Exploration of the Effect of Oxygen on Superconductivity in MgB2 Bulk by Using Boron Powder with Different Particle and Purification
Abstract
:1. Introduction
2. Materials and Methods
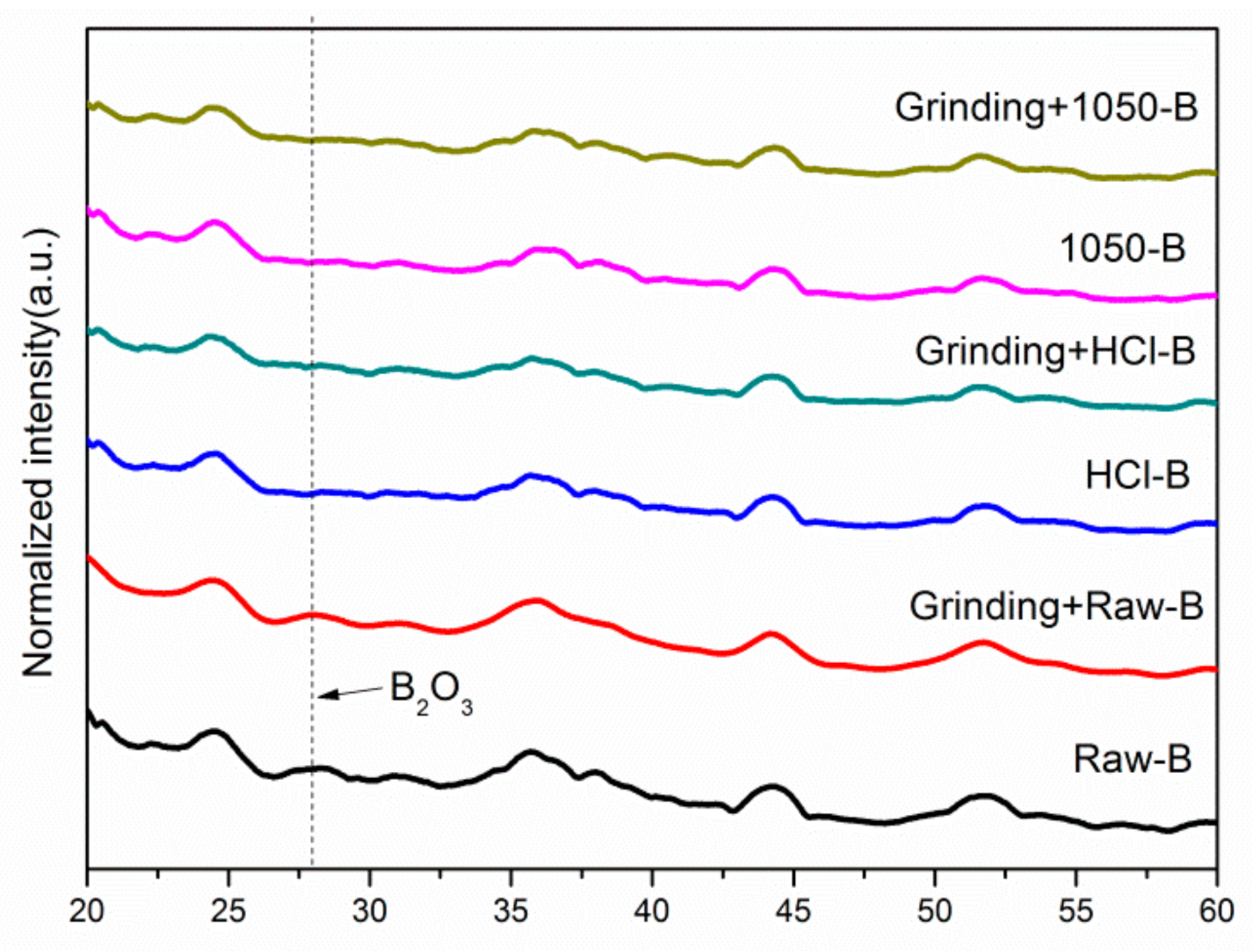
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 63–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Suo, H.; Ma, L.; Liu, M.; Zhang, T.; Zhou, M. The Effect of Doping with the Polymer Metallic Complex Poly Zinc Acrylate on Superconducting Bulk MgB2. J. Electron. Mater. 2011, 40, 1369–1376. [Google Scholar] [CrossRef]
- Acharya, N.; Wolak, M.A.; Tan, T.; Lee, N.; Lang, A.C.; Taheri, M.; Cunnane, D.; Karasik, B.S.; Xi, X.X. MgB2 ultrathin films fabricated by hybrid physical chemical vapor deposition and ion milling. Appl. Mater. 2016, 4, 086114. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.H.; Pogrebnyakov, A.V.; Kotcharov, A.; Jones, J.E.; Xi, X.X.; Lysczek, E.M.; Redwing, J.M.; Xu, S.; Li, Q.; Lettieri, J.; et al. In situ epitaxial MgB2 thin films for superconducting electronics. Nat. Mater. 2002, 1, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Wolak, M.A.; Acharya, N.; Krick, A.; Lang, A.C.; Sloppy, J.; Taheri, M.L.; Civale, L.; Chen, K.; Xi, X.X. Enhancement of lower critical field by reducing the thickness of epitaxial and polycrystalline MgB2 thin films. Appl. Mater. 2015, 3, 041101. [Google Scholar] [CrossRef] [Green Version]
- Ağıl, H.; Çiçek, O.; Ertekin, E.; Motaman, A.; AHossain, M.S.; Dou, S.X.; Gencer, A. Effects of MgO on the Electronic and Superconducting Properties in Succinic Acid (C4H6O4) Doped MgB2 Bulks. J. Supercond. Nov. Magn. 2013, 26, 1525–1529. [Google Scholar] [CrossRef]
- Hong, Z.; Yong, Z.; Yong, Z. The Effects of Excess Mg Addition on the Superconductivity of MgB2. J. Supercond. Nov. Magn. 2015, 28, 2711–2714. [Google Scholar]
- Fujii, H.; Ishitoy, A.; Itoh, S.; Ozawa, K.; Kitaguchi, H. Effect of additions of Ca compounds to the filling powder on the reduction of MgO and the critical current density properties of ex situ processed MgB2 tapes. J. Alloys Compd. 2016, 64, 650–656. [Google Scholar] [CrossRef]
- Jiang, C.H.; Hatakeyama, H.; Kumakura, H. Effect of nanometer MgO addition on the in situ PIT processed MgB2/Fe tapes. Physics C 2005, 423, 45–50. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, M.; Ma, L.; Liu, M.; Suo, H.; Wang, Q. A novel gas-solid reaction process to synthesize low oxygen MgB2 powder using Mg vapour. Supercond. Sci. Technol. 2019, 32, 015015. [Google Scholar] [CrossRef]
- Jiang, J.; Senkowicz, B.J.; Larbalestier, D.C.; Hellstrom, E.E. Influence of boron powder purification on the connectivity of bulk MgB2. Supercond. Sci. Technol. 2006, 19, L33–L36. [Google Scholar] [CrossRef]
- Hishinuma, Y.; Kikuchi, A.; Shimada, Y.; Hata, S.; Takeuchi, T.; Yamada, S.; Sagara, A. Effect of boron particle size on microstructure and superconducting properties of in-situ Cu addition MgB2 multifilamentary wire. J. Phys. Conf. Ser. 2014, 507, 022009. [Google Scholar] [CrossRef] [Green Version]
- Rosova, A.; Kulic, M.; Kovac, P.; Brunner, B.; Scheiter, J.; Hassler, W. The effect of boron powder on the microstructure of MgB2 filaments prepared by the modified internal magnesium diffusion technique. Supercond. Sci. Technol. 2017, 30, 055001. [Google Scholar] [CrossRef]
- Zhou, S.H.; Zhang, Y.; Dou, S.X. Effects of ball-mill processing on the superconductivity of sucrose doped MgB2. Physics C 2010, 470, S635–S636. [Google Scholar] [CrossRef]
- Xu, X.; Qin, M.J.; Konstantinov, K.; dos Santos, D.I.; Yeoh, W.K.; Kim, J.H.; Dou, S.X. Effect of boron powder purity on superconducting properties of MgB2. Supercond. Sci. Technol. 2006, 19, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Tian, M.; Ma, L.; Liu, M.; Suo, H.; Wang, Q. A Systematic investigation into the effect of boron powder purified by different methods on the microstructures and superconductivity of MgB2 bulks. Supercond. Sci. Technol. 2019, 32, 055009. [Google Scholar] [CrossRef]
- Dew-Hugh, D. Flux pinning mechanisms in type II superconductors. Philos. Mag. 1974, 30, 293–305. [Google Scholar] [CrossRef]
- Eisterer, M. Calculation of the volume pinning force in MgB2 superconductors. Phys. Rev. B 2008, 77, 144524. [Google Scholar] [CrossRef]
- Rowell, J.M. The widely variable resistivity of MgB2 samples. Supercond. Sci. Technol. 2003, 16, R17–R27. [Google Scholar] [CrossRef]
- Wilke, R.H.T.; Bud’ko, S.L.; Canfield, P.C.; Finnemore, D.K.; Suplinskas, R.J.; THannahs, S. Synthesis and optimization of Mg(B1−xCx)2 wire segments. Physics C 2005, 424, 1–16. [Google Scholar] [CrossRef]
- Canfield, P.C.; KFinnemore, D.; LBud’ko, S.; EOstenson, J.; Lapertot, G.; Cunningham, C.E.; Petrovic, C. Superconductivity in dense MgB2 wires. Phys. Rev. Lett. 2001, 86, 2423–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susner, M.A.; Yang, Y.; Sumption, M.D.; Wcollings, E.; Rindfleisch, M.A.; Tomsic, M.J.; Marzik, J.V. Enhanced critical fields and superconducting properties of pre-doped B powder-type MgB2 strands. Supercond. Sci. Technol. 2011, 24, 12001. [Google Scholar] [CrossRef]
- Saglietti, L.; Perini, E.; Ripamonti, G.; Bassani, E.; Carcano, G.; Giunchi, G. Boron Purity Effects on Structural Properties of the MgB2 Obtained by Mg-Reactive Liquid Infiltration. IEEE Trans. Appl. Supercond. 2009, 19, 2739–2743. [Google Scholar] [CrossRef]
- Yi, H.C.; Moore, J.J. Self-propagating high-temperature (combustion) synthesis (SHS) of powder-compacted materials. J. Mater. Sci. 1990, 25, 1159–1168. [Google Scholar] [CrossRef]
- Vignolo, M.; Romano, G.; Martinelli, A.; Bernini, C.; Antonio, S. A Novel Process to Produce Amorphous Nanosized Boron Useful for MgB2 Synthesis. IEEE Trans. Appl. Supercond. 2012, 22, 6200606. [Google Scholar] [CrossRef]
- Aladdin-e. Available online: https://www.aladdin-e.com/zh_cn/catalogsearch/result/?form_key=Kg8JWOGO65dFQRNE&q=%E7%A1%BC%E7%B2%89 (accessed on 9 March 2021).
- Specialty Materials, Inc. Available online: http://www.specmaterials.com (accessed on 9 March 2021).
- Pavezyum Advanced Chemicals. Available online: http://www.pavezyum.com (accessed on 9 March 2021).




| Sample Name | Purification Method | Oxygen Content (ppm) | Particle Size | |
|---|---|---|---|---|
| Average Size (μm) | Standard Deviation (%) | |||
| Raw B | Without any purification | 37,692 ± 112 | 0.5855 | 0.4217 |
| HCl B | Purified B powder by 15 vol% HCl solution | 22,408 ± 104 | 0.7064 | 0.6375 |
| Grinding+HCl B | Grinding powder and purified B powder by 15 vol% HCl solution | 16,022 ± 113 | 0.3407 | 0.5396 |
| 1050 B | Heat treatment at 1050 °C for 24 h | 9519 ± 120 | 0.7169 | 0.3479 |
| Grinding+1050 B | Grinding powder and heat treatment at 1050 °C for 24 h | 9532 ± 112 | 0.5636 | 0.4547 |
| Sample Name | Lattice Parameter (Å) | Grain Size (nm) | MgB2 (%) | MgO (%) | |
|---|---|---|---|---|---|
| a | c | ||||
| Raw B | 3.083 | 3.520 | 24.5 | 81.9 | 19.1 |
| Grinding+1050 B | 3.085 | 3.525 | 38.7 | 92.12 | 7.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Suo, H.; Ma, L.; Liu, M.; Zhao, W.; Liu, J.; Wang, L.; Zhang, Z.; Wang, Q. Exploration of the Effect of Oxygen on Superconductivity in MgB2 Bulk by Using Boron Powder with Different Particle and Purification. Crystals 2021, 11, 278. https://doi.org/10.3390/cryst11030278
Yang L, Suo H, Ma L, Liu M, Zhao W, Liu J, Wang L, Zhang Z, Wang Q. Exploration of the Effect of Oxygen on Superconductivity in MgB2 Bulk by Using Boron Powder with Different Particle and Purification. Crystals. 2021; 11(3):278. https://doi.org/10.3390/cryst11030278
Chicago/Turabian StyleYang, Liangqun, Hongli Suo, Lin Ma, Min Liu, Wanli Zhao, Jianhua Liu, Lei Wang, Zili Zhang, and Qiuliang Wang. 2021. "Exploration of the Effect of Oxygen on Superconductivity in MgB2 Bulk by Using Boron Powder with Different Particle and Purification" Crystals 11, no. 3: 278. https://doi.org/10.3390/cryst11030278
APA StyleYang, L., Suo, H., Ma, L., Liu, M., Zhao, W., Liu, J., Wang, L., Zhang, Z., & Wang, Q. (2021). Exploration of the Effect of Oxygen on Superconductivity in MgB2 Bulk by Using Boron Powder with Different Particle and Purification. Crystals, 11(3), 278. https://doi.org/10.3390/cryst11030278






