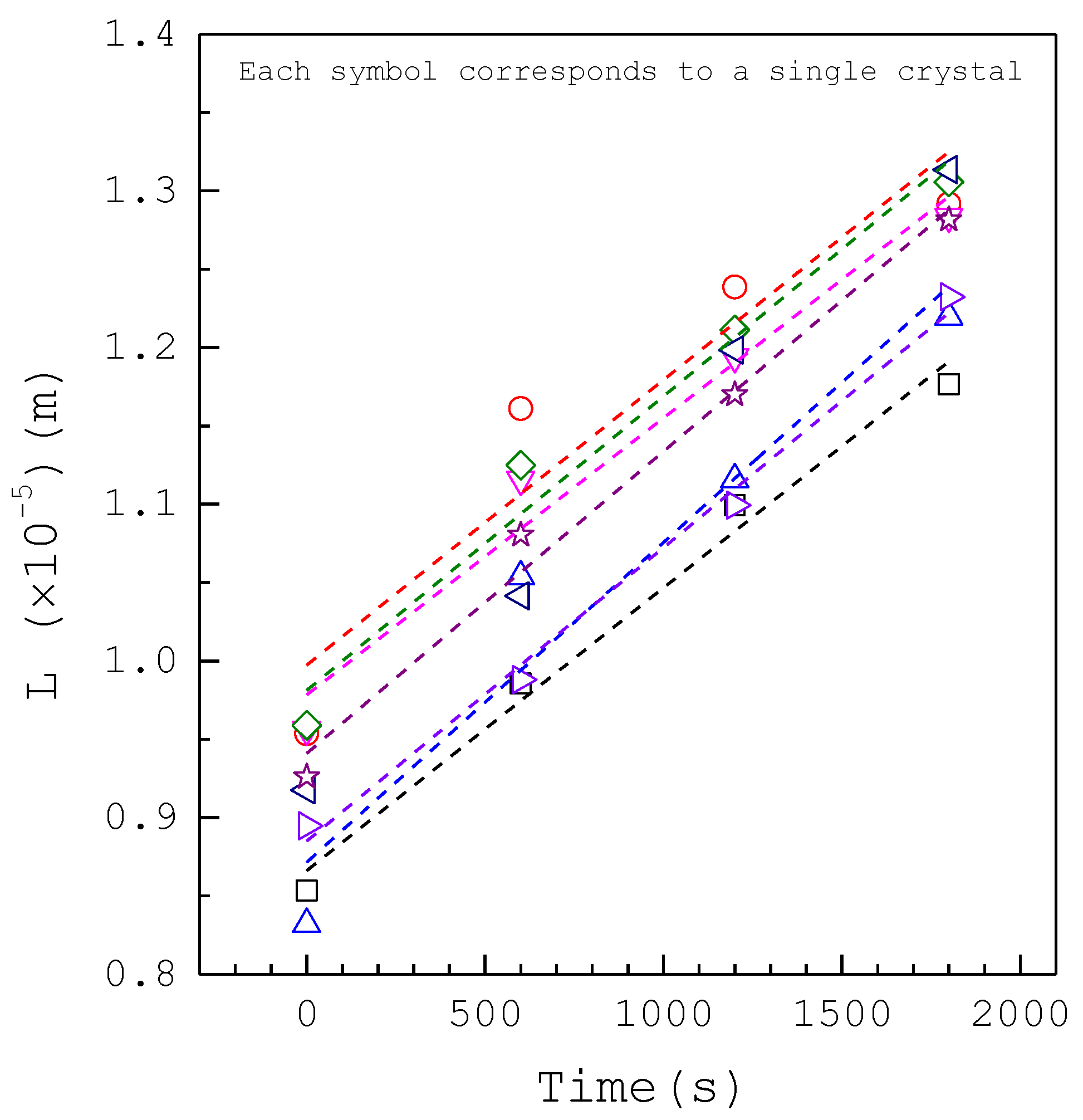
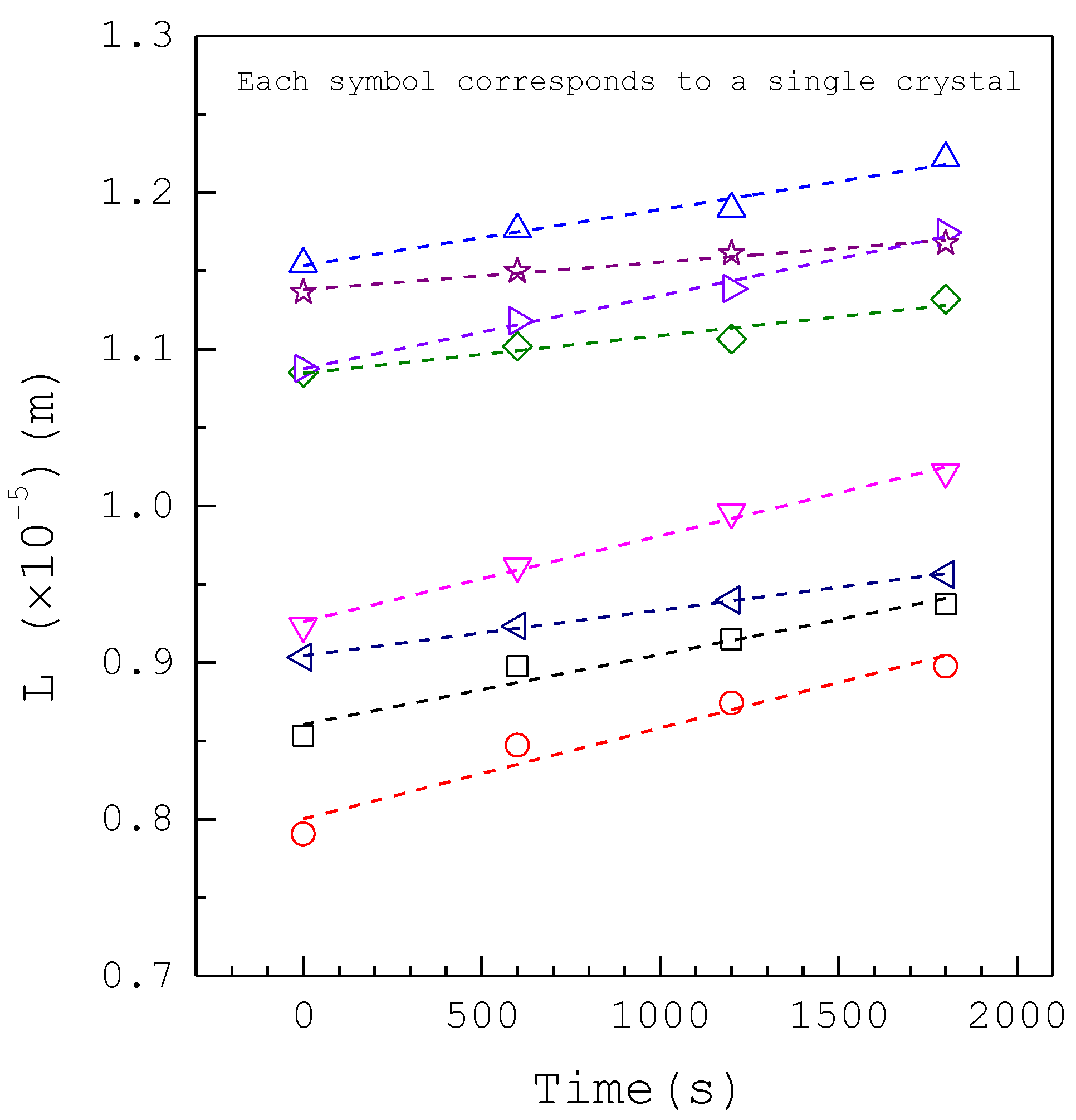
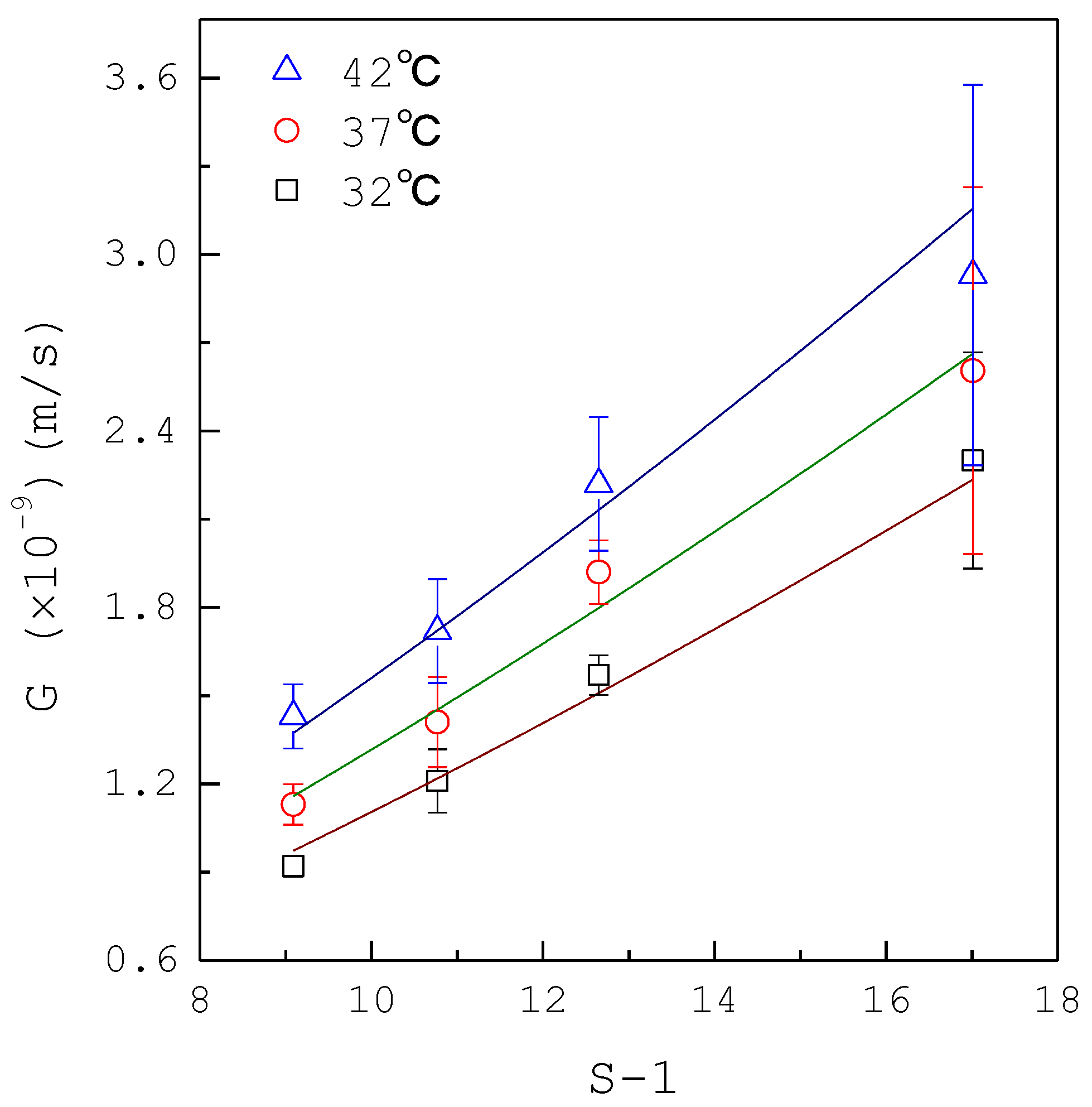
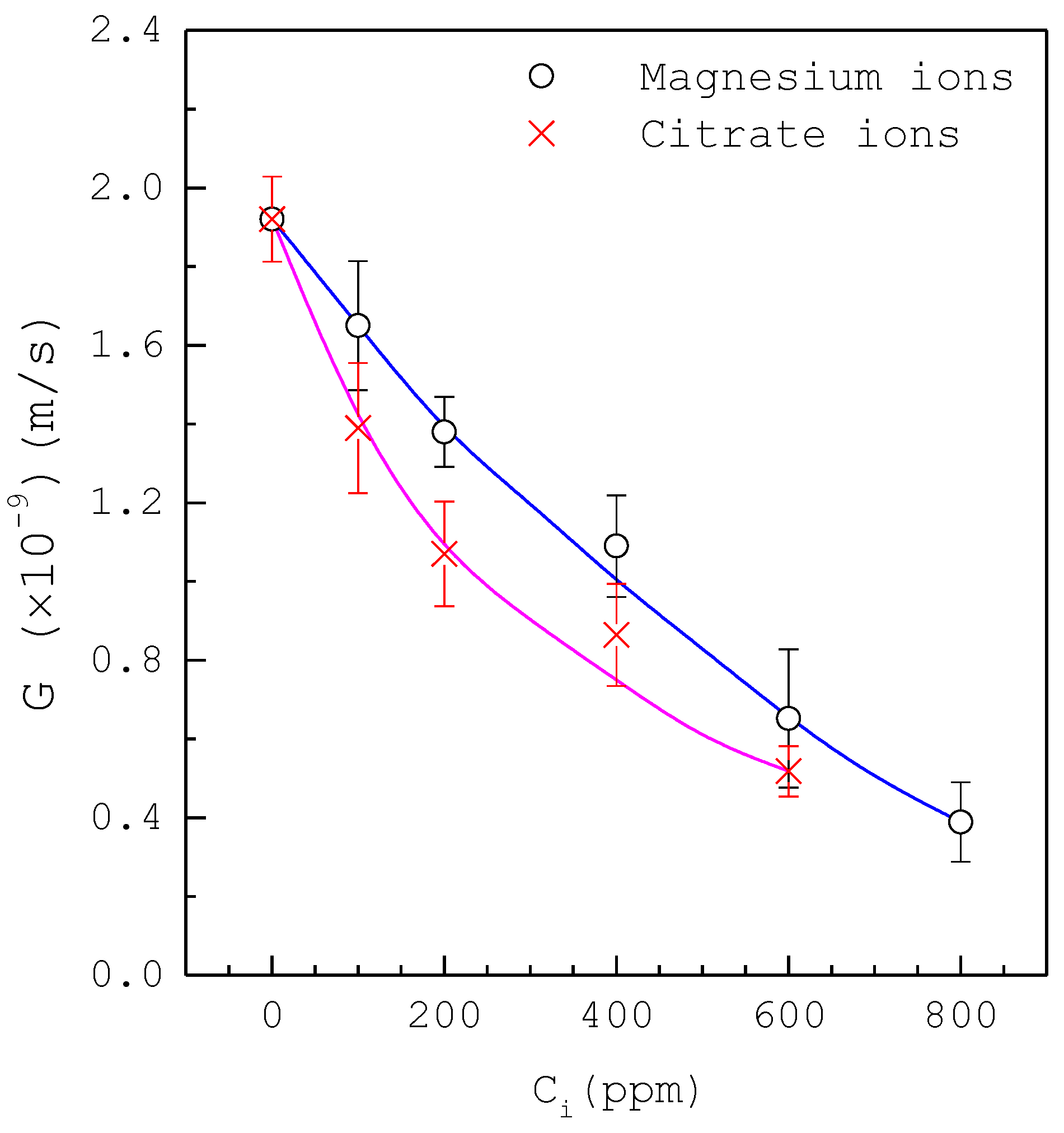
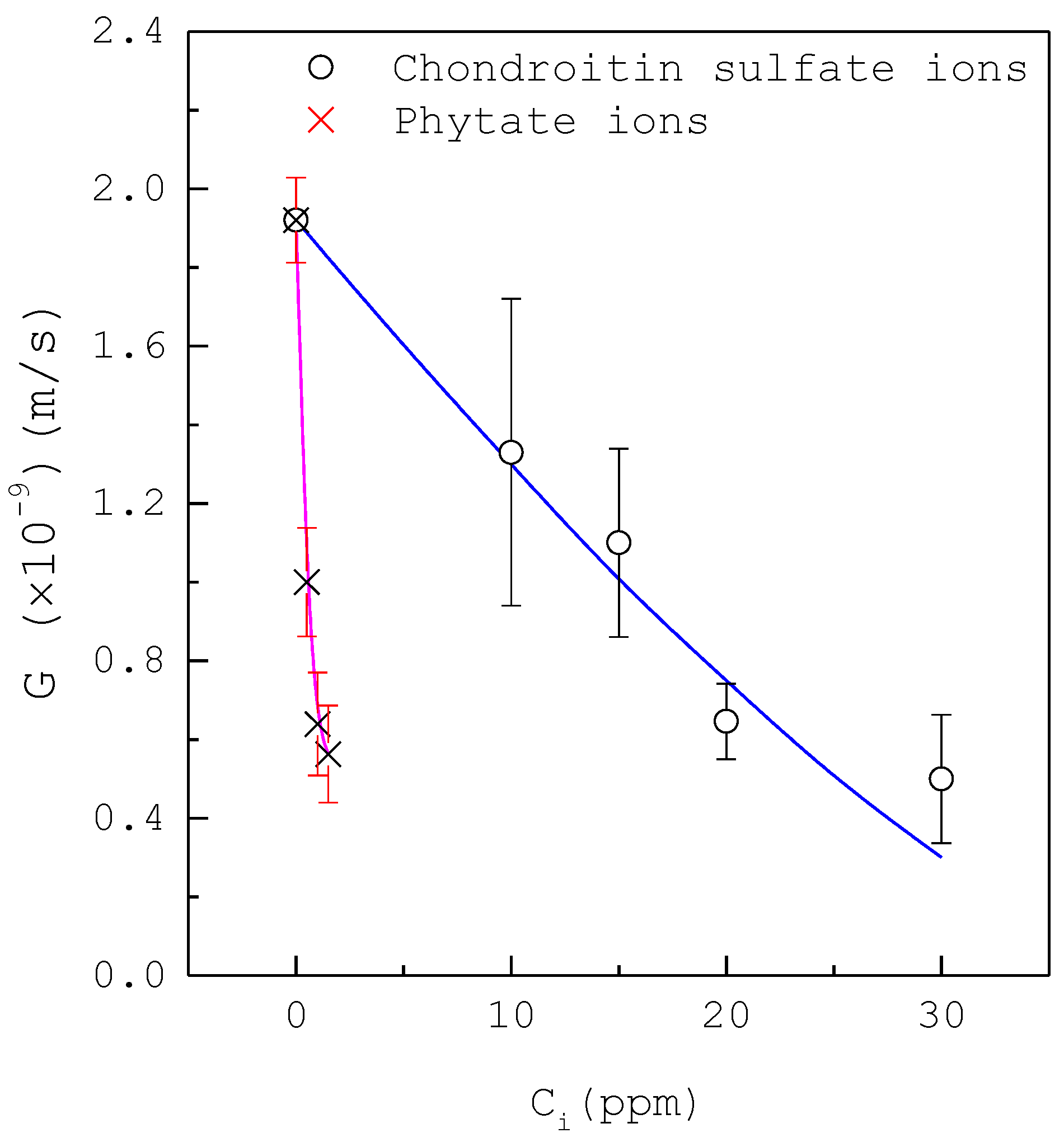
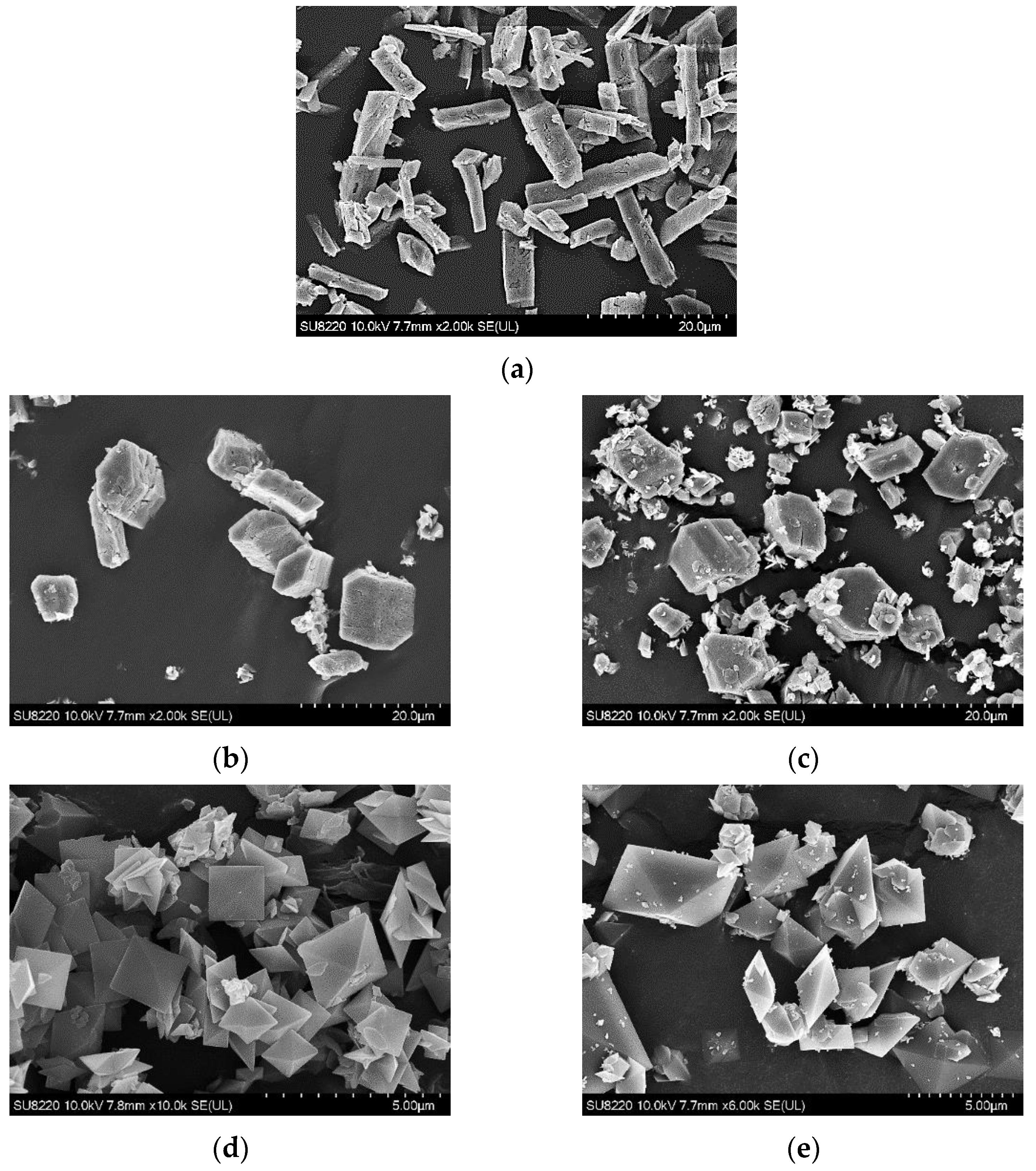
| Composition | Concentration (mM) |
|---|---|
| Solution 1 (100 mL): | |
| Na2SO4·10H2O | 9.67 |
| NH4Cl | 43.37 |
| KCl | 81.30 |
| CaCl2 | 5.00 |
| Solution 2 (100 mL): | |
| NaH2PO4·2H2O | 7.73 |
| Na2HPO4·12H2O | 7.82 |
| NaCl | 111.54 |
| Urea | 200 |
| Uric acid | 1.00 |
| Creatinine | 4.00 |
| Na2C2O4 | 0.76, 0.96, 1.2, 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Pan, L.-C.; Shiau, L.-D. A Photomicroscopic Study on the Growth Rates of Calcium Oxalate Crystals in a New Synthetic Urine without Inhibitors and with Various Inhibitors. Crystals 2021, 11, 223. https://doi.org/10.3390/cryst11030223
Hsu Y-C, Pan L-C, Shiau L-D. A Photomicroscopic Study on the Growth Rates of Calcium Oxalate Crystals in a New Synthetic Urine without Inhibitors and with Various Inhibitors. Crystals. 2021; 11(3):223. https://doi.org/10.3390/cryst11030223
Chicago/Turabian StyleHsu, Yu-Chao, Li-Cheng Pan, and Lie-Ding Shiau. 2021. "A Photomicroscopic Study on the Growth Rates of Calcium Oxalate Crystals in a New Synthetic Urine without Inhibitors and with Various Inhibitors" Crystals 11, no. 3: 223. https://doi.org/10.3390/cryst11030223
APA StyleHsu, Y.-C., Pan, L.-C., & Shiau, L.-D. (2021). A Photomicroscopic Study on the Growth Rates of Calcium Oxalate Crystals in a New Synthetic Urine without Inhibitors and with Various Inhibitors. Crystals, 11(3), 223. https://doi.org/10.3390/cryst11030223






