Structures of Impurity Defects in Lithium Niobate and Tantalate Derived from Electron Paramagnetic and Electron Nuclear Double Resonance Data
Abstract
:1. Introduction
- high quality conventional samples with crystal composition xC are usually grown from the congruent melt with the composition xm ≈ 48.4%, for LN and xm ≈ 48.7% for LT (x = [Li]/([Li] + [Nb/Ta]); this means that the congruent crystals with xm = xC contain many intrinsic (non-stoichiometric) defects, causing a broadening of the observable spectral lines and ambiguities in their interpretation;
- the crystal composition xC of the undoped samples depends on both melt composition and growth conditions;
- the most probable positions for impurity incorporation, the Li and Nb sites as well as the octahedral structural vacancy, have the same local symmetry C3; this means that they are not distinguishable by many spectroscopic techniques.
- Mössbauer spectroscopy demands the presence of special nuclei,
- channeling investigations are more successful in the case of heavy, many-electron ions,
- channeling methods are not sensitive to the charge state of the impurity and do not distinguish centers with C3 and C1 symmetry,
- due to the relatively low sensitivity the EXAFS needs high levels of crystal dopants (about 3–5 mol.%), for which clustering and occupation of both Li and Nb positions become very probable,
- EPR/ENDOR techniques are applicable to systems with non-zero spins only.
2. Materials and Methods
3. Basics of EPR and ENDOR Spectra Interpretation
3.1. Symmetry Considerations
3.2. Isotropic and Dipole-Dipole Interactions
- to calculate for the several nearest nuclei around the impurity taking lattice distances from X-ray data and gjm from EPR measurements,
- to calculate ENDOR frequencies, to plot patterns of their angular dependencies, and
- to measure the road map of angular dependencies in three perpendicular planes (Figure 4a),
- to determine all components of tensors by fitting observed angular dependencies,
- to separate isotropic and anisotropic parts,
- to find principal values of the anisotropic part, and finally,
- to compare these principal values with calculated ones on the base of Equation (3).
3.3. Charge Compensation and Intrinsic Defects
3.4. Di-Vacancy Models for Trivalent Impurities
- Impurity ion like Nd3+ has an off-axis lattice defect (charge compensator) in the immediate neighborhood.
- Impurity ion substitutes for O2− (this is very improbable for cations).
- Impurity ion incorporates into a small tetrahedral void (this is possible, but often unlikely due to larger charge misfit than for Li substitution).
4. Structures of Impurity Centers
4.1. Monovalent Cations
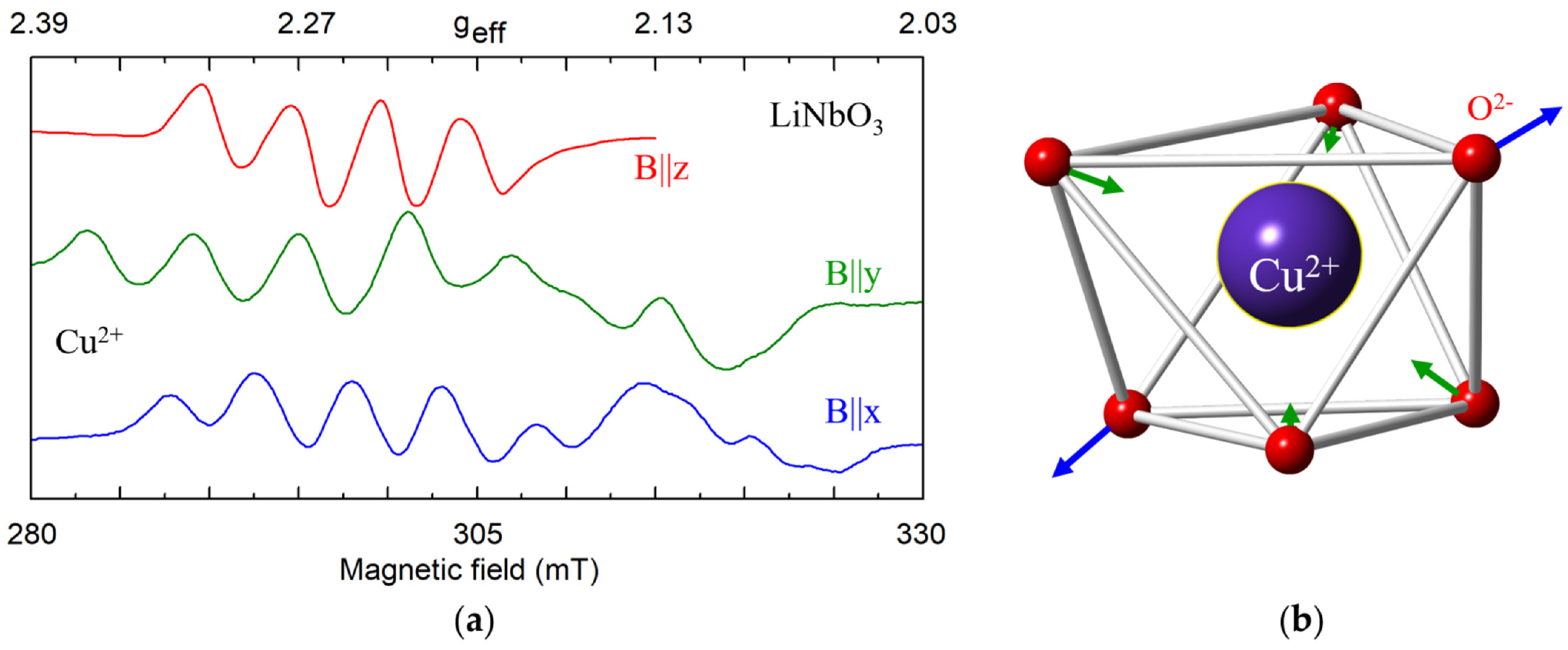
4.2. Divalent Cations
4.3. Trivalent Cations
- Lines of allowed transitions in LNK become symmetric—intensities of left (up) and right (down) wings become equal.
- Lines of forbidden transitions (see yellow box on Figure 18) disappear in LNK.
4.4. Tetra-, Penta- and Heptavalent Cations
4.5. Radiation and Reduction Defects
5. Impurity Identification
6. Conclusions
- the compensation by nonstoichiometric defects (vLi, vNb) for small concentrations of MeLi in crystals,
- the partial self-compensation of charges of MeLi by MeNb mainly for the MeLi concentration that exceeds the concentration of vLi,
- the partial compensation of MeNb by interstitial Li+ in voct,
- the compensation by other impurities (H, co-dopants like Mg, Zn, etc.).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Räuber, A. Chemistry and Physics of Lithium Niobate. In Current Topics in Materials Science, 1st ed.; Kaldis, E., Ed.; North-Holland: Amsterdam, The Netherlands, 1978; Volume 1, pp. 481–601. [Google Scholar]
- Volk, T.; Wöhlecke, M. Point defects in LiNbO3. In Lithium Niobate. Defects, Photorefraction and Ferroelectric Switching, 1st ed.; Hull, R., Osgood, R.M., Jr., Parisi, J., Warlimont, H., Eds.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2009; Volume 115, pp. 1–50. [Google Scholar]
- Ilyenkov, A.V.; Khiznyak, A.I.; Kreminskaya, L.V.; Soskin, M.S.; Vasnetsov, M.V. Birth and evolution of wave-front dislocations in a laser beam passed through a photorefractive LiNbO3: Fe crystal. Appl. Phys. B 1996, 62, 465–471. [Google Scholar] [CrossRef]
- Prokhorov, A.M.; Kuz’minov, Y.S. Physics and Chemistry of Crystalline Lithium Niobate; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Properties of Lithium Niobate; Wong, K.K. (Ed.) EMIS Data Review Series No. 5; The Institution of Electrical Engineers, INSPEC: London, UK, 2002. [Google Scholar]
- Toney, J.E. Lithium Niobate Photonics; Artech House Publishers: London, UK, 2015; pp. 1–265. [Google Scholar]
- Streque, J.; Aubert, T.; Kokanyan, N.; Bartoli, F.; Taguett, A.; Polewczyk, V.; Kokanyan, E.; Hage-Ali, S.; Boulet, P.; Elmazria, O. Stoichiometric Lithium Niobate Crystals: Towards Identifiable Wireless Surface Acoustic Wave Sensors Operable up to 600 °C. IEEE Sens. Lett. 2019, 3, 2501204. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Reddy, J.M.; Bernstein, J.L. Ferroelectric lithium niobate. 3. Single crystal X-ray diffraction study at 24 °C. J. Phys. Chem. Solids 1966, 27, 997–1012. [Google Scholar] [CrossRef]
- Peterson, G.E.; Bridenbaugh, P.M.; Green, P. NMR Study of Ferroelectric LiNbO3 and LiTaO3. J. Chem. Phys. 1967, 46, 4009–4014. [Google Scholar] [CrossRef]
- Vianden, R. Hyperfine techniques for defects studies. In Insulating Materials for Optoelectronics. New Developments; Agulló-López, F., Ed.; World Scientific Publishing: Singapore; Hackensack, NJ, USA; London, UK; Hong Kong, China, 1995; pp. 125–146. [Google Scholar]
- Kling, A.; Soares, J.C.; da Silva, M.F. Channeling Investigation of Oxide materials for Optoelectronic Applications. In Insulating Materials for Optoelectronics. New Development; Agulló-López, F., Ed.; World Scientific Publishing: Singapore; Hackensack, NJ, USA; London, UK; Hong Kong, China, 1995; pp. 175–200. [Google Scholar]
- Gog, T.; Marerlik, G. X-ray Standing Wave Determination of Lattice Positions of Impurities in Lithium Niobate. In Insulating Materials for Optoelectronics. New Developments; Agulló-López, F., Ed.; World Scientific Publishing: Singapore; Hackensack, NJ, USA; London, UK; Hong Kong, China, 1995; pp. 201–220. [Google Scholar]
- Spaeth, J.-M.; Niklas, J.R.; Bartram, R.H. Structural Analysis of Point Defects in Solids: An Introduction to Multiple Magnetic Resonance Spectroscopy; Springer: Singapore, 1992; pp. 1–367. [Google Scholar]
- Prieto, C. Influence of lithium niobate stoichiometry on the lattice position of Nd3+ in Nd:LiNbO3. Opt. Mater. 1999, 12, 135–142. [Google Scholar] [CrossRef]
- Lorenzo, A.; Jaffrezic, H.; Roux, B.; Boulon, G.; Garcia-Sole, J. Lattice location of rare-earth ions in LiNbO3. Appl. Phys. Lett. 1995, 67, 3735–3737. [Google Scholar] [CrossRef]
- Lorenzo, A.; Loro, H.; Muñoz Santiuste, J.E.; Terrile, M.C.; Boulon, G.; Bausa, L.E.; Garcia Sole, J. RBS/channeling to locate active ions in laser materials: Application to rare earth activated LiNbO3. Opt. Mater. 1997, 8, 55–63. [Google Scholar] [CrossRef]
- Rebouta, L.; Smulders, P.J.M.; Boerma, D.O.; Agullo-Lopez, F.; da Silva, M.F.; Soares, J.C. Ion-beam channeling yields of host and impurity atoms in LiNbO3: Computer simulations. Phys. Rev. B 1993, 48, 3600–3610. [Google Scholar] [CrossRef]
- Rebouta, L.; da Silva, M.F.; Soares, J.C.; Santos, M.T.; Dieguez, E.; Agulló-López, F. Ion-beam/channeling characterization of LiNbO3: Interaction between impurity sites. Opt. Mater. 1995, 4, 174–178. [Google Scholar] [CrossRef]
- Garcia Sole, J.; Petith, T.; Jaffrez, H.; Boulon, G. Lattice Location of the Non-Equivalent Nd3+ Ions in LiNbO3: Nd and LiNbO3: MgO:Nd. Europhys. Lett. 1993, 24, 719–724. [Google Scholar] [CrossRef]
- Vila, M.; de Bernabe, A.; Prieto, C. EXAFS determination of the Nd lattice position in Nd:LiNbO3: Influence of lithium niobate stoichiometry and Mg and Zn co-doping. J. Alloys Compd. 2001, 323–324, 331–335. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Chadwick, A.V.; Cole, M.; Tomlinson, S.M. An EXAFS study of dopant substitution in LiNbO3 and LiTaO3. Radiat. Eff. Defects Solids 1991, 119–121, 565–570. [Google Scholar] [CrossRef]
- Bush, T.S.; Catlow, C.R.A.; Chadwick, A.V.; Cole, M.; Geatches, R.M.; Greaves, G.N.; Tomlinson, S.M. Studies of cation dopant sites in metal oxides by EXAFS and computer-simulation techniques. J. Mater. Chem. 1992, 2, 309–316. [Google Scholar] [CrossRef]
- Prieto, C.; Zaldo, C. Determination of the lattice site of Fe in photorefractive LiNbO3. Solid State Commun. 1992, 83, 819–821. [Google Scholar] [CrossRef]
- Zaldo, C.; Prieto, C. Lattice site of transition metal and rare-earth impurities in LiNbO3 single crystals: An exafs study. Ferroelectrics 1992, 134, 47–51. [Google Scholar] [CrossRef]
- Corradi, G.; Chadwick, A.V.; West, A.R.; Cruickshankand, K.; Paul, M. On the substitution site of Cr and Fe in LiNbO3: An exafs study. Radiat. Eff. Defects Solids 1995, 134, 219–222. [Google Scholar] [CrossRef]
- Sanson, A.; Zaltron, A.; Argiolas, N.; Sada, C.; Bazzan, M.; Schmidt, W.G.; Sanna, S. Polaronic deformation at the Fe2+/3+ impurity site in Fe:LiNbO3 crystals. Phys. Rev. B 2015, 91, 094109. [Google Scholar] [CrossRef]
- Rebouta, L.; da Silva, M.F.; Soares, J.C.; Hage-Ali, M.; Stoquert, J.P.; Siffert, P.; Sanz-García, J.A.; Diéguez, E.; Agulló-López, F. Lattice Site of Iron in LiNbO3(Fe3+) by the PIXE/Channelling Technique. Europhys. Lett. 1991, 14, 557–561. [Google Scholar] [CrossRef]
- Gog, T.; Schotters, P.; Falta, J.; Materlik, G.; Grodzicki, M. The lattice position of Fe in Fe-doped LiNbO3. J. Phys. Condens. Matter 1995, 7, 6971–6980. [Google Scholar] [CrossRef]
- Marques, J.G.; Kling, A.; Soares, J.C.; Rebouta, L.; da Silva, M.F.; Diéguez, E.; Agulló-López, F. Lattice site location of Hf in LiNbO3: Influence of dopant concentration and crystal stoichiometry. Nucl. Instrum. Methods Phys. Res. B 1998, 136–138, 431–435. [Google Scholar] [CrossRef]
- Mignotte, C. Determination of lattice site locations of erbium ions implanted into LiNbO3 single crystals after annealing at moderate and high temperature. Appl. Surf. Sci. 2001, 185, 11–26. [Google Scholar] [CrossRef]
- Kovacs, L.; Rebouta, L.; Soaresh, J.C.; da Silva, M.F.; Hage-Ali, M.; Stoquert, J.P.; Siffert, P.; Sanz-Garcia, J.A.; Corradi, G.; Szaller, Z. On the lattice site of trivalent dopants and the structure of Mg2+-OH--M3+ defects in LiNbO3:Mg crystals. J. Phys. Condens. Matter 1993, 5, 781–794. [Google Scholar] [CrossRef]
- Kling, A.; Soares, J.C.; da Silva, M.F.; Sanz-Garcia, J.A.; Dieguez, E.; Agullo-Lopez, F. Lattice site determination of Cr in low doped lithium niobate single crystals using PIXE/channeling. Nucl. Instrum. Methods Phys. Res. B 1998, 136–138, 426–430. [Google Scholar] [CrossRef]
- Engelmann, H.; Mouahid, F.; Dezsi, I.; Molnar, B.; Gonser, U.; Siebert, D.; Dahlem, J.; Tuczek, F. Mössbauer and ESR study of LiNbO3-Fe2O3 for low Fe2O3 concentrations. Appl. Phys. A 1989, 48, 211–217. [Google Scholar] [CrossRef]
- Tomov, T.; Engelmann, H.; Dezci, I.; Gonser, U. Investigation of the ferroelectric phase transition in LiNbO3: Fe by Mössbauer spectroscopy. Solid State Commun. 1989, 69, 41–44. [Google Scholar] [CrossRef]
- Mignoni, S.; Fontana, M.D.; Bazzan, M.; Ciampolillo, M.V.; Zaltron, A.M.; Argiolas, N.; Sada, C. Micro-Raman analysis of Fe-diffused lithium niobate waveguides. Appl. Phys. B 2010, 101, 541. [Google Scholar] [CrossRef]
- Kamińska, A.; Suchocki, A.; Kobyakov, S.; Arizmendi, L.; Potemski, M.; Teran, F.J. High-pressure and magneto-optical studies of Cr-related defects in the lithium-rich LiNbO3:Cr, Mg crystal. Phys. Rev. B 2007, 76, 144117. [Google Scholar] [CrossRef]
- Basun, S.; Kaplyanskii, A.; Kutsenko, A.; Dierolf, V.; Troester, T.; Kapphan, S.E.; Polgar, K. Optical characterization of Cr3+ centers in LiNbO3. Appl. Phys. B 2001, 73, 453–461. [Google Scholar] [CrossRef]
- Sothe, H.; Rowan, L.G.; Spaeth, J.-M. Sites of Fe3+ impurities in a congruent LiTaO3 crystal. J. Phys. Condens. Matter 1989, 1, 3591–3600. [Google Scholar] [CrossRef]
- Sothe, H.; Spaeth, J.-M. The site and local environment of Fe3+ in LiNbO3 investigated with ENDOR. J. Phys. Condens. Matter 1992, 4, 9901–9906. [Google Scholar] [CrossRef]
- Spaeth, J.-M. Determination of atomic positions of point defects in solids by ENDOR. Appl. Magn. Reson. 1992, 3, 257–281. [Google Scholar] [CrossRef] [Green Version]
- Lerner, P.; Legras, C.; Dumas, J.P. Stoechiometrie des monocristaux de metaniobate de lithium. J. Cryst. Growth 1968, 3, 231–235. [Google Scholar] [CrossRef]
- Byer, R.L.; Young, J.F.; Feigelson, R.S. Growth of High-Quality LiNbO3 Crystals from the Congruent Melt. J. Appl. Phys. 1970, 41, 2320–2325. [Google Scholar] [CrossRef]
- Malovichko, G.I.; Grachev, V.G.; Gabrielyan, V.T.; Kokanyan, E.P. Widths and intensities of ESR lines of iron-group impurities in nonstoichiometric lithium niobate crystals. Sov. Phys. Solid State 1986, 28, 1453–1458. [Google Scholar]
- Iyi, N.; Kitamura, K.; Izumi, F.; Yamamoto, J.; Hayashi, T.; Asano, H.; Kimura, S. Comparative of defect structures in lithium niobate with different compositions. J. Solid State Chem. 1992, 101, 340–352. [Google Scholar] [CrossRef]
- Holman, R.L. Processing of Crystalline Ceramics; Palmour, H., Davis, R.F., Eds.; Plenum: New York, NY, USA, 1978; p. 343. [Google Scholar]
- Jundt, D.H.; Fejer, M.M.; Byer, R.L. Optical properties of lithium-rich lithium niobate fabricated by vapor transport equilibration. IEEE J. Quantum Electron. 1990, 26, 135–138. [Google Scholar] [CrossRef]
- Bordui, P.F.; Norwood, R.G.; Bird, C.D.; Calvert, G.D. Compositional uniformity in growth and poling of large-diameter lithium niobate crystals. J. Cryst. Growth 1991, 113, 61–68. [Google Scholar] [CrossRef]
- Bordui, P.F.; Norwood, R.G.; Jundt, D.H.; Fejer, M.M. Preparation and characterization of off-congruent lithium niobate crystals. J. Appl. Phys. 1992, 71, 875–879. [Google Scholar] [CrossRef] [Green Version]
- Jundt, D.H.; Fejer, M.M.; Norwood, R.G.; Bordui, P.F. Composition dependence of lithium diffusivity in lithium niobate at high temperature. J. Appl. Phys. 1992, 72, 3468–3473. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Kapphan, S.; Feng, X.-Q.; Cheng, N. Sharp R-lines in absorption and emission of Cr3+ in stoichiometric (VTE) LiNbO3. Radiat. Eff. Defects Solids 1995, 135, 199–202. [Google Scholar] [CrossRef]
- Furukawa, Y.; Sato, M.; Kitamura, K.; Yajima, Y.; Minakata, M. Optical damage resistance and crystal quality of LiNbO3 single crystals with various [Li]/[Nb] ratios. J. Appl. Phys. 1992, 72, 3250–3254. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamamoto, J.; Iyi, N.; Kirnura, S.; Hayashi, T. Stoichiometric LiNbO3 single crystal growth by double crucible Czochralski method using automatic powder supply system. J. Cryst. Growth 1992, 116, 327–332. [Google Scholar] [CrossRef]
- Kitamura, K.; Furukawa, Y.; Iyi, N. Progress in single crystal growth of LiNbO3 using double crucible Czochralski method. Ferroelectrics 1997, 202, 21–28. [Google Scholar] [CrossRef]
- Kitamura, K.; Furukawa, Y.; Ji, Y.; Zgonik, M.; Medrano, C.; Montemezzani, G.; Guenter, P. Photorefractive effect in LiNbO3 crystals enhanced by stoichiometry control. J. Appl. Phys. 1997, 82, 1006–1009. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kitamura, K.; Suzuki, E.; Niwa, K. Stoichiometric LiTaO3 single crystal growth by double crucible Czochralski method using automatic powder supply system. J. Cryst. Growth 1999, 197, 889–895. [Google Scholar] [CrossRef]
- Malovichko, G.I.; Grachev, V.G.; Yurchenko, L.P.; Proshko, V.Y.; Kokanyan, E.P.; Gabrielyan, V.T. Improvement of LiNbO3 microstructure by crystal growth with potassium. Phys. Status Solidi (a) 1992, 133, K29–K33. [Google Scholar] [CrossRef]
- Malovichko, G.I.; Grachev, V.G.; Kokanyan, E.; Schirmer, O.F.; Betzler, K.; Gather, B.; Jermann, F.; Klauer, S.; Schlarb, U. Characterization of stoichiometric LiNbO3 grown from melts containing K2O. Appl. Phys. A 1993, 56, 103–108. [Google Scholar] [CrossRef]
- Bermudez, V.; Dutta, P.S.; Serrano, M.D.; Dieguez, E. The effect of native defects on the domain structures of Fe—A case study by means of the addition of MgO and K2O to the congruent melt. J. Phys. Condens. Matter 1997, 9, 6097–6101. [Google Scholar] [CrossRef]
- Polgar, K.; Peter, A.; Kovacs, L.; Corradi, G.; Szaller, Z. Growth of stoichiometric LiNbO3 single crystals by top seeded solution growth method. J. Cryst. Growth 1997, 177, 211–216. [Google Scholar] [CrossRef]
- Balasanyan, R.N.; Vartanyan, E.S.; Gabrielyan, V.T.; Kazaryan, L.M. Method for Growing Lithium Niobate Single Crystals. Patent SU 845506 A1, 29 March 1979. Available online: https://www.elibrary.ru/item.asp?id=41087794 (accessed on 27 February 2000).
- Serrano, M.D.; Bermudez, V.; Arizmendi, L.; Dieguez, E. Determination of the Li/Nb ratio in LiNbO3 crystals grown by Czochralski method with K2O added to the melt. J. Cryst. Growth 2000, 210, 670–675. [Google Scholar] [CrossRef]
- Kokanyan, E.; Dieguez, E. New perspectives of lithium niobate crystals. J. Optoelectron. Adv. Mater. 2000, 2, 205–214. [Google Scholar]
- Polgar, K.; Peter, A.; Foldvari, I.; Szaller, Z. Structural defects in flux-grown stoichiometric LiNbO3 single crystals. J. Cryst. Growth 2000, 218, 327–333. [Google Scholar] [CrossRef]
- Polgár, K.; Péter, Á.; Földvári, I. Crystal growth and stoichiometry of LiNbO3 prepared by the flux method. Opt. Mater. 2002, 19, 7–11. [Google Scholar] [CrossRef]
- Polgár, K.; Péter, Á.; Pöppl, L.; Ferriol, M.; Földvári, I. Chemical and thermal conditions for the formation of stoichiometric LiNbO3. J. Crystal Growth 2002, 237–239, 682–686. [Google Scholar] [CrossRef]
- Grunskij, O.S.; Denisov, A.V.; Badmaev, T.V. A Method for Growing Doped Crystals of Lithium Niobate with a Composition close to Stoichiometric, and a Device for Its Implementation. Patent RU 2367730 C2, 29 November 2007. Available online: https://www.elibrary.ru/item.asp?id=37690005 (accessed on 20 September 2009).
- Lengyel, K.; Péter, Á.; Kovács, L.; Corradi, G.; Pálfalvi, L.; Hebling, J.; Unferdorben, M.; Dravecz, G.; Hajdara, I.; Szaller, Z.; et al. Growth, defect structure, and THz application of stoichiometric lithium niobate. Appl. Phys. Rev. 2015, 2, 040601. [Google Scholar] [CrossRef] [Green Version]
- Malovichko, G.; Grachev, V.; Schirmer, O. Interrelation of intrinsic and extrinsic defects—Congruent, stoichiometric, and regularly ordered lithium niobate. Appl. Phys. B 1999, 68, 785–793. [Google Scholar] [CrossRef]
- Malovichko, G.I.; Grachev, V.G.; Schirmer, O.F.; Faust, B. New axial Fe3+ centers in stoichiometric lithium niobate crystals. J. Phys. Condens. Matter 1993, 5, 3971–3976. [Google Scholar] [CrossRef]
- de Bernabe, A.; Prieto, C.; de Andres, A. Effect of stoichiometry on the dynamic mechanical properties of LiNbO3. J. Appl. Phys. 1995, 79, 143–148. [Google Scholar] [CrossRef]
- Garret, M.H.; Mnushkina, I.; Furukawa, Y.; Kitamura, K.; Halliburton, L.E.; Giles, N.C.; Setzler, S.D. Photorefractive Properties of Iron-Doped Congruent Lithium Niobate, and Iron-Doped K2O Flux Grown Stoichiometric Lithium Niobate. Proceedings of 1997 Topical Meeting on Photorefractive Materials, Effects and Devices, Chiba, Japan, 11–13 June 1997. [Google Scholar]
- Abdi, F.; Aillerie, M.; Bourson, P.; Fontana, M.D.; Polgar, K. Electro-optic properties in pure LiNbO3 crystals from the congruent to the stoichiometric composition. J. Appl. Phys. 1998, 84, 2251–2254. [Google Scholar] [CrossRef]
- Choi, Y.N.; Choh, S.H.; Park, I.W.; Koh, E.K.; Kim, S.S. Characterization of Stoichiometry of LiNbO3 Crystals by EPR and Raman Scattering Measurements. J. Korean Phys. Soc. 1998, 32, S643. [Google Scholar]
- Malovichko, G. Nonstoichiometry as a powerful tool for photorefractive material optimization. Lithium Niobate Crystals. In Advances in Photorefractive Materials, Effects and Devices; Andersen, P.E., Johansen, P.M., Pedersen, H.C., Petersen, P.M., Saffman, M., Eds.; Trends in Optics and Photonics; Optical Society of America: Washington, DC, USA, 1999; Volume 27. [Google Scholar] [CrossRef]
- Malovichko, G.; Petersen, R.; Bäuman, C.; Grachev, V. Second axial Fe3+ center in stoichiometric lithium tantalate. J. Appl. Phys. 2006, 100, 023911. [Google Scholar] [CrossRef]
- Razdobarin, A.G.; Basun, S.A.; Bursian, V.É.; Sochava, L.S.; Evans, D.R. A Fe[Nb]-Li center in stoichiometric LiNbO3 crystals: Mechanism of formation. Phys. Solid State 2010, 52, 706–711. [Google Scholar] [CrossRef]
- Schlarb, U.; Klauer, S.; Wesselmann, M.; Betzler, K. Determination of the Li/Nb ratio in lithium niobate by means of birefringence and Raman measurements. Appl. Phys. A 1993, 56, 311–315. [Google Scholar] [CrossRef]
- Kling, A.; Marques, J.G.; Correia, J.G.; da Silva, M.F.; Dieguez, E.; Agullo-Lopez, F.; Soares, J.C. Study of structural differences between stoichiometric and congruent lithium niobate. Nucl. Instrum. Methods Phys. Res. B 1996, 113, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, L.; Ruschhaupt, G.; Polgar, K.; Corradi, G.; Woehlecke, M. Composition dependence of the ultraviolet absorption edge in lithium niobate. Appl. Phys. Lett. 1997, 70, 2801–2803. [Google Scholar] [CrossRef]
- Dravecz, G.; Kovács, L. Determination of the crystal composition from the OH- vibrational spectrum in lithium niobate. Appl. Phys. B 2007, 88, 305–307. [Google Scholar] [CrossRef]
- Grachev, V.G. Visual EPR, Visual ENDOR Packages, Version 20.11. Available online: www.visual-epr.com (accessed on 1 June 2013).
- CrystalMaker Program, Version 2.7.7; CrystalMaker Software Ltd.: Oxford, UK; Available online: www.crystalmaker.com (accessed on 31 December 2011).
- Boysen, H.; Altorfer, F. A neutron powder investigation of the high-temperature structure and phase transition in LiNbO3. Acta Crystallogr. B 1994, 50, 405–414. [Google Scholar] [CrossRef]
- Hsu, R.; Maslen, E.N.; du Boulay, D.; Ishizawa, N. Synchrotron X-ray Studies of LiNbO3 and LiTaO3. Acta Crystallogr. B 1997, 53, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Malovichko, G.; Cerclier, O.; Estienne, J.; Grachev, V.; Kokanyan, E.; Boulesteix, C. Lattice constants of K- and Mg-doped LiNbO3. Comparison with nonstoichiometric lithium niobate. J. Phys. Chem. Sol. 1995, 56, 1285–1289. [Google Scholar] [CrossRef]
- Lehnen, H.; Boysen, H.; Frey, F.; Hewat, A.; Radaelli, P. A neutron powder investigation of the high-temperature structure and phase transition in stoichiometric LiNbO3. Zeitschrift für Kristallographie 1997, 212, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Malovichko, G.; Grachev, V.; Kokanyan, E.; Schirmer, O. Axial and low-symmetry centers of trivalent impurities in lithium niobate. Chromium in congruent and stoichiometric crystals. Phys. Rev. B 1999, 59, 9113–9125. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Hamilton, W.C.; Reddy, J.M. Ferroelectric lithium niobate. 4. Single crystal neutron diffraction study at 24 °C. J. Phys. Chem. Solids 1966, 27, 1013–1018. [Google Scholar] [CrossRef]
- Megaw, H.D. A note on the structure of lithium niobate, LiNbO3. Acta Crystallogr. A 1968, 24, 583–588. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Levinstein, H.J.; Reddy, J.M. Ferroelectric lithium niobate. 5. Polycrystal X-ray diffraction study between 24° and 1200 °C. J. Phys. Chem. Solids 1966, 27, 1019–1026. [Google Scholar] [CrossRef]
- Malovichko, G.; Grachev, V.; Okulov, S.; Kokanyan, E.; Henecker, F.; Hofstaetter, A.; Schirmer, O. EPR of Nd3+ in congruent and nearly stoichiometric lithium niobate. Phys. Status Solidi (b) 2005, 243, 1–7. [Google Scholar] [CrossRef]
- Malovichko, G.; Bratus, V.; Munro, M.; Kokanyan, E.; Okulov, S.; Grachev, V. Multifrequency spectroscopy of laser active centers Nd3+ and Yb3+ in nearly stoichiometric LiNbO3. Phys. Status Solidi (c) 2007, 4, 1346–1351. [Google Scholar] [CrossRef]
- Grachev, V.; Munro, M.; Kokanyan, E.; Malovichko, G. Determination of g-tensors of low-symmetry Nd3+ centers in LiNbO3 by rectification of angular dependence of electron paramagnetic resonance spectra. J. Appl. Phys. 2015, 118, 044103. [Google Scholar] [CrossRef]
- Blümel, J.; Born, E.; Metzger, T. Solid state NMR study supporting the lithium vacancy defect model in congruent lithium niobate. J. Phys. Chem. Solids 1994, 55, 589–593. [Google Scholar] [CrossRef]
- Zotov, N.; Boysen, H.; Frey, F.; Metzger, T.; Born, E. Cation substitution models of congruent LiNbO3 investigated by X-ray and neutron powder diffraction. J. Phys. Chem. Solids 1994, 55, 145–152. [Google Scholar] [CrossRef]
- Leroux, C.; Nihoul, G.; Malovichko, G.; Grachev, V.; Boulesteix, C. High resolution electron microscopy investigation of correlated defects in non-stoichiometric lithium niobate. J. Phys. Chem. Solids 1998, 59, 311–319. [Google Scholar] [CrossRef]
- Kim, S.; Gopalan, V.; Kitamura, K.; Furukawa, Y. Domain reversal and nonstoichiometry in lithium tantalate. J. Appl. Phys. 2001, 90, 2949–2963. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Marsh, P. Defect structure dependence on composition in lithium niobate. Acta Crystallogr. B 1986, 42, 61–68. [Google Scholar] [CrossRef]
- Peterson, G.E.; Carnevale, A. 93Nb NMR linewidths in nonstoichiometric lithium niobate. J. Chem. Phys. 1972, 56, 4648–4851. [Google Scholar] [CrossRef]
- Araujo, R.M.; dos Santos Mattos, E.F.; Valerio, M.E.G.; Jackson, R.A. Computer Simulation of the Incorporation of V2+, V3+, V4+, V5+ and Mo3+, Mo4+, Mo5+, Mo6+ Dopants in LiNbO3. Crystals 2020, 10, 457. [Google Scholar] [CrossRef]
- Kuzminov, Y.; Osiko, V.V. Disorder of stoichiometry in lithium niobate crystals. Cryst. Rep. 1994, 39, 471–476. [Google Scholar]
- Fay, H.; Alford, W.J.; Dess, H.M. Temperature in LiNbO3 Crystals on Melt Composition. Appl. Phys. Lett. 1968, 12, 89. [Google Scholar] [CrossRef]
- Suzuki, T. Oxygen partial pressure dependence of optical absorption in lithium niobate. J. Cryst. Growth 1996, 163, 403–410. [Google Scholar] [CrossRef]
- Sweeney, K.L.; Halliburton, L.E.; Bryan, D.A.; Rice, R.R.; Gerson, R.; Tomaschke, H.E. Point defects in Mg-doped lithium niobate. J. Appl. Phys. 1985, 57, 1036–1044. [Google Scholar] [CrossRef]
- Erdei, S.; Ainger, F.W. Trends in the growth of stoichiometric single crystals. J. Cryst. Growth 1997, 174, 293–300. [Google Scholar] [CrossRef]
- Kovács, L.; Wohlecke, M.; Jovanović, A.; Polgár, K.; Kapphan, S. Infrared absorption study of the OH vibrational band in LiNbO3 crystals. J. Phys. Chem. Solids 1991, 52, 797–803. [Google Scholar] [CrossRef]
- Engelsberg, M.; de Souza, R.E.; Pacobahyba, L.H.; do Nascimento, G.C. Structural determination of hydrogen site occupation in proton-exchanged LiNbO3 by nuclear magnetic resonance. Appl. Phys. Lett. 1995, 67, 359–361. [Google Scholar] [CrossRef]
- Yevdokimov, S.V.; Yatsenko, A.V. Investigation of the Localization of H+ Ions in Stoichiometric LiNbO3. Crystallogr. Rep. 2003, 48, 542–546. [Google Scholar] [CrossRef]
- Rakitina, L.G.; Zaritskii, I.M.; Polgar, K. New hydrogen associated paramagnetic centre in LiNbO3. Appl. Magn. Reson. 1990, 1, 149–154. [Google Scholar] [CrossRef]
- Rosa, J.; Polak, K.; Kubatova, J. ESR and optical studies of impurity centres in γ- and X-irradiated LiNbO3. Phys. Status Solidi (b) 1982, 111, K85–K87. [Google Scholar] [CrossRef]
- Corradi, G.; Polgar, K.; Bugai, A.A.; Grachev, V.G.; Deryugina, N.I.; Rakitina, L.M.; Zaritskii, I.M. Models of Ni+ and Cu2+ impurity centers in LiNbO3. Sov. Phys. Solid State 1986, 28, 739–748. [Google Scholar]
- Zhang, H.-M.; Xiao, W.-B. Investigations on the EPR parameters and defect structures due to Jahn-Teller effect for the Cu2+ and Ni+ centers in LiNbO3. J. Alloys Compd. 2018, 745, 586–591. [Google Scholar] [CrossRef]
- Zaritskii, I.M.; Rakitina, L.G.; Polgar, K. Annealing and gamma irradiation effects on the ESR in LiNbO3:Mg crystals. Sov. Phys. State Solid 1995, 37, 1073–1978. [Google Scholar]
- Faust, B.; Müller, H.; Schirmer, O.F. Free small polarons in LiNbO3. Ferroelectrics 1994, 153, 297–302. [Google Scholar] [CrossRef]
- Mirzakhanyan, A.A.; Petrosyan, A.K. EPR and optical absorption of Co2+ impurity ions in single crystal α-LiIO3 and LiNbO3. Sov. Phys. Solid State 1986, 904–906, 1593–1595. [Google Scholar]
- Donnerberg, H.J.; Shirmer, O.F. The paramagnetic properties of Co2+ in LiNbO3 and LiTaO3. Solid State Commun. 1987, 63, 29–32. [Google Scholar] [CrossRef]
- Choi, Y.N.; Park, I.-W.; Kim, S.S.; Park, S.S.; Choh, S.H. Electron paramagnetic resonance studies of Co2+ ions in congruent and nearly stoichiometric LiNbO3 single crystals. J. Phys. Condens. Matter 1999, 11, 4723–4730. [Google Scholar] [CrossRef]
- Grachev, V.G.; Hansen, K.; Meyer, M.; Kokanyan, E.P.; Malovichko, G.I. Substitution mechanisms and location of Co2+ ions in congruent and stoichiometric lithium niobate crystals derived from electron paramagnetic resonance data. Mater. Res. Express 2017, 4, 036204. [Google Scholar] [CrossRef]
- Paul, M.; Tabuchi, M.; Weast, A.R. Defect Structure of Ni, Co-doped LiNbO3 and LiTaO3. Chem. Mater. 1997, 9, 3206–3214. [Google Scholar] [CrossRef]
- Mei, Y.; Wu, X.-X.; Zheng, W.-C. EPR parameters and defect structures for the Co2+ ions in LiNbO3 and LiTaO3 crystals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 714–719. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Lu, G.D.; Zhang, Z.H.; Wei, L.H.; Hu, Y.-X. Theoretical investigations of the local structures and spin Hamiltonian parameters for the trigonal Co2+ centers in LiNbO3 and LiTaO3. J. Alloys Compd. 2009, 1–2, 1–5. [Google Scholar] [CrossRef]
- Setser, G.G. An Investigation by Electron Paramagnetic Resonance of the Jahn-Teller Effect in LiNbO3:Ni3+ and LiNbO3:Cu2+. Ph.D. Thesis, Rice University, Houston, TX, USA, 1974; pp. 1–237. [Google Scholar]
- Palatnikov, M.N.; Sidorov, N.V.; Skiba, V.I.; Biryukova, I.V.; Serebryakov, Y.A.; Kravchenko, O.E.; Balabanov, Y.I.; Kalinnikov, V.T. Effects of nonstoichiometry and doping on the curie temperature and defect structure of lithium niobate. Inorg. Mater. 2000, 36, 489–493. [Google Scholar] [CrossRef]
- Pape, M.; Reyher, H.-J.; Schirmer, O.F. Optically detected magnetic resonance of Cu, Fe and Mn defects in LiNbO3. J. Phys. Condens. Matter 2005, 17, 6835–6847. [Google Scholar] [CrossRef]
- Mirzakhanyan, A.A. Zero-field splitting of energy levels of Ni2+ ground state in α-LiIO3 and LiNbO3. Sov. Phys. State Solid 1981, 23, 2452. [Google Scholar]
- Khalil, A.; Masaif, N.; Jennane, A.; Maaider, K. Substitution Mechanism of Ni:LiTaO3. J. Mater. Environ. Sci. 2011, 2, 196–200. [Google Scholar]
- Yang, Z.Y.; Rudowicz, C.; Yeung, Y.Y. Microscopic spin-Hamiltonian parameters and crystal field energy levels for the low C3 symmetry Ni2+ centre in LiNbO3 crystals. Physica B 2004, 348, 151–159. [Google Scholar] [CrossRef]
- Petrov, M.P. Spectrum of Electron Paramagnetic Resonance of Mn2+ in ferroelectric LiNbO3. Sov. Phys. Solid State 1968, 10, 2574. [Google Scholar]
- Takeda, T.; Watanabe, A.; Sugihara, K. Spacing of the hyperfine sextet in Mn2+ ESR in LiNbO3. Phys. Lett. A 1968, 27, 114–115. [Google Scholar] [CrossRef]
- Danner, J.C.; Ranon, U.; Stamires, D.N. ESR of Mn2+ in ferroelectric LiTaO3 and LiNbO3 single crystals. Chem. Phys. Lett. 1968, 2, 605–606. [Google Scholar] [CrossRef]
- Herrington, J.B.; Dischler, B.; Schneider, J. An EPR investigation of Fe3+ and Mn2+ in LiNbO3. Solid State Commun. 1972, 10, 509–511. [Google Scholar] [CrossRef]
- Rexford, D.J.; Kim, Y.M. Electron-Spin Resonance Studies of Crystal Field Parameters in Mn2+:LiNbO3. J. Chem. Phys. 1972, 57, 3094–3098. [Google Scholar] [CrossRef]
- Malovichko, G.I.; Grachev, V.G. Use of nuclear quadrupole splitting in determination of positions of iron group impurities in ferroelectric LiNbO3. Sov. Phys. Solid. State 1985, 27, 1678–1679. [Google Scholar]
- Malovichko, G.I.; Grachev, V.G.; Lukin, S.N. EPR of axial and low symmetry Cr3+, Fe3+, and Mn2+ centers in LiNbO3. Sov. Phys. Solid State 1986, 28, 553–557. [Google Scholar]
- Malovichko, G.I.; Karmazin, A.A.; Bykov, I.P.; Laguta, V.V.; Yarunichev, V.P. Electron spin resonance investigation of the temperature dependence of the axial crystal field parameters in Mn2+ and Cr3+ doped LiNbO3. Sov. Phys. Solid State 1983, 25, 2038–2041. [Google Scholar]
- Park, I.W.; Choh, S.H.; Song, K.J. The hyperfine structure of Mn2+ in LiNbO3 and the sign of the second-order axial zero-field splitting. J. Korean Phys. Soc. 1993, 26, 77–80. [Google Scholar]
- Park, I.-W. Three-fold Symmetry Effect on Mn2+ Centers in a LiNbO3 Crystal. J. Korean Magn. Res. Soc. 2008, 12, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Yeom, T.H.; Lee, S.H. Temperature Dependence of Mn2+ Paramagnetic Ion in a Stoichiometric LiNbO3 Single Crystal. J. Magn. 2013, 18, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Corradi, G.; Söthe, H.; Spaeth, J.-M.; Polgar, K. Mn2+ defects in LiNbO3: An electron nuclear double resonance (ENDOR) investigation of the Mn2+ site and the local disorder. J. Phys. Condens. Matter. 1990, 2, 6603–6618. [Google Scholar] [CrossRef]
- Malovichko, G.; Grachev, V.; Hofstaetter, A.; Kokanyan, E.; Scharmann, A.; Schirmer, O. ENDOR study of Cr3+ centers substituting for lithium in lithium niobate. Phys. Rev. B 2002, 65, 224116. [Google Scholar] [CrossRef]
- Grachev, V.; Malovichko, G. EPR, ENDOR, and optical-absorption study of Cr3+ centers substituting for niobium in Li-rich lithium niobate crystals. Phys. Rev. B 2000, 62, 7779–7790. [Google Scholar] [CrossRef]
- Grachev, V.; Malovichko, G.; Schirmer, O. Structures of point defects in lithium niobate. Ukr. J. Phys. 2004, 49, 438–448. [Google Scholar]
- Evlanova, N.F.; Kornienko, L.S.; Rashkovich, L.N.; Rybaltovskii, A.O. EPR of some rare-earth ions and Cr3+ in LiNbO3. Sov. JETP 1967, 53, 1920–1926. [Google Scholar]
- Burns, G.; O’Kane, D.F.; Title, R.S. Optical and Electron-Spin-Resonance Spectra of Yb3+, Nd3+, and Cr3+ in LiNbO3 and LiTaO3. Phys. Rev. 1968, 167, 314–318. [Google Scholar] [CrossRef]
- Rexford, D.J.; Kim, Y.M.; Story, H.S. Electron-Spin resonance studies of Cr3+ in LiNbO3. J. Chem. Phys. 1970, 52, 860–863. [Google Scholar] [CrossRef]
- Choh, S.H.; Kim, H.T.; Choh, H.K.; Han, C.S.; Choi, D.; Kim, J.N. Characterization of Stoichiometry of LiNbO3 Crystals by EPR and Raman Scattering Measurements. Bull. Magn. Res. 1989, 11, 371. [Google Scholar]
- Salley, G.M.; Basun, S.A.; Imbusch, G.F.; Kaplyanskii, A.A.; Kapphan, S.; Meltzer, R.S.; Happek, U. Chromium centers in LiNbO3 revisited. J. Luminescence 1999, 83–84, 423–427. [Google Scholar] [CrossRef]
- Grachev, V.; Malovichko, G.; Schirmer, O. Single, dimer and trimer chromium centers in lithium niobate. Ferroelectrics 1996, 185, 5–8. [Google Scholar] [CrossRef]
- Grachev, V.G.; Malovichko, G.I.; Troitskii, V.V. Investigation of the charge compensation mechanisms of Cr3+ ions in LiNbO3 by EPR and ENDOR methods. Sov. Phys. Solid State 1987, 29, 349–350. [Google Scholar]
- Siu, G.G.; Zhao, M.-G. Electron-spin-resonance studies of Cr-ion pairs in LiNbO3: Cr3+. Phys. Rev. B 1991, 43, 13575–13578. [Google Scholar] [CrossRef] [PubMed]
- Corradi, G.; Sothe, H.; Spaeth, J.-M.; Polgar, K. Electron spin resonance and electron-nuclear double-resonance investigation of a new Cr3+ defect on an Nb site in LiNbO3:Mg:Cr. J. Phys. Condens. Matter 1991, 3, 1901–1908. [Google Scholar] [CrossRef]
- Corradi, G.; Sothe, H.; Spaeth, J.-M.; Polgar, K. ENDOR for characterizing transition metal centres in LiNbO3. Radiat. Eff. Defects Solids 1991, 119–121, 583–587. [Google Scholar] [CrossRef]
- Corradi, G.; Söthe, H.; Spaeth, J.-M.; Polgar, K. Local environment of Mn2+ and Cr3+ centres in LiNbO3: Discussion of recent ENDOR results. Ferroelectrics 1992, 125, 295–299. [Google Scholar] [CrossRef]
- Diazcaro, J.; Garcia-Sole, J.; Bravo, D.; Han, T.P.J.; Jaque, F.; Henderson, B. EPR and infrared absorption study in MgO Co-Doped LiNbO3 crystals. Ferroelectr. Lett. Sect. 1997, 23, 27–35. [Google Scholar] [CrossRef]
- Thiemann, O.; Corradi, G.; Reyher, H.-J. ODMR-investigations of transition metals in LiNbO3. Ferroelectrics 1992, 125, 283–288. [Google Scholar] [CrossRef]
- Camarillo, E.; Garcia-Sole, J.; Cusso, F.; Agullo-Lopez, F.; Sanz-Garcia, J.A.; Han, T.P.J.; Jaque, F.H.; Henderson, B. Polarization spectroscopy of Cr3+ ions in LiNbO3 single crystals: Effect of Mg2+ ions. Chem. Phys. Lett. 1991, 185, 505–510. [Google Scholar] [CrossRef]
- Camarillo, E.; Tocho, J.; Vergara, I.; Dieguez, E.; Garcia-Sole, J.; Jaque, F. Optical bands of Cr3+ induced by Mg2+ ions in LiNbO3:Cr,Mg. Phys. Rev. B 1992, 45, 4600–4604. [Google Scholar] [CrossRef]
- Martin, A.; Lopez, F.J.; Agullo-Lopez, F. Cr3+ in pure and Mg-doped LiNbO3: Analysis of the EPR and optical spectra. J. Phys. Condens. Matter 1992, 4, 847–853. [Google Scholar] [CrossRef]
- Macfarlane, P.I.; Holliday, K.; Nicholls, J.F.H.; Henderson, B. Characterization of Cr3+ centres in LiNbO3 using fluorescence line narrowing. J. Phys. Condens. Matter. 1995, 7, 9643–9656. [Google Scholar] [CrossRef]
- Diaz-Caro, J.; Garcia-Sole, J.; Bravo, D.; Sanz-Garcia, J.A.; Lopez, F.J.; Jaque, F. MgO codoping-induced change in the site distribution of Cr3+ ions in LiNbO3. Phys. Rev. B 1996, 54, 13042–13046. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Caro, J.; Garcia-Sole, J.; Martinez, J.L.; Henderson, B.; Jaque, F.; Han, T.P.J. Redistribution of Cr3+ defect centres in LiNbO3 crystals: The MgO effect. Opt. Mater. 1998, 10, 69–77. [Google Scholar] [CrossRef]
- Torchia, G.A.; Martinez Matos, O.; Vaveliuk, P.; Tocho, J.O. Influence of the electron-lattice coupling for Cr3+ ions in Nb5+ site into congruent co-doped LiNbO3: Cr3+: ZnO crystal. Solid State Commun. 2003, 127, 535–539. [Google Scholar] [CrossRef]
- Ahn, S.W.; Rudowicz, C.; Choh, S.H.; Han, S.Y. EPR study of two Cr3+defect centers in LiTaO3 single crystals. J. Korean Phys. Soc. 1997, 30, 99–102. [Google Scholar]
- Ahn, S.W.; Kim, J.S.; Choh, S.H.; Yeom, T.H. An Induced Cr3+ Center in gamma-Irradiated LiTaO3. J. Korean Phys. Soc. 1994, 27, 535–538. [Google Scholar]
- Loyo-Menoyo, M.; Keeble, D.J.; Furukawa, Y.; Kitamura, K. Electron paramagnetic resonance of Cr3+ in near-stoichiometric LiTaO3. J. Appl. Phys. 2005, 97, 123905. [Google Scholar] [CrossRef]
- Bourson, P.; Malovichko, G.; Ridah, A.; Kokanyan, E. Effect of chromium concentration on site selective luminescence in nearly stoichiometric lithium niobate crystals. Ferroelectrics 1996, 155, 273–276. [Google Scholar] [CrossRef]
- Lhomme, F.; Bourson, P.; Fontana, M.D.; Malovichko, G.; Aillerie, M.; Kokanyan, E. Luminescence of Cr3+ in lithium niobate: Influence of the chromium concentration and crystal composition. J. Phys. Condens. Matter 1998, 10, 1137–1146. [Google Scholar] [CrossRef]
- Lhomme, F.; Bourson, P.; Moncorge, R.; Burlot, R.; Fontana, M.D.; Aillerie, M.; Boulon, G.; Malovichko, G. New study of the 720–750 nm range in the emission spectra of LiNbO3:Cr3+ crystals with different compositions. Radiat. Eff. Defects Solids 1999, 150, 259–263. [Google Scholar] [CrossRef]
- Kaminska, A.; Suchocki, A.; Grinberg, M.; Garcia-Sole, J.; Jaque, F.; Arizmendi, L. High-pressure spectroscopy of LiNbO3:MgO, Cr3+ crystals. J. Lumin. 2000, 87–89, 571–573. [Google Scholar] [CrossRef]
- Salley, G.M.; Basun, S.A.; Kaplyanskii, A.A.; Meltzer, R.S.; Polgar, K.; Happek, U. Chromium centers in stoichiometric LiNbO3. J. Lumin 2000, 87–89, 1133–1135. [Google Scholar] [CrossRef]
- Dierolf, V.; Kaplyanskii, A.A.; Kapphan, S.; Kutsenko, A.B. The fine structure of spectral response of Cr3+ sites in LiNbO3. In Radiat. Eff. Defects Solids; 2001; Volume 155, pp. 241–246. [Google Scholar] [CrossRef]
- Basun, S.A.; Salley, G.M.; Kaplyanskii, A.A.; Gallagher, H.G.; Polgar, K.; Lu, L.; Happek, U. A novel luminescent center in LiNbO3:Cr:Mg crystals. J. Lumin. 1999, 83–84, 435–439. [Google Scholar] [CrossRef]
- Lhomme, F.; Bourson, P.; Boulon, G.; Guyot, Y.; Fontana, M.D. Comparative analysis of the Cr3+ centre spectroscopic properties in LiNbO3 crystals from congruent to nearly stoichiometric compositions. Eur. Phys. J. AP 2002, 20, 29–40. [Google Scholar] [CrossRef]
- Han, T.P.J.; Jaque, F.; Bermudez, V.; Dieguez, E. Luminescence of the Cr3+ R-lines in pure and MgO co-doped near stoichiometric LiNbO3:Cr crystals. Chem. Phys. Lett. 2003, 369, 519–524. [Google Scholar] [CrossRef]
- Han, T.P.J.; Jaque, F. Optical stability of the Cr3+ centres in codoped stoichiometric and congruent LiNbO3:Cr:Mg. Opt. Mater. 2007, 29, 1041–1043. [Google Scholar] [CrossRef]
- Kaczmarek, S.M.; Lukasiewicz, T.; Pracka, I.; Jablonski, R.; Boulon, G.; Kaczmarek, B.; Warchol, S. Radiation defects in Dy3+ doped LiNbO3 single crystals. J. Alloys Compd. 1998, 275–277, 105–108. [Google Scholar] [CrossRef]
- Jablonski, R.; Pracka, I.; Swirkowicz, M. Electron spin resonance spectra of Nd3+, Dy3+, Er3+ and Yb3+ in LiNbO3. In Solid State Crystals: Growth and Characterization, Proceedings of the XII Conference on Solid State Crystals: Materials Science and Applications, Zakopane, Poland, 7–11 October 1996; SPIE: Bellingham, WA, USA, 1997. [Google Scholar] [CrossRef]
- Milori, D.M.B.P.; Moraes, I.J.; Hernandes, A.C.; de Souza, R.R.; Li, M.S.; Terrile, M.C.; Barberis, G.E. Optical and ESR study of Er3+ in LiNbO3. Phys. Rev. B 1995, 51, 3206—3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, T.; Pawlik, T.; Spaeth, J.-M. EPR study of Er3+ in congruent LiNbO3. Solid State Commun. 1997, 104, 535–539. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M. EPR study of low symmetry Er centers in congruent lithium niobate. Phys. Status Solidi (b) 2008, 245, 998–1002. [Google Scholar] [CrossRef]
- Kaczmarek, S.M.; Bodziony, T. Low symmetry centers in LiNbO3 doped with Yb and Er. J. Non-Cryst. Solids 2008, 354, 4202–4210. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M. Temperature dependence of the EPR spectra and optical measurements of LiNbO3: Er, Tm single crystal. J. Alloys Compd. 2009, 468, 581–585. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M.; Kruk, R. Low temperature magnetic measurements of LiNbO3 single crystal weakly doped with Er and codoped with Tm ions. J. Spectrosc. Dyn. 2011, 1, 1–8. [Google Scholar]
- Vrable, I.; Malovichko, G.; Grachev, V.; Meyer, M. EPR/ENDOR Studies of Erbium Centers in Stoichiometric Lithium Niobate Crystals. In Proceedings of the 49th Rocky Mountain Conference on Analytical Chemistry, Breckenridge, CO, USA, 22–26 July 2007; Abstract C 143. p. 71. Available online: http://rockychem.com/links/past-conferences.html (accessed on 1 March 2007).
- Malovichko, G.; Grachev, V.; Jorgensen, J.; Meyer, M.; Munro, M.; Todt, B.; Vrable, I.; Kokanyan, E.; Bratus, V.; Okulov, S. Magnetic resonance study of non-equivalent centers created by 4f-ions in congruent and stoichiometric lithium niobate. MRS Online Proc. Libr. 2008, 1111, 103. [Google Scholar] [CrossRef]
- Mackova, A.; Groetzschel, R.; Eichhorn, F.; Nekvindova, P.; Spirkova, J. Characterization of Er:LiNbO3 and APE:Er:LiNbO3 by RBS–channeling and XRD techniques. Surf. Interface Anal. 2004, 36, 949–951. [Google Scholar] [CrossRef]
- Gog, T.; Griebenow, M.; Materlik, G. X-ray standing wave determination of the lattice location of Er diffused into LiNbO3. Phys. Lett. A 1993, 181, 417–420. [Google Scholar] [CrossRef]
- Rebouta, L.; da Silva, M.F.; Soares, J.C.; Serrano, D.; Diéguez, E.; Agulló-López, F.; Tornero, J. Nonaxial sites for Er in LiNbO3. J. Appl. Phys. Lett. 1997, 70, 1070–1072. [Google Scholar] [CrossRef]
- Malovichko, G.; Vrable, I.; Meyer, M.; Kokanyan, E.; Grachev, V. Ordering magnetic moments of Er3+ in ferroelectric lithium niobate. In Proceedings of the International Conference “Oxide Materials for Electronic Engineering—Fabrication, Properties and applica$tion” (OMEE-2014), Lviv, Ukraine, 26–30 May 2014; pp. 35–36. [Google Scholar]
- Gill, D.M.; Wright, J.C.; McCaughan, L. Site characterization of rare-earth-doped LiNbO3 using total site selective spectroscopy. J. Appl. Phys. Lett. 1994, 64, 2483–2485. [Google Scholar] [CrossRef] [Green Version]
- Gill, D.M.; McCaughan, L.; Wright, J.C. Spectroscopic site determinations in erbium-doped lithium niobate. Phys. Rev. B 1996, 53, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Muntoz, J.A.; Cantelar, E.; Sanz-garcia, J.A.; Duchowicz, R.; Serrano, D.; Dieguez, E.; Lifante, G.; Cusso, F. Site-selective spectroscopy of Er3+ and Er3+ Yb3+ doped stoichiometric and congruent lithium niobate. Radiat. Eff. Defects Solids 1999, 150, 221–225. [Google Scholar] [CrossRef]
- Dierolf, V.; Koerdt, M. Combined excitation-emission spectroscopy of Er3+ ions in stoichiometric LiNbO3: The site selectivity of direct and up conversion excitation processes. Phys. Rev. B 2000, 61, 8043–8048. [Google Scholar] [CrossRef]
- Dierolf, V.; Kutsenko, A.B.; Ostendorf, A.; von der Osten, W.; Sohler, W.; Suche, H. Site-selective spectroscopy of Er3+:Ti:LiNbO3 waveguides. Appl. Phys. B 2001, 72, 803–810. [Google Scholar] [CrossRef]
- Dierolf, V.; Kutsenko, A.B.; Sandmann, C.; Troester, T.; Corradi, G. High-resolution site selective optical spectroscopy of rare earth and transition metal defects in insulators. J. Lumin. 2000, 87–89, 989–991. [Google Scholar] [CrossRef]
- Dierolf, V.; Sandmann, C. Combined excitation emission spectroscopy of defects for site-selective probing of ferroelectric domain inversion in lithium niobate. J. Lumin. 2007, 125, 67–79. [Google Scholar] [CrossRef]
- Bravo, D.; Martin, A.; Lopez, F.J. A new centre of Er3+ in MgO or ZnO co-doped LiNbO3 single crystals. Solid State Commun. 1999, 112, 541–544. [Google Scholar] [CrossRef]
- Mehran, F.; Scott, B.A. Electron paramagnetic resonance of LiNbO3: Fe3+. Solid State Commun. 1972, 11, 15–19. [Google Scholar] [CrossRef]
- Towner, H.H.; Kim, Y.M.; Story, H.S. EPR studies of crystal field parameters in Fe3+: LiNbO3. J. Chem. Phys. 1972, 56, 3676–3679. [Google Scholar] [CrossRef]
- Dikantova, Z. EPR study of impurities centers in LiNbO3. Acta Phys. Slov. 1973, 23, 245–250. [Google Scholar]
- Keune, W.; Date, S.K.; Gonser, U.; Bunzel, H. Mossbauer effect study of Fe57 doped LiNbO3 and LiTaO3. Ferroelectrics 1976, 13, 443–445. [Google Scholar] [CrossRef]
- Kurz, H.; Krätzig, E.; Keune, W.; Engelmann, H.; Gonser, U.; Dischler, B.; Räuber, A. Photorefractive centers in LiNbO3, studied by optical-, Mössbauer- and EPR-methods. Appl. Phys. 1977, 12, 355–368. [Google Scholar] [CrossRef]
- Grachev, V.G.; Malovichko, G.I. Determination of the point symmetry of defects in crystals exhibiting structural phase transitions, using the temperature dependencies of the EPR spectra. Impurities in LiNbO3. Sov. Phys. Solid State 1985, 27, 686–689. [Google Scholar]
- Pfannes, H.D.; Putzka, A.; Sampaio, J.F. Electronic structure and spin relaxation of Fe(III) in LiNbO3. Hyperfine Interact. 1986, 28, 785–788. [Google Scholar] [CrossRef]
- Jablonski, R.; Kaczmarek, S.M.; Praska, I.; Surma, B.; Swirkowicz, M.; Lukasiewicz, T. ESR and optical measurements of LiNbO3 and LiTaO3 single crystals doped with ions of the first transition series. Spectrochim. Acta A 1998, 54, 1701–1709. [Google Scholar] [CrossRef]
- Yeom, T.H.; Choh, S.H. Magnetic Resonance Investigations of LiNbO3 and LiTaO3 Single Crystals. J. Korea Phys. Soc. 1998, 32, S672–S674. [Google Scholar]
- Santana, R.C.; Terrile, M.C.; Hernandes, A.C.; Andreeta, M.R.B.; Barberis, G.E. Electron spin resonance study of Fe3+ in LiNbO3 single crystals: Bulk and fibres. Solid State Commun. 1997, 103, 61–64. [Google Scholar] [CrossRef]
- Keeble, D.J.; Loyo-Menoyo, M.; Furukawa, Y.; Kitamura, K. Electron paramagnetic resonance of Fe3+ in LiNbO3. Phys. Rev. B 2005, 71, 224111. [Google Scholar] [CrossRef]
- Agullò-López, F.; Müller, K.A. Superposition Model Calculations for Fe3+ in LiNbO3. Cryst. Latt. Def. Amorph. Mater. 1987, 15, 89. [Google Scholar]
- Zhao, M.G.; Chiu, M. Substitution site of the Fe3+ impurity in crystalline LiNbO3. Phys. Rev. B 1994, 49, 12556–12558. [Google Scholar] [CrossRef]
- Wang, H.; Kuang, X.Y.; Die, D.; Tan, X.-M.; Yang, X. EPR spectra and local lattice structure of Fe3+ impurity ions in ferroelectric LiNbO3. Chem. Phys. 2006, 330, 212–215. [Google Scholar] [CrossRef]
- Wang, H.; Kuang, X.Y.; Die, D.; Yang, X.; Zhou, K.-W. EPR investigation of substitution position for Fe3+ in LiNbO3:Fe3+ system. Physica B 2005, 367, 53–60. [Google Scholar] [CrossRef]
- Yeom, T.H.; Chang, Y.M.; Choh, S.H.; Rudowicz, C. Experimental and Theoretical Investigation of Spin-Hamiltonian Parameters for the Low Symmetry Fe3+ Centre in LiNbO3. Phys. Status Solidi (b) 1994, 185, 409–415. [Google Scholar] [CrossRef]
- Xue, D.; He, X. Dopant occupancy and structural stability of doped lithium niobate crystals. Phys. Rev. B 2006, 73, 064113. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, D. Local Lattice Structure and Dopant Occupancy Of Doped Lithium Niobate Crystals. Mod. Phys. Lett. B 2009, 23, 3687–3694. [Google Scholar] [CrossRef]
- Basun, S.A.; Bursian, V.E.; Evans, D.R.; Kaplyanskii, A.A.; Razdobarin, A.G.; Sochava, L.S. Ferroelectric-Specific Stark Effect in Stoichiometric LiNbO3:Fe at Room Temperature. Phys. Rev. Lett. 2008, 100, 057602. [Google Scholar] [CrossRef]
- Grachev, V.G.; Petersen, R.T.; Kokanyan, E.P.; Schirmer, O.F.; Malovichko, G.I. Structural analysis of the dominant axial Fe3+ center in LiNbO3 crystal by electron nuclear double resonance. J. Appl. Phys. 2016, 120, 193901. [Google Scholar] [CrossRef]
- Boker, A.; Donnerberg, H.; Schirmer, O.F.; Feng, X. Two sites of Fe3+ in highly Mg-doped LiNBO3. J. Phys. Condens. Matter 1990, 2, 6865–6868. [Google Scholar] [CrossRef]
- Volk, T.R.; Rubinina, N.M.; Pryalkin, V.I.; Krasnikov, V.V.; Volkov, V.V. Optical and non-linear optical investigations in LiNbO3:Mg and LiNbO3:Zn. Ferroelectrics 1990, 109, 345–350. [Google Scholar] [CrossRef]
- Yeom, T.H.; Lee, S.H.; Choh, S.H.; Choi, D. Electron paramagnetic resonance study of Fe3+ in LiNbO3:Mg:Fe crystal. J. Korean Phys. Soc. 1998, 32, S647. [Google Scholar]
- Malovichko, G.I.; Grachov, V.G.; Kokanyan, E.P. Low-symmetry Fe-Mg complexes in LiNbO3:Mg. Ferroelectrics 1992, 125, 289–294. [Google Scholar] [CrossRef]
- Grachev, V.; Malovichko, G.; Kokanyan, E. Optimization of lithium niobate for advanced applications by variation of extrinsic and intrinsic defect subsystems. Ferroelectrics 2001, 258, 131–140. [Google Scholar] [CrossRef]
- Volk, T.; Wöhlecke, M.; Rubinina, N.; Razumovskii, N.V.; Jermann, F.; Fischer, C.; Böwer, R. LiNbO3 with the damage-resistant impurity indium. Appl. Phys. A 1995, 60, 217–225. [Google Scholar] [CrossRef]
- Volk, T.R.; Rubinina, N.M. Non photorefractive impurities in lithium niobate: Magnesium and zinc. Sov. Phys. Solid State 1991, 33, 674–680. [Google Scholar]
- Vazhenin, V.A.; Guseva, V.B.; Artyomov, M.Y.; Route, R.K.; Fejer, M.M.; Byer, R.L. Fine structure of the electron paramagnetic resonance spectrum of Fe3+ centres in LiTaO3. J. Phys. Condens. Matter 2003, 15, 275–280. [Google Scholar] [CrossRef]
- Loyo-Menoyo, M.; Keeble, D.J.; Furukawa, Y.; Kitamura, K. Electron paramagnetic resonance of Fe3+ in near-stoichiometric LiTaO3. J. Phys. Condens. Matter 2004, 16, 9047–9057. [Google Scholar] [CrossRef]
- McDonald, P.F.; Tam, C.P.; Mok, Y.W. EPR Gd3+ in LiNbO3. J. Chem. Phys. 1972, 56, 1007–1008. [Google Scholar] [CrossRef]
- Dischler, B.; Herrington, J.R.; Räuber, A.; Schneider, J.; Urban, W. An EPR study of different Gd3+ centers in LiNbO3. Solid State Communs. 1973, 12, 737–740. [Google Scholar] [CrossRef]
- Park, I.-W.; Kim, M.; Choh, S.H.; Kim, J.N. EPR study of Gd3+ Paramagnetic Centers in Congruent LiNbO3 Single Crystal. New Phys. (Korean Phys. Soc.) 1994, 34, 464–469. [Google Scholar]
- Bonardi, C.; Carvalho, R.A.; Basso, H.C.; Terrile, M.C.; Cruz, G.K.; Bausa, L.E.; Sole, J.G. Magnetic circular dichroism of Nd3+ and Yb3+ ions in LiNbO3 crystals. J. Chem. Phys. 1999, 111, 6042–6046. [Google Scholar] [CrossRef]
- Camarillo, E.; Hernandez, J.; Garcia Sole, J.; Caldino, U.; Munoz Santiuste, J.E.; Loro, H.; Voda, M.; Jaque, F.; Murrieta, H. Nd3+ centres induced by ZnO or MgO codoping LiNbO3. J. Phys. Condens. Matter 1995, 7, 96359641. [Google Scholar] [CrossRef]
- Ziling, K.K.; Nadolinnii, V.A.; Shashkin, V.V. Sov.: Reports of AS of USSR. Inorg. Mater. 1980, 16, 701. [Google Scholar]
- Juppe, S.; Schirmer, O.F. EPR Ti3+ in LiNbO3. Phys. Lett. A 1986, 117, 150–155. [Google Scholar] [CrossRef]
- Rakitina, L.G.; Zaritskii, I.M.; Corradi, G.; Polgar, K. Mechanisms of broadening of ESR lines of polaron centers in LiNbO3: Ti subjected to irradiation and heat treatments. Sov. Phys. Solid State 1990, 32, 654–660. [Google Scholar]
- Thiemann, O.; Donnerberg, G.H.; Wöhlecke, M.; Schirmer, O. Vibronic structure, energy level, and incorporation mechanism of Ti3+ in LiNbO3 and LiTaO3. Phys. Rev. B 1994, 49, 5845–5851. [Google Scholar] [CrossRef]
- Corradi, G.; Polgar, K.; Zaritskii, I.M.; Rakitina, L.G.; Deryugina, N.I. Defect formation under γ-irradiation and heat treatment in pure and doped LiNbO3 monocrystals. Sov. Phys. State Solid 1989, 31, 115–122. [Google Scholar]
- Corradi, G.; Zaritskii, I.M.; Hofstaetter, A.; Polgar, K.; Rakitina, L.G. Ti3+ on Nb site: A paramagnetic Jahn-Teller center in vacuum-reduced LiNbO3:Mg:Ti single crystals. Phys. Rev. B 1998, 58, 8329–8337. [Google Scholar] [CrossRef]
- Corradi, G. Nb4+ Polaron and Ti3+ Shallow Donor Jahn-Teller Centers in LiNbO3 Systems. In Defects and Surface-Induced Effects in Advanced Perovskites; Borstel, G., Krumins, A., Millers, D., Eds.; NATO Science Series (Series 3. High Technology); Springer: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2000; Volume 77, pp. 89–100. [Google Scholar] [CrossRef]
- Corradi, G.; Meyer, M.; Kovács, L.; Polgar, K. Gap levels of Ti3+ on Nb or Li sites in LiNbO3:(Mg):Ti crystals and their effect on charge transfer processes. Appl. Phys. B 2004, 78, 607–614. [Google Scholar] [CrossRef]
- Corradi, G.; Meyer, M.; Polgár, K. Bipolarons localised by Ti dopants in reduced LiNbO3 crystals double-doped by Ti and Mg. Phys. Status Solidi (c) 2005, 2, 132–135. [Google Scholar] [CrossRef]
- Bonardi, C.; Magon, C.J.; Vidoto, E.A.; Terrile, M.C.; Bausa, L.E.; Montoya, E.; Bravo, D.; Martin, A.; Lopez, F.J. EPR spectroscopy of Yb3+ in LiNbO3 and Mg:LiNbO3. J. Alloys Compd. 2001, 323, 340–343. [Google Scholar] [CrossRef]
- Dohnke, I.; Trusch, B.; Klimm, D.; Hulliger, J. A study of influence of ytterbium and impurities on lattice parameters and phase transition temperature of Czochralski grown LiNbO3. J. Phys. Chem. Solids 2004, 65, 1297–1305. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M. New low symmetry centres of Yb3+ impurities in lithium niobate single crystal. Opt. Mater. 2007, 29, 1440–1446. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M. EPR and optical study of coupled Yb3+ ion pairs in weakly doped LiNbO3:Yb single crystal. Res. Chem. Intermed. 2007, 33, 885–899. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M.; Rudowicz, C. Temperature dependence of the EPR lines in weakly doped LiNbO3:Yb—Possible evidence of Yb3+ ion pairs formation. Physica B 2008, 403, 207–218. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M.; Hanuza, J. EPR and optical studies of LiNbO3:Yb and LiNbO3:Yb, Pr single crystals. J. Alloys Compd. 2008, 451, 240–247. [Google Scholar] [CrossRef]
- Bodziony, T.; Kaczmarek, S.M.; Kruk, R. Magnetic properties of LiNbO3 single crystals weakly doped by Yb and/or codoped by Pr. Rev. Adv. Mater. Sci. 2010, 23, 1–7. [Google Scholar]
- Malovichko, G.; Grachev, V.; Kokanyan, E.; Schirmer, O. EPR, NMR and ENDOR study of intrinsic and extrinsic defects in disordered and regularly ordered lithium niobate crystals. Ferroelectrics 2000, 239, 357–366. [Google Scholar] [CrossRef]
- Malovichko, G.; Bratus, V.; Grachev, V.; Kokanyan, E. Electron paramagnetic resonance and electron-nuclear double resonance study of nonequivalent Yb3+ centers in congruent and nearly stoichiometric lithium niobate. Phys. Status Solidi (b) 2009, 246, 215–225. [Google Scholar] [CrossRef]
- Xu, H.; Lee, D.; He, J.; Sinnott, S.B.; Gopalan, V.; Dierolf, V.; Phillpot, S.R. Stability of intrinsic defects and defect clusters in LiNbO3 from density functional theory calculations. Phys. Rev. B 2008, 78, 174103. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Chernatynskiy, A.; Lee, D.; Sinnott, S.B.; Gopalan, V.; Dierolf, V.; Phillpot, S.R. Stability and charge transfer levels of extrinsic defects in LiNbO3. Phys. Rev. B 2010, 82, 184109. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lee, D.; Sinnott, S.B.; Dierolf, V.; Gopalan, V.; Phillpot, S.R. Structure and diffusion of intrinsic defect complexes in LiNbO3 from density functional theory calculations. J. Phys. Condens. Matter 2010, 22, 135002. [Google Scholar] [CrossRef] [Green Version]
- Vyalikh, A.; Zschornak, M.; Köhler, T.; Nentwich, M.; Weigel, T.; Hanzing, J.; Zaripov, R.; Vavilova, E.; Gemming, S.; Brendler, E.; et al. Analysis of the defect clusters in congruent lithium tantalate. Phys. Rev. Mater. 2018, 2, 013804. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-R.; Feng, X.-Q. Defect structures and the MgO-doping-level-threshold effect on the optical absorption of reduced MgO-doped lithium niobate. Phys. Rev. B 1991, 43, 12019–12024. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, O.F.; Thiemann, O.; Wohlecke, M. Defects in LiNbO3—I. experimental aspects. J. Phys. Chem. Solids 1991, 52, 185–200. [Google Scholar] [CrossRef]
- Schirmer, O.F.; Imlau, M.; Merschjann, C.; Schoke, B. Electron small polarons and bipolarons in LiNbO3. J. Phys. Condens. Matter 2009, 21, 123201. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, O.F.; von der Linde, D. Two photon and x-ray induced Nb4+ and O− small polarons in LiNbO3. Appl. Phys. Lett. 1978, 33, 35–38. [Google Scholar] [CrossRef]
- Ketchum, J.L.; Sweeney, K.L.; Halliburton, L.E.; Armington, A.F. Vacuum annealing effects in lithium niobate. Phys. Lett. A 1983, 94, 450–453. [Google Scholar] [CrossRef]
- Sweeney, K.L.; Halliburton, L.E. Oxygen vacancies in lithium niobate. Appl. Phys. Lett. 1983, 43, 336–338. [Google Scholar] [CrossRef]
- Halliburton, L.E.; Sweeney, K.L.; Chen, C.Y. Electron spin resonance and optical studies of point defects in lithium niobate. Nucl. Instrum. Methods Phys. Res. B 1984, 1, 344–347. [Google Scholar] [CrossRef]
- Dutt, D.A.; Feigl, F.J.; DeLeo, G.G. Optical absorption and electron paramagnetic resonance studies of chemically reduced congruent lithium niobate. J. Phys. Chem. Solids 1990, 51, 407–415. [Google Scholar] [CrossRef]
- Müller, H.; Schirmer, O.F. Microscopic structure of NbLi related defects in reduced undoped LiNbO3. Ferroelectrics 1992, 125, 319–324. [Google Scholar] [CrossRef]
- Zaritskii, I.M.; Rakitina, L.G.; Corradi, G.; Polgar, K.; Bugai, A.A. A new trapped-hole radiation defect in heavily Mg-doped LiNbO3. J. Phys. Condens. Matter 1991, 3, 8457–8465. [Google Scholar] [CrossRef]
- Halliburton, L.E.; Sweeney, K.L.; Kappers, L.A. Self-trapped electrons in lithium tantalate. Phys. Lett. A 1986, 116, 81–84. [Google Scholar] [CrossRef]
- Lee, M.; Gyoo Kim, I.; Takekawa, S.; Furukawa, Y.; Uchida, Y.; Kitamura, K.; Hatano, H. Electron paramagnetic resonance investigation of the photochromic effect in near-stoichiometric LiNbO3 with applications to holographic storage. J. Appl. Phys. 2001, 89, 5311. [Google Scholar] [CrossRef]
- Miki, T.; Hantehzadeh, M.R.; Halliburton, L.E. A new trapped-hole center in irradiated LiNbO3. J. Phys. Chem. Solids 1989, 50, 1003–1007. [Google Scholar] [CrossRef]
























| Center vLi Site | Me1 | Me2 | Me3 | Me4 | Me5 | Me6 | Me7 | Me8 |
|---|---|---|---|---|---|---|---|---|
| First | 5a/5b | 1a/1b | 2a/2b | 1a | 1b | 1a/1b | 2a/2b | 5a |
| Second | distant | 5a/5b | 5b/5b | 2a | 2b | 3a/3b | 3a/3b | 5b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grachev, V.G.; Malovichko, G.I. Structures of Impurity Defects in Lithium Niobate and Tantalate Derived from Electron Paramagnetic and Electron Nuclear Double Resonance Data. Crystals 2021, 11, 339. https://doi.org/10.3390/cryst11040339
Grachev VG, Malovichko GI. Structures of Impurity Defects in Lithium Niobate and Tantalate Derived from Electron Paramagnetic and Electron Nuclear Double Resonance Data. Crystals. 2021; 11(4):339. https://doi.org/10.3390/cryst11040339
Chicago/Turabian StyleGrachev, Valentin G., and Galina I. Malovichko. 2021. "Structures of Impurity Defects in Lithium Niobate and Tantalate Derived from Electron Paramagnetic and Electron Nuclear Double Resonance Data" Crystals 11, no. 4: 339. https://doi.org/10.3390/cryst11040339






