Rapid Microwave Synthesis of β-SnWO4 Nanoparticles: An Efficient Anode Material for Lithium Ion Batteries
Abstract
1. Introduction
2. Experiment
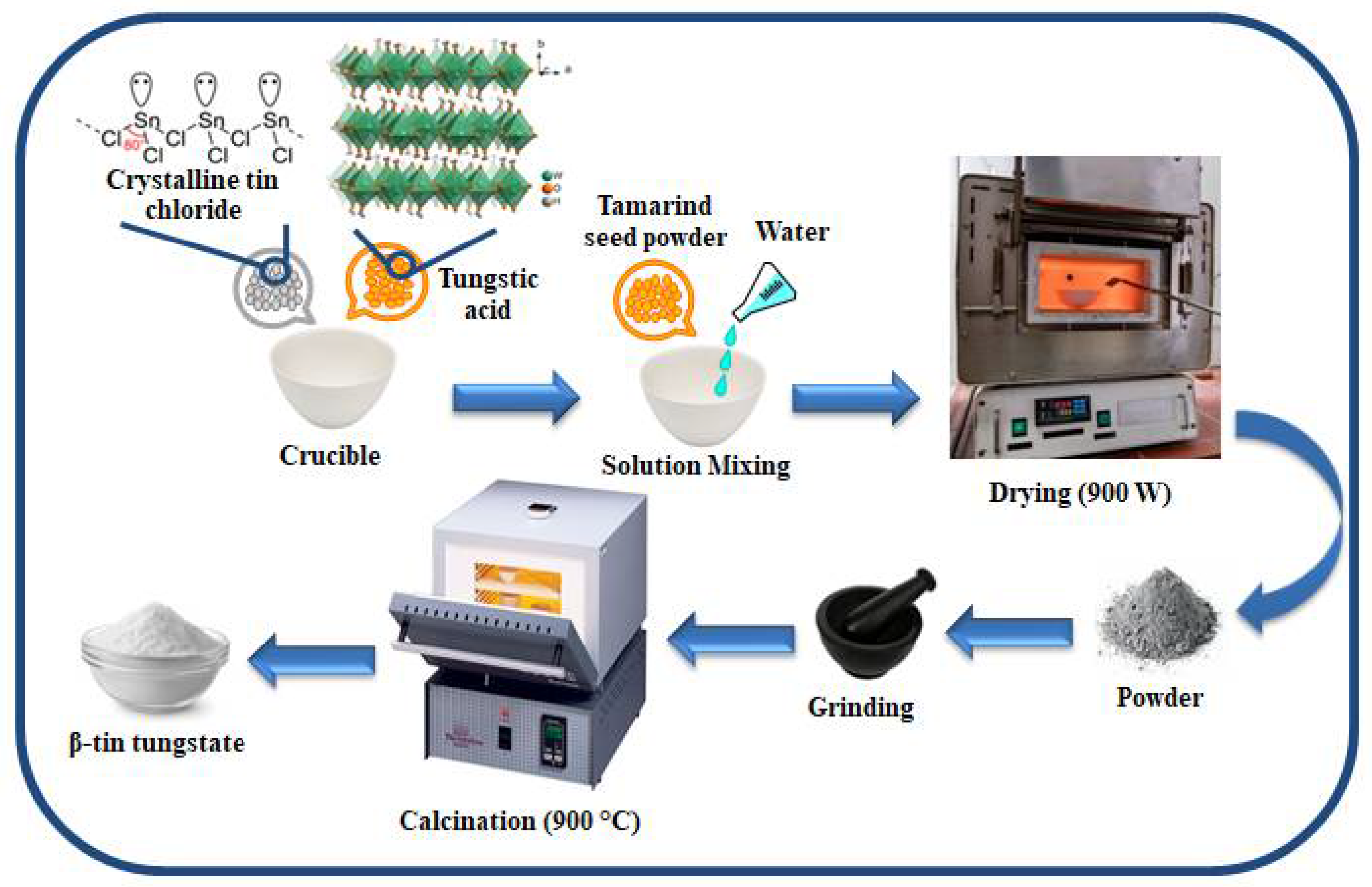
2.1. Synthesis of β-SnWO4 Nanoparticles
2.2. Characterization
2.3. Electrode Fabrication for the Lithium Ion Battery
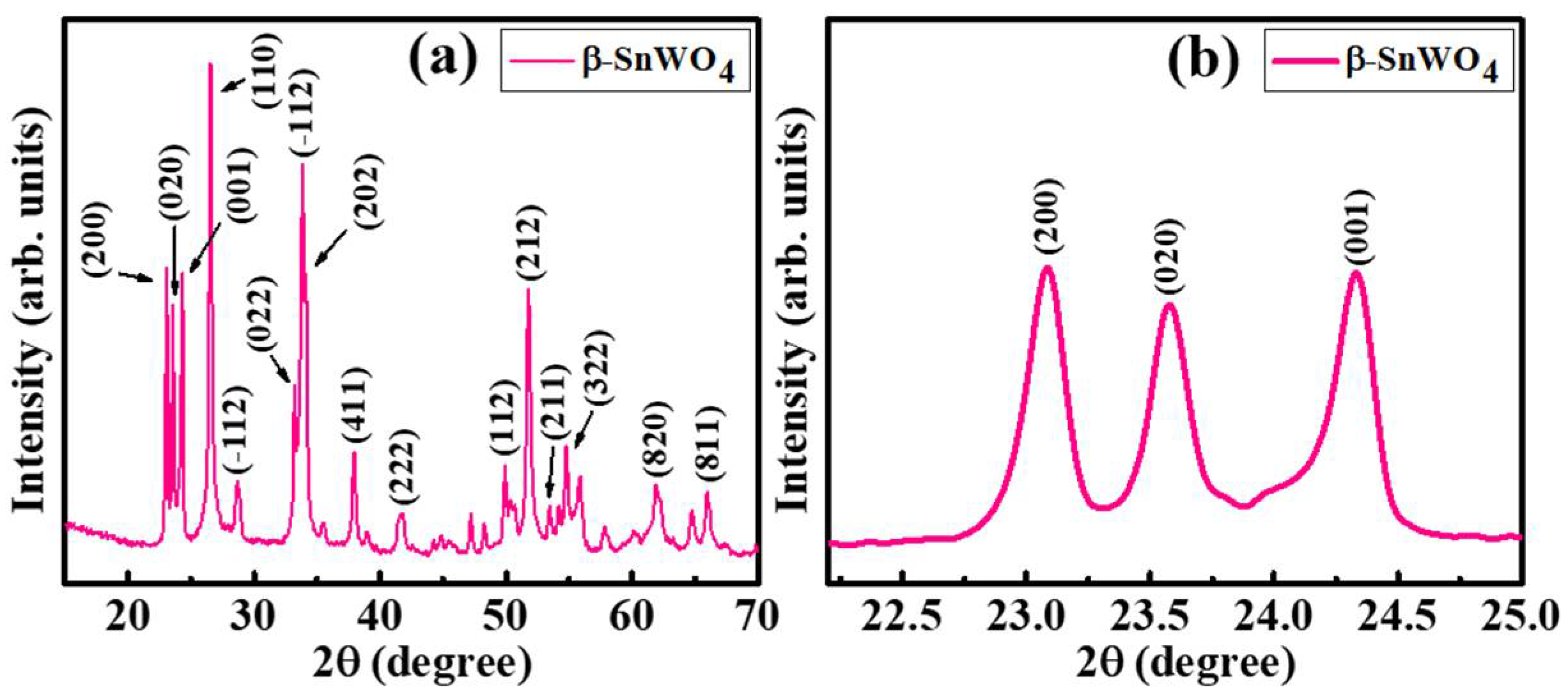
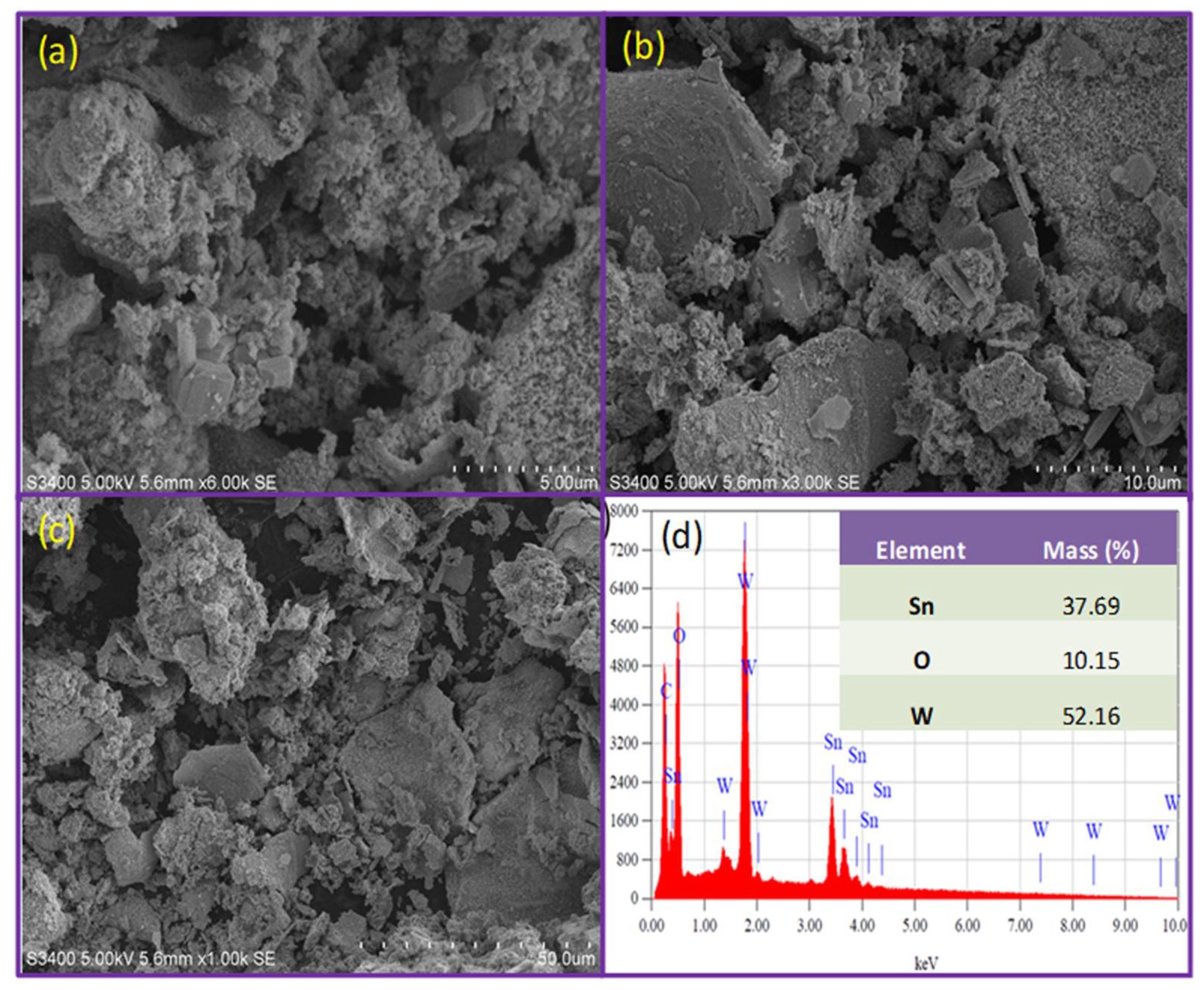
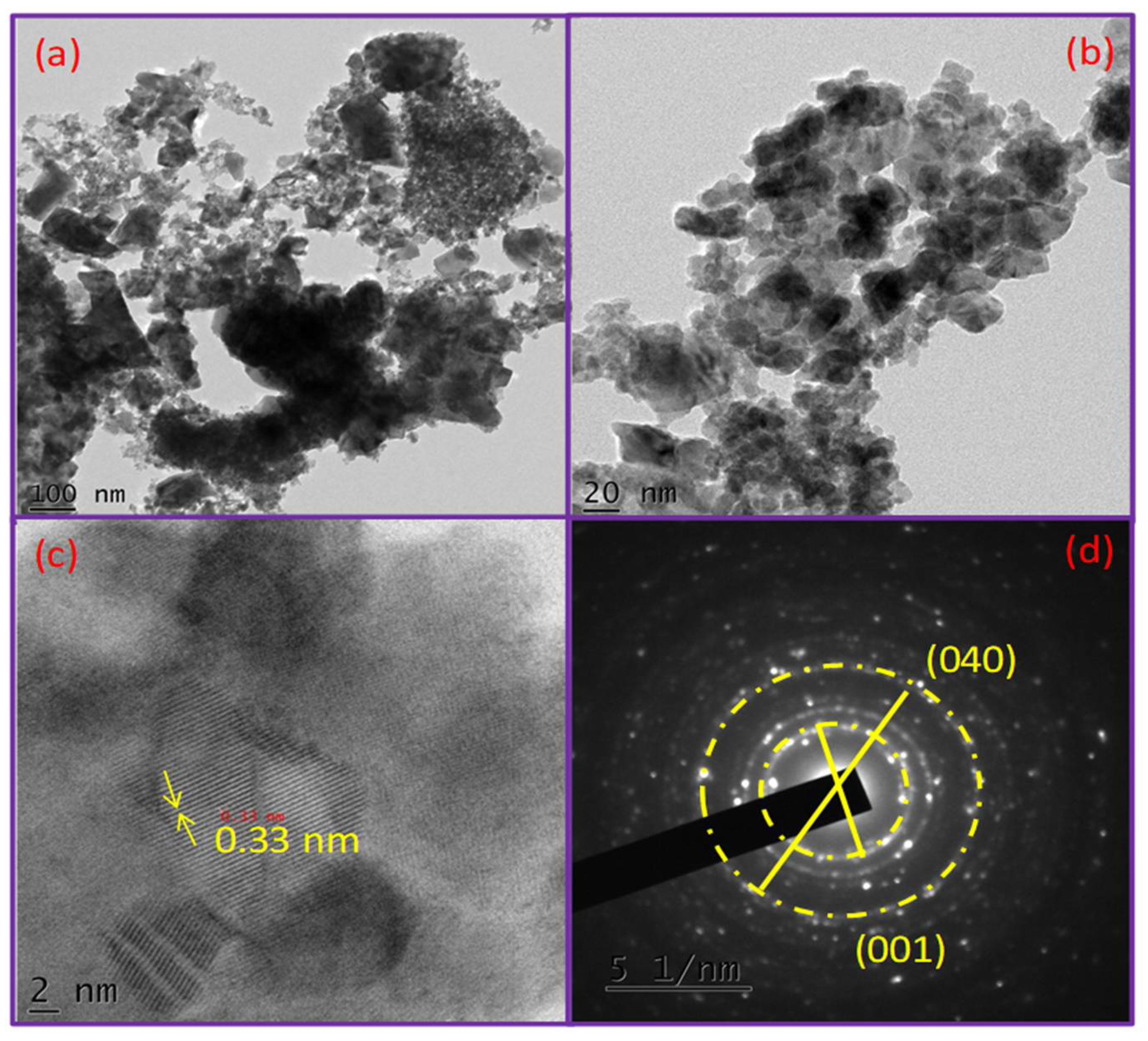
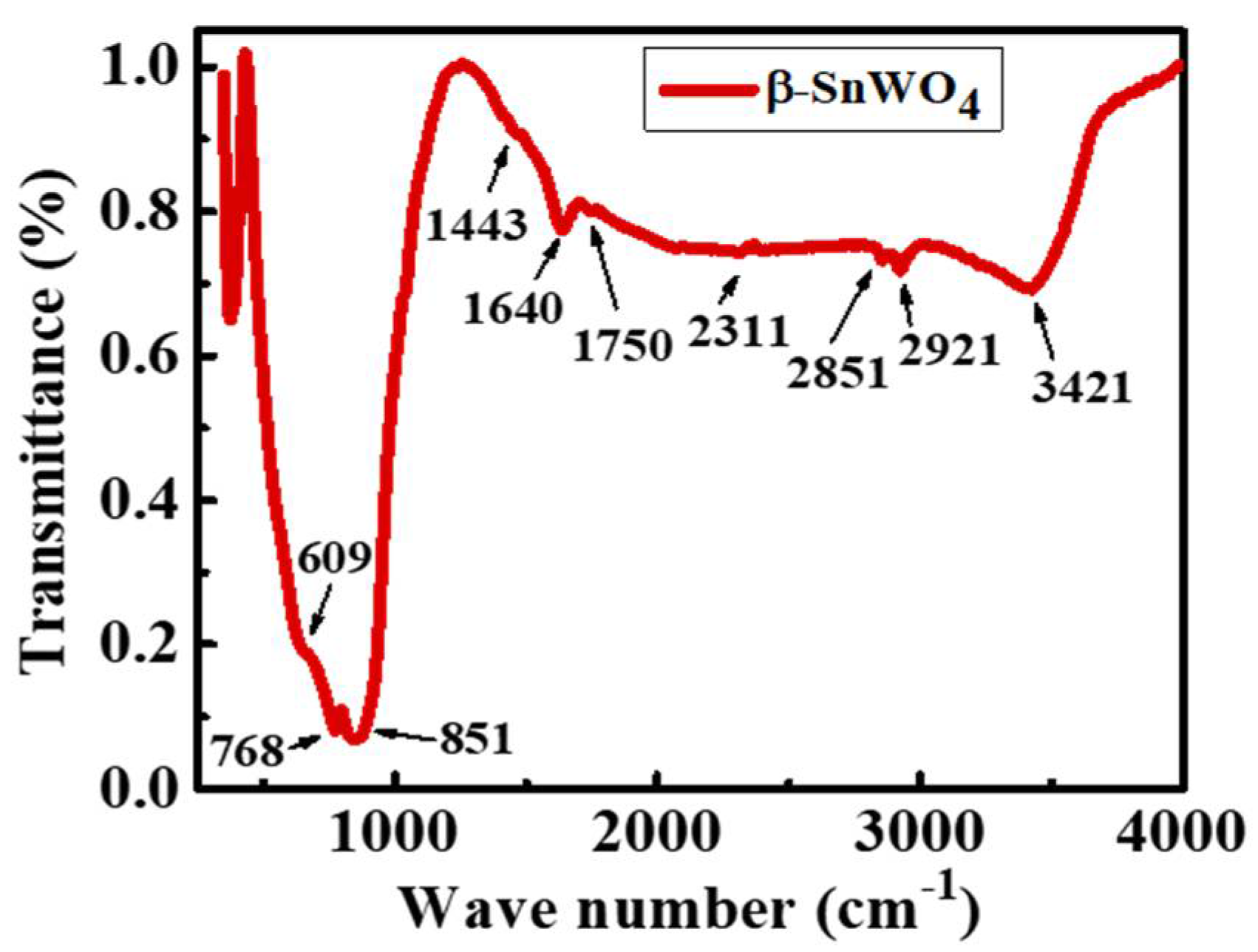
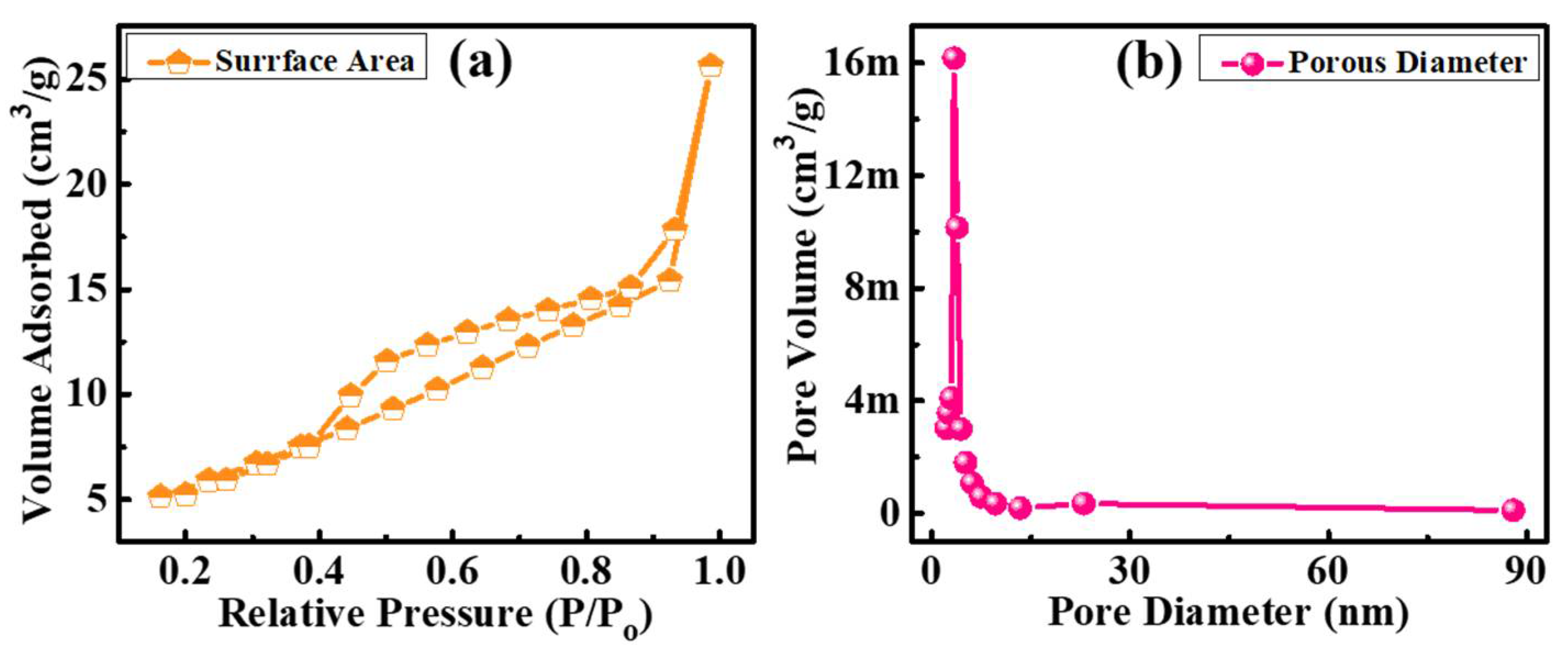
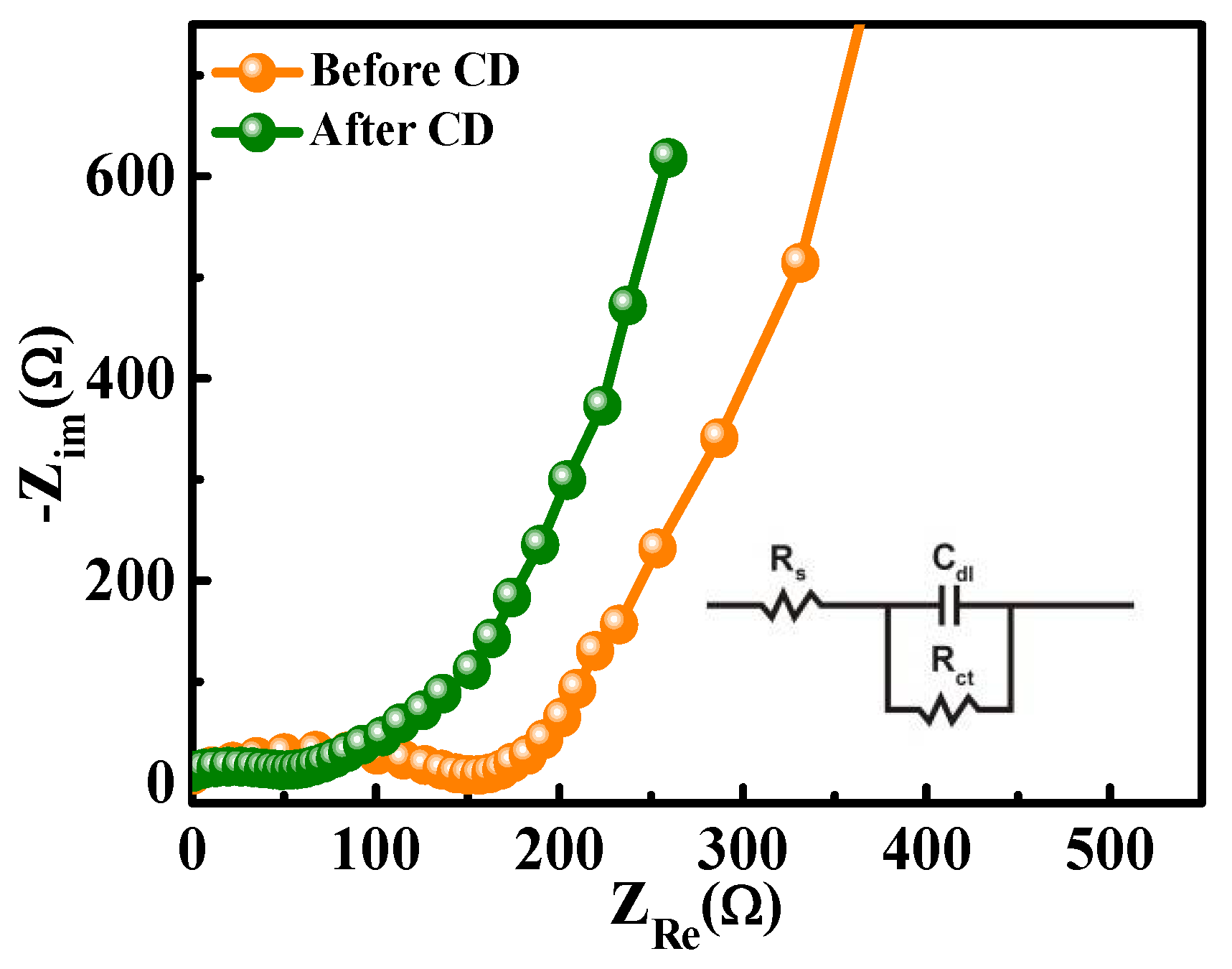
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, P.A.; Jun, S.C. Review on recent progress in the development of tungsten oxide based electrodes for electrochemical energy storage. ChemSusChem 2020, 13, 11–38. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xiao, X.; Pang, H. Transition metal (Fe, Co, Ni) fluoride-based materials for electrochemical energy storage. Nanoscale Horizons 2019, 4, 99–116. [Google Scholar] [CrossRef]
- Borzutzki, K.; Thienenkamp, J.; Diehl, M.; Winter, M.; Brunklaus, G. Fluorinated polysulfonamide based single ion conducting room temperature applicable gel-type polymer electrolytes for lithium ion batteries. J. Mater. Chem. A 2019, 7, 188–201. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.K.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Liu, D.; Xiong, H. ZnWO4 nanocrystals/reduced graphene oxide hybrids: Synthesis and their application for Li ion batteries. Sci. China Ser. B Chem. 2013, 57, 122–126. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Tan, Q.; Yang, J. Ruthenium oxide-based nanocomposites with high specific surface area and improved capacitance as a supercapacitor. RSC Adv. 2014, 4, 42839–42845. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Ji, T.; Lokhande, C.D. Facile synthesis of hierarchical mesoporous weirds-like morphological MnO2 thin films on carbon cloth for high performance supercapacitor application. J. Colloid Interface Sci. 2017, 498, 202–209. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, J.; Li, C.; Li, B.; Li, D.; Liu, Z.; Liu, Y. Fabrication of novel ternary three-dimensional RuO2/graphitic-C3N4@ reduced graphene oxide aerogel composites for supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 4982–4991. [Google Scholar] [CrossRef]
- Prabakar, S.R.; Han, S.C.; Jeong, J.; Sohn, K.S.; Pyo, M. CoSn (OH)6 hybridized with anionic and cationic graphenes as a new high-capacity anode for lithium ion batteries. Mater. Des. 2017, 118, 294–303. [Google Scholar] [CrossRef]
- Prabakar, S.R.; Babu, R.S.; Oh, M.; Lah, M.S.; Han, S.C.; Jeong, J.; Pyo, M. Dense CoO/graphene stacks via self-assembly for improved reversibility as high performance anode in lithium ion batteries. J. Power Sources 2014, 272, 1037–1045. [Google Scholar] [CrossRef]
- Manukumar, K.N.; Kishore, B.; Manjunath, K.; Nagaraju, G. Mesoporous Ta2O5 nanoparticles as an anode material for lithium ion battery and an efficient photocatalyst for hydrogen evolution. Int. J. Hydrogen Energy 2018, 43, 18125–18135. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Pei, Y.; Li, S.; Cao, X.; Masse, R.C.; Cao, G. Novel carbon-encapsulated porous SnO2 anode for lithiumion batteries with much improved cyclic stability. Small 2016, 12, 1945–1955. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Shen, D.; Zhao, D.; Wang, G. Graphitic carbon conformal coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 2015, 137, 13161–13166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Que, W.; Zhang, J.; Zhong, P. Photocatalytic activity of SnWO4 and SnW3O9 nanostructures prepared by a surfactant-assisted hydrothermal process. Mater. Sci. Eng. B 2011, 176, 1448–1455. [Google Scholar] [CrossRef]
- Ede, S.R.; Kundu, S. Microwave synthesis of SnWO4 nanoassemblies on DNA scaffold: A novel material for high performance supercapacitor and as catalyst for butanol oxidation. ACS Sustain. Chem. Eng. 2015, 3, 2321–2336. [Google Scholar] [CrossRef]
- Solis, J.L.; Lantto, V. Gas-sensing properties of different α-SnWO4-based thick films. Phys. Scripta 1997, 1997, 281. [Google Scholar] [CrossRef]
- Warmuth, L.; Feldmann, C. β-SnWO4 with morphology-controlled synthesis and facet-depending photocatalysis. ACS Omega 2019, 4, 13400–13407. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Kwak, C.H.; Kim, D.W.; Lee, S.; Hong, K.S. Photophysical, photoelectrochemical, and photocatalytic properties of novel SnWO4 oxide semiconductors with narrow band gaps. J. Phys. Chem. C 2009, 113, 10647–10653. [Google Scholar] [CrossRef]
- Huang, R.; Ge, H.; Lin, X.; Guo, Y.; Yuan, R.; Fu, X.; Li, Z. Facile one-pot preparation of α-SnWO4/reduced graphene oxide (RGO) nanocomposite with improved visible light photocatalytic activity and anode performance for Li-ion batteries. RSC Adv. 2012, 3, 1235–1242. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alsaiari, M.A.; Jalalah, M.S.; Shashank, M.; Alghamdi, A.A.; Algethami, J.S.; Ganganagappa, N. Combustion synthesis of β-SnWO4-rGO: Anode material for Li-ion battery and photocatalytic dye degradation. Ceram. Int. 2020, 47, 10291–10300. [Google Scholar] [CrossRef]
- Ansar, M.T.; Ali, A.; Mustafa, G.M.; Afzal, F.; Ishaq, S.; Kanwal, F.; Atiq, S. Polypyrrole-based nanocomposites architecture as multifunctional material for futuristic energy storage applications. J. Alloy. Compd. 2021, 855, 157341. [Google Scholar] [CrossRef]
- Mustafa, G.M.; Atiq, S.; Abbas, S.K.; Riaz, S.; Naseem, S. Tunable structural and electrical impedance properties of pyrochlores based Nd doped lanthanum zirconate nanoparticles for capacitive applications. Ceram. Int. 2018, 44, 2170–2177. [Google Scholar] [CrossRef]
- Pavithra, N.S.; Patil, S.B.; Kumar, S.K.; Alharthi, F.A.; Nagaraju, G. Facile synthesis of nanocrystalline β-SnWO4: As a photocatalyst, biosensor and anode for Li-ion battery. SN Appl. Sci. 2019, 1, 1123. [Google Scholar] [CrossRef]
- Quader, A.; Mustafa, G.M.; Abbas, S.K.; Ahmad, H.; Riaz, S.; Naseem, S.; Atiq, S. Efficient energy storage and fast switching capabilities in Nd-substituted La2Sn2O7 pyrochlores. Chem. Eng. J. 2020, 396, 125198. [Google Scholar] [CrossRef]
- Garadkar, K.M.; Ghule, L.A.; Sapnar, K.B.; Dhole, S.D. A facile synthesis of ZnWO4 nanoparticles by microwave assisted technique and its application in photocatalysis. Mater. Res. Bull. 2013, 48, 1105–1109. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X.; Wan, L.; Deng, Y.; Zhan, S. Synthesis of ZnFe2O4 nanoplates by succinic acid-assisted hydrothermal route and their photocatalytic degradation of rhodamine B under visible light. J. Environ. Chem. Eng. 2014, 2, 123–130. [Google Scholar] [CrossRef]
- Mani, S.; Vediyappan, V.; Chen, S.M.; Madhu, R.; Pitchaimani, V.; Chang, J.Y.; Liu, S.B. Hydrothermal synthesis of NiWO4 crystals for high performance non-enzymatic glucose biosensors. Sci. Rep. 2016, 6, 24128. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chen, J.S.; Lou, X.W. One-dimensional hierarchical structures composed of novel metal oxide nanosheets on a carbon nanotube backbone and their lithium-storage properties. Adv. Funct. Mater. 2011, 21, 4120–4125. [Google Scholar] [CrossRef]
- Shim, H.W.; Cho, I.S.; Hong, K.S.; Lim, A.H.; Kim, D.W. Wolframite-type ZnWO4 nanorods as new anodes for Li-ion batteries. J. Phys. Chem. C 2011, 115, 16228–16233. [Google Scholar] [CrossRef]
- Patil, S.B.; Kishore, B.; Nagaraju, G.; Dupont, J. High capacity MoO3/rGO nanocomposite anode for lithium ion batteries: An intuition into the conversion mechanism of MoO3. New J. Chem. 2018, 42, 18569–18577. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Kumar, J.V.; Chen, S.M.; Karthik, R.; Elangovan, A.; Muthuraj, V. One-step sonochemical synthesis of 1D β-stannous tungstate nanorods: An efficient and excellent electrocatalyst for the selective electrochemical detection of antipsychotic drug chlorpromazine. Ultrason. Sonochem. 2018, 44, 231–239. [Google Scholar] [CrossRef] [PubMed]

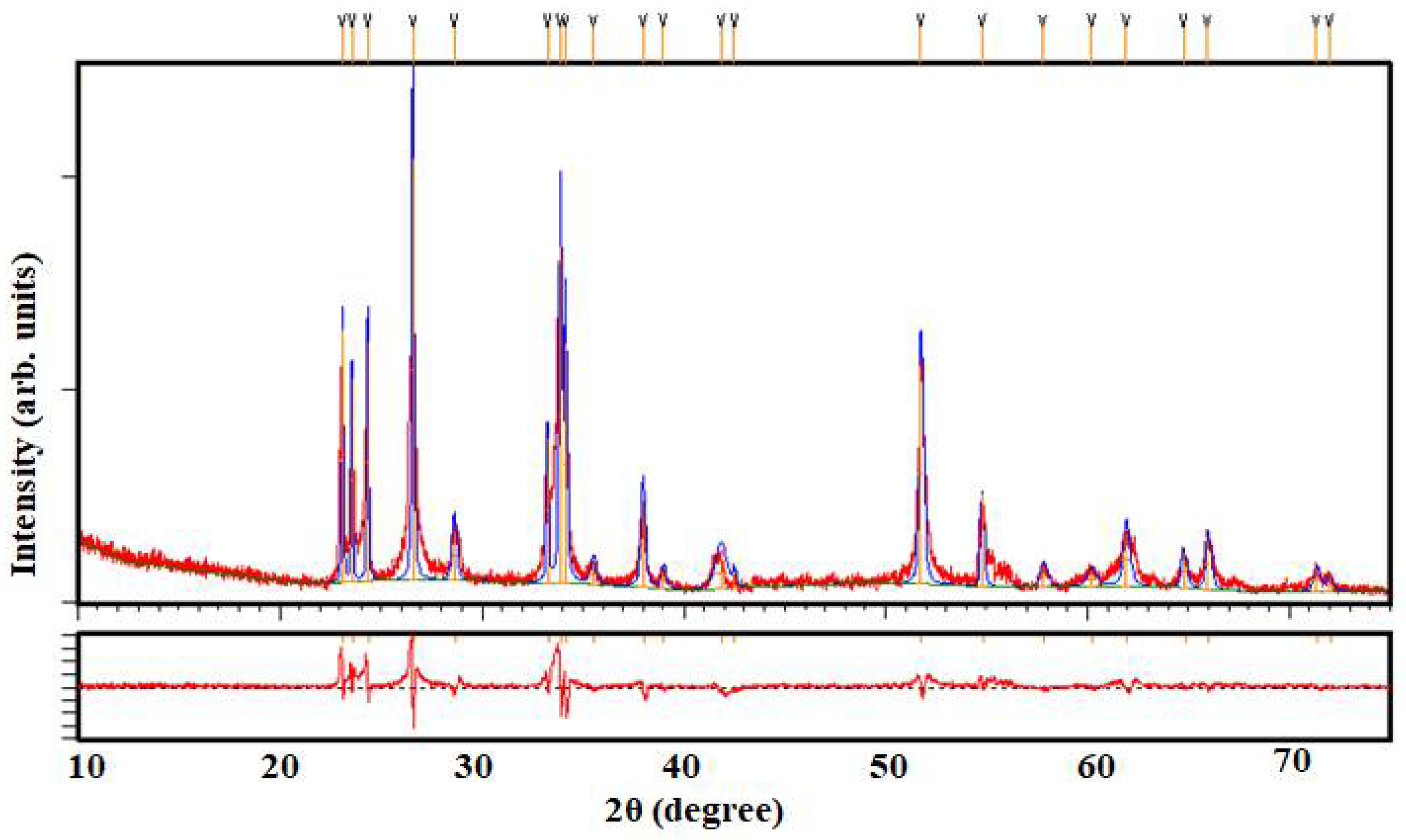

| Rexp | Rp | Rwp | Dstatics | Wt. Dstatics | χ2 |
|---|---|---|---|---|---|
| 10.84 | 13.73 | 18.04 | 0.44 | 0.56 | 2.77 |
| Composition | Synthesis Method | C-Rates | Specific Capacity (mAhg−1) | References |
|---|---|---|---|---|
| β-SnWO4–rGO | Solution combustion method followed by a low temperature hydrothermal method | 0.1 C | 578 | [21] |
| β-SnWO4 | ~ | 0.1 C | 271 | [21] |
| β-SnWO4–rGO | ~ | 3 C | 165 | [21] |
| β-SnWO4 | ~ | 3 C | 35 | [21] |
| β-SnWO4 | Rapid microwave heat treatment | 0.1 C | 600 | Current work |
| β-SnWO4 | ~ | 3 C | 54 | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumedha, H.N.; Alsaiari, M.A.; Jalalah, M.S.; Shashank, M.; Alharthi, F.A.; Ahmad, N.; Algethami, J.S.; Vishwanth, V.; Ganganagappa, N. Rapid Microwave Synthesis of β-SnWO4 Nanoparticles: An Efficient Anode Material for Lithium Ion Batteries. Crystals 2021, 11, 334. https://doi.org/10.3390/cryst11040334
Sumedha HN, Alsaiari MA, Jalalah MS, Shashank M, Alharthi FA, Ahmad N, Algethami JS, Vishwanth V, Ganganagappa N. Rapid Microwave Synthesis of β-SnWO4 Nanoparticles: An Efficient Anode Material for Lithium Ion Batteries. Crystals. 2021; 11(4):334. https://doi.org/10.3390/cryst11040334
Chicago/Turabian StyleSumedha, H. N., Mabkhoot A. Alsaiari, Mohammed S. Jalalah, M. Shashank, Fahad A. Alharthi, Naushad Ahmad, Jari S. Algethami, Vishwanth Vishwanth, and Nagaraju Ganganagappa. 2021. "Rapid Microwave Synthesis of β-SnWO4 Nanoparticles: An Efficient Anode Material for Lithium Ion Batteries" Crystals 11, no. 4: 334. https://doi.org/10.3390/cryst11040334
APA StyleSumedha, H. N., Alsaiari, M. A., Jalalah, M. S., Shashank, M., Alharthi, F. A., Ahmad, N., Algethami, J. S., Vishwanth, V., & Ganganagappa, N. (2021). Rapid Microwave Synthesis of β-SnWO4 Nanoparticles: An Efficient Anode Material for Lithium Ion Batteries. Crystals, 11(4), 334. https://doi.org/10.3390/cryst11040334







