Three-Dimensional Flower-like Fe, C-Doped-MoS2/Ni3S2 Heterostructures Spheres for Accelerating Electrocatalytic Oxygen and Hydrogen Evolution
Abstract
:1. Introduction
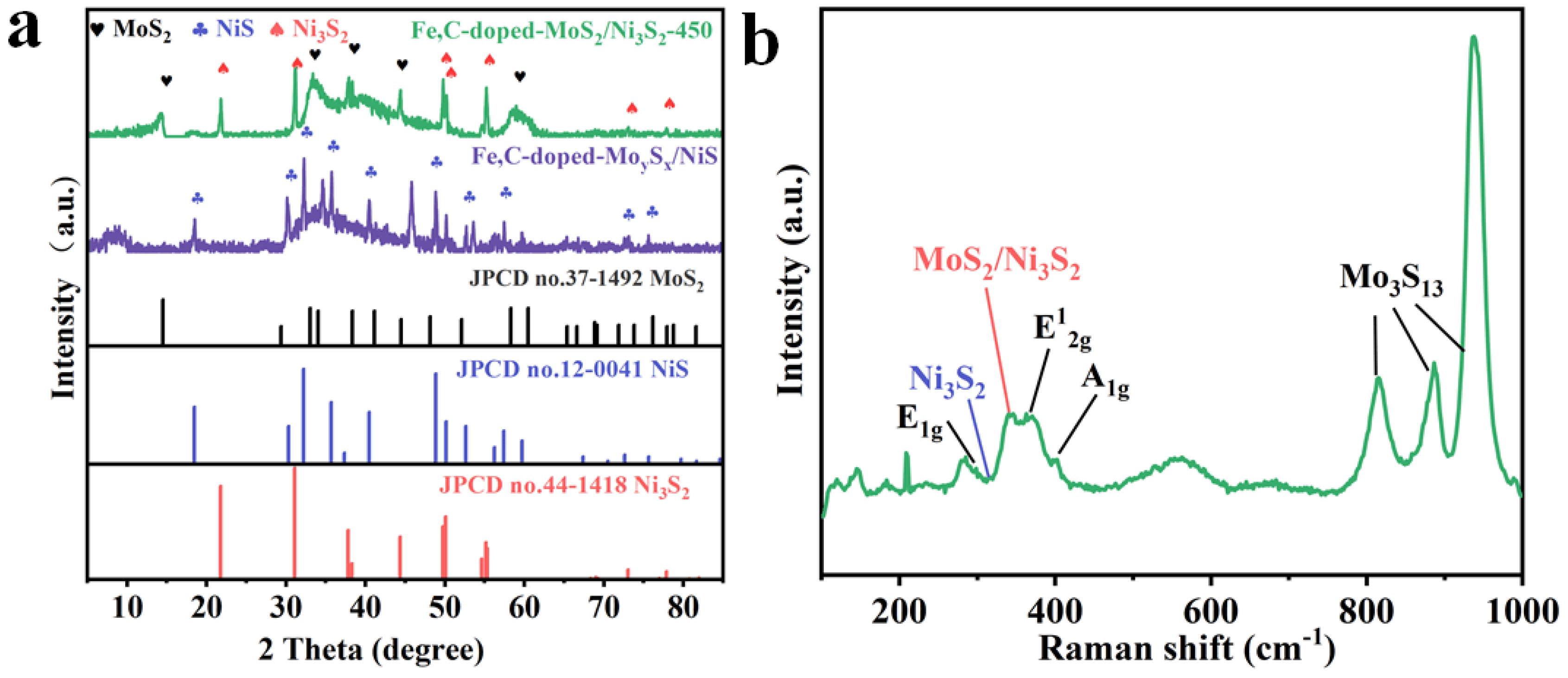
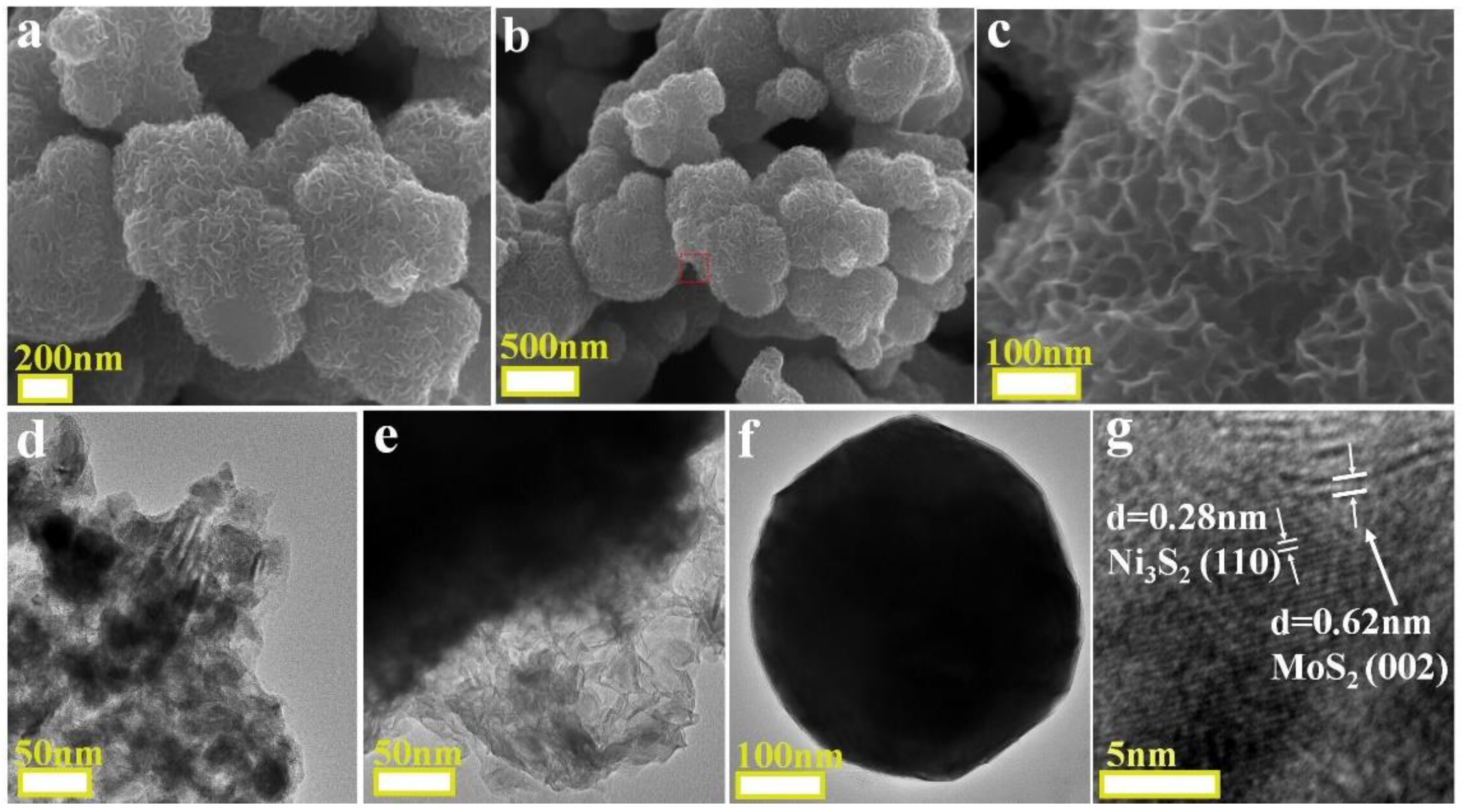
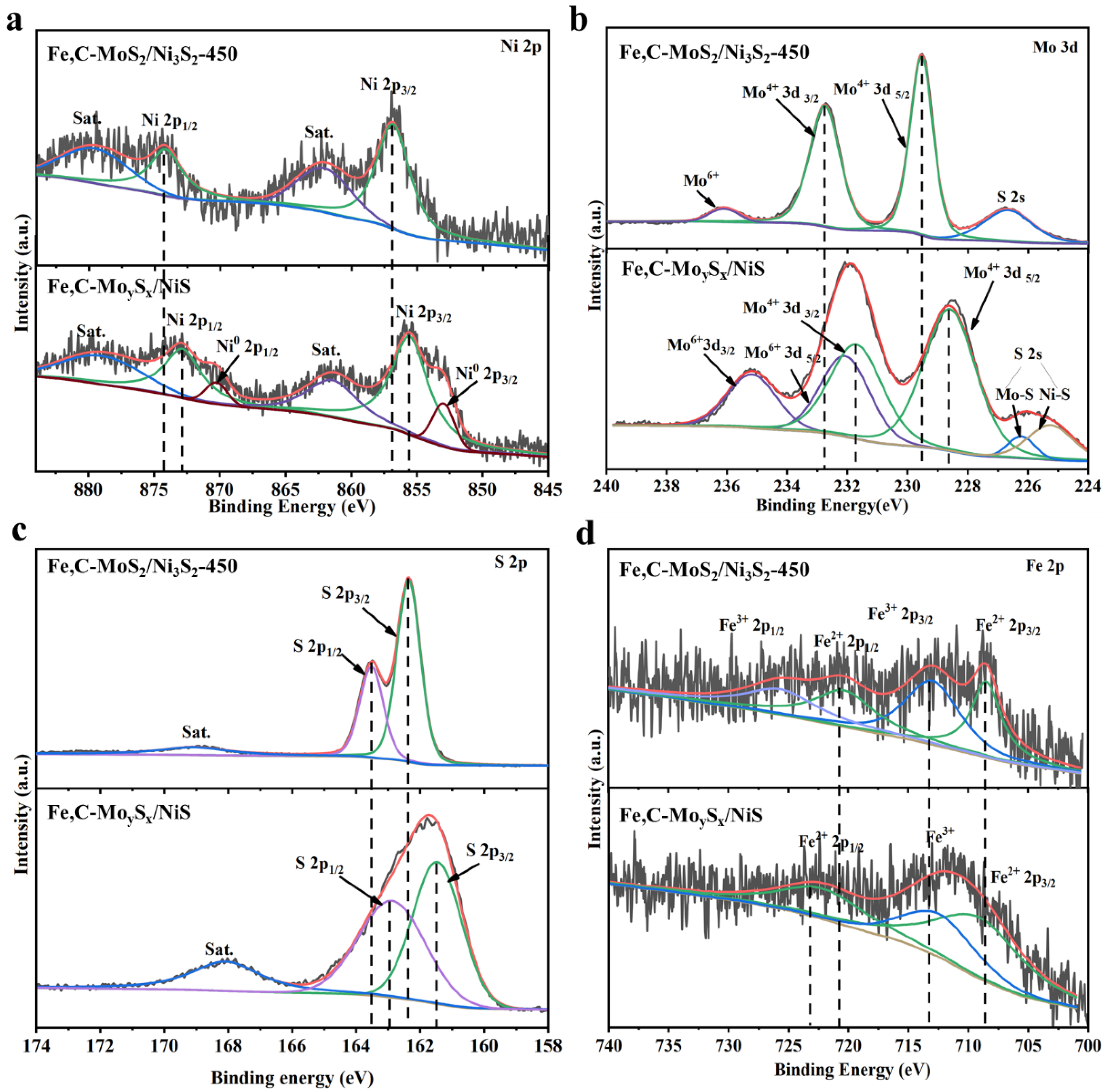
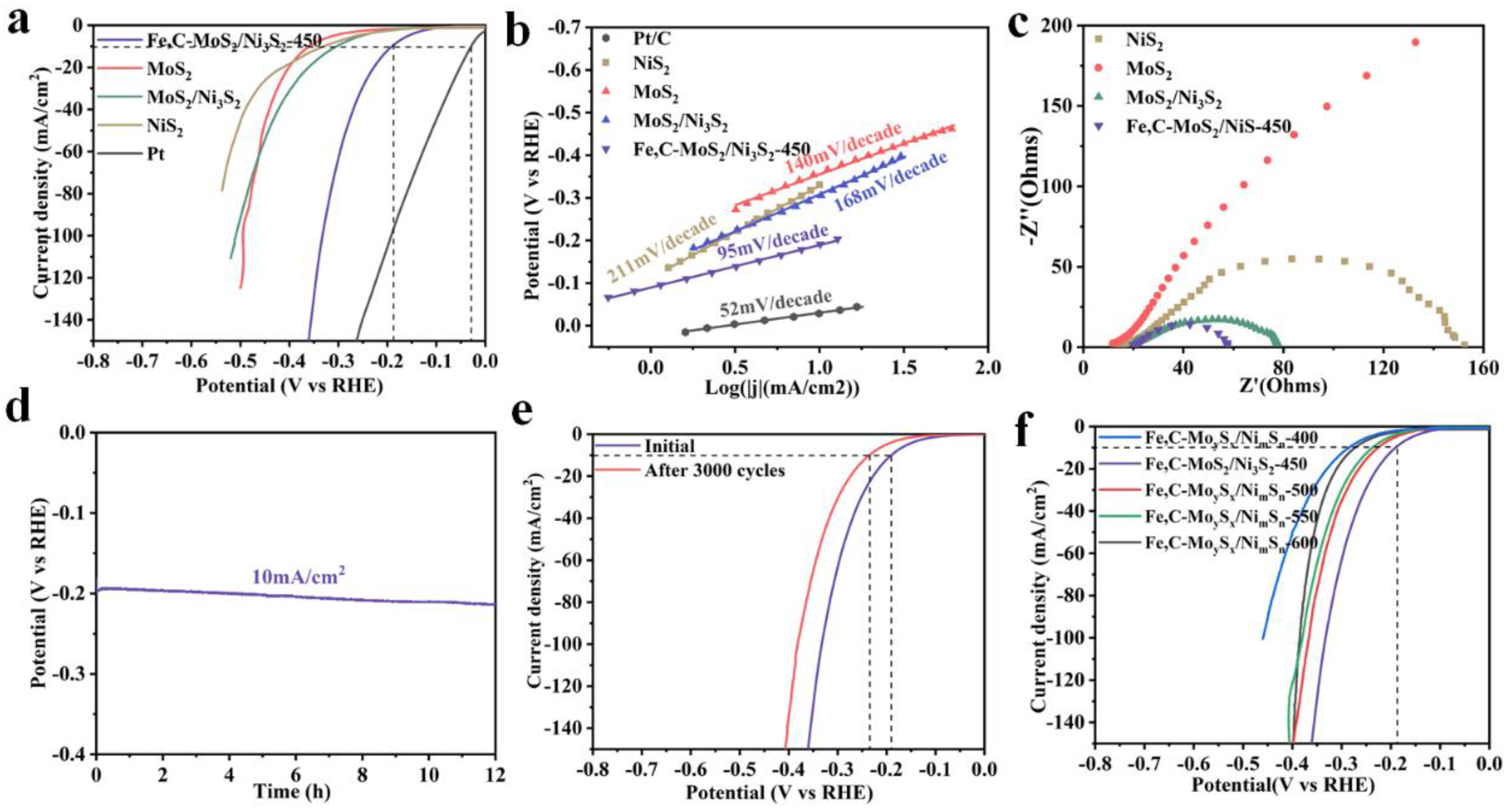
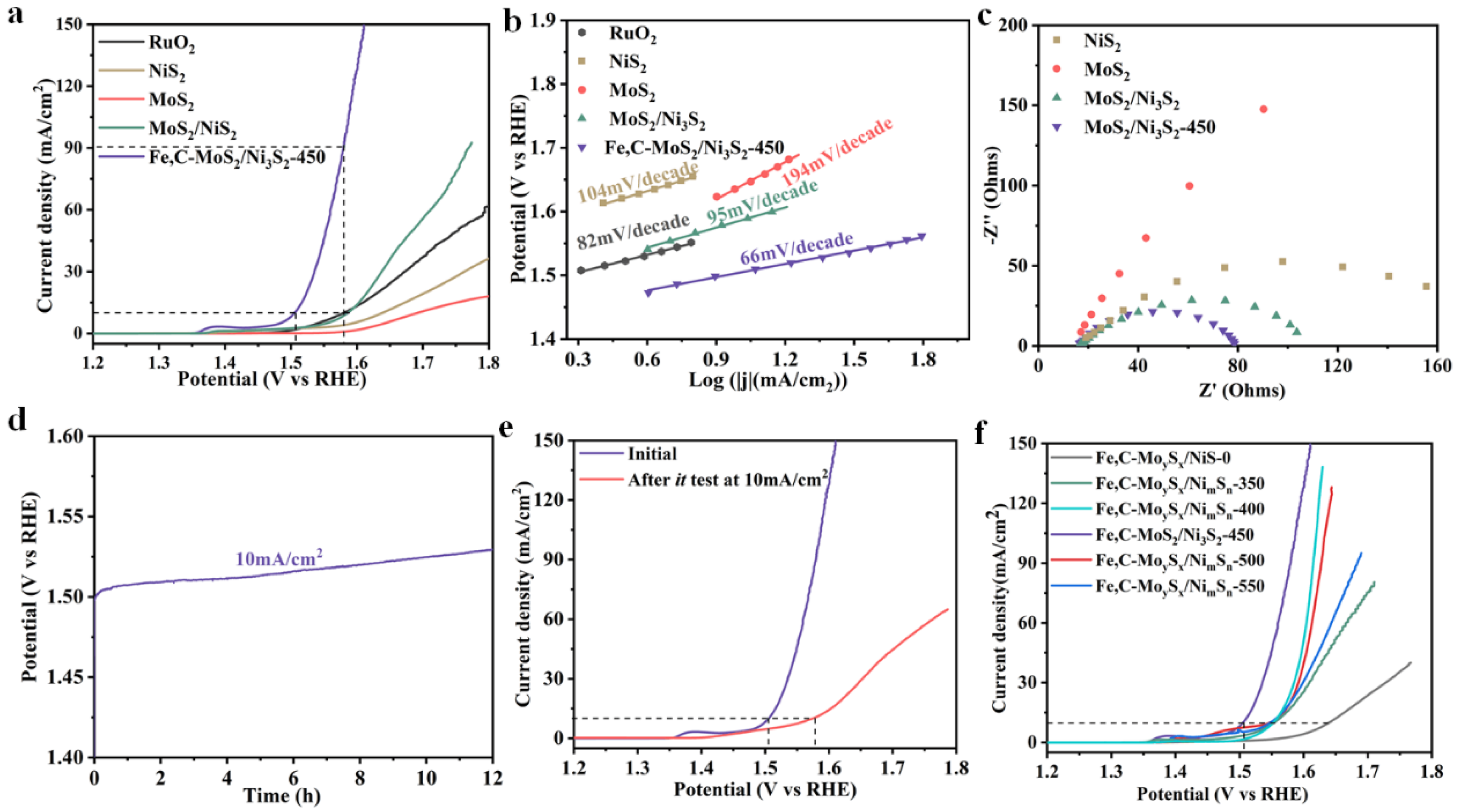
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Reagents and Chemicals
4.2. Preparation of Ni(OH)2 Precursor
4.3. Preparation of Fe, C-MoySx/NiS and MoySx/NiS
4.4. Preparation of Fe, C-MoS2/Ni3S2 or MoS2/Ni3S2
4.5. Preparation of MoS2
4.6. Preparation of NiS2
5. Materials Characterization
6. Electrochemical Measurement
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, P.; Li, W.; Chen, R.; Lin, Y. Boosting the Oxygen Evolution Electrocatalysis Performance of Iron Phosphide via Architectural Design and Electronic Modulation. ACS Sustain. Chem. Eng. 2020, 8, 9206–9216. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.M.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Lin, J.; Wang, P.; Wang, H.; Li, C.; Si, X.; Qi, J.; Cao, J.; Zhong, Z.; Fei, W.; Feng, J. Defect-Rich Heterogeneous MoS2/NiS2 Nanosheets Electrocatalysts for Efficient Overall Water Splitting. Adv. Sci. 2019, 6, 1900246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, J.; Zhou, Y.; Zhang, Y.; Sheng, X.; Wang, Y.; Liang, S.; Guo, C.; Yang, W.; Zhuang, T.; Hu, Y. Engineering water splitting sites in three-dimensional flower-like Co–Ni–P/MoS2 heterostructural hybrid spheres for accelerating electrocatalytic oxygen and hydrogen evolution. J. Mater. Chem. A 2020, 8, 22181–22190. [Google Scholar] [CrossRef]
- Deng, X.; Kang, X.; Li, M.; Xiang, K.; Wang, C.; Guo, Z.; Zhang, J.; Fu, X.-Z.; Luo, J.-L. Coupling efficient biomass upgrading with H2 production via bifunctional CuxS@NiCo-LDH core–shell nanoarray electrocatalysts. J. Mater. Chem. A 2019, 8, 1138–1146. [Google Scholar] [CrossRef]
- Zhong, C.; Han, Z.; Wang, T.; Wang, Q.; Shen, Z.; Zhou, Q.; Wang, J.; Zhang, S.; Jin, X.; Li, S.; et al. Aliovalent fluorine doping and anodization-induced amorphization enable bifunctional catalysts for efficient water splitting. J. Mater. Chem. A 2020, 8, 10831–10838. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A., 3rd; Chen, S.; Ren, Z. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nat. Commun. 2018, 9, 2551. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhou, Y.-T.; Yao, R.-Q.; Wan, W.-B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.-Y.; Zheng, W.-T.; Jiang, Q. Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Qiu, Y.; Liu, S.; Wang, W.; Chen, K.; Li, H.; Yuan, W.; Qu, Y.; Guo, X. Interfacial Engineering of W2 N/WC Heterostructures Derived from Solid-State Synthesis: A Highly Efficient Trifunctional Electrocatalyst for ORR, OER, and HER. Adv. Mater. 2019, 32, e1905679. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, Y.; Wang, H.; Chen, Q.; Wan, J. Robust Half-Metallic Magnetism in Two-Dimensional Fe/MoS2. J. Phys. Chem. C 2018, 122, 21617–21622. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of Intercalated Metals on the Electrocatalytic Activity of 1T-MoS2 for the Hydrogen Evolution Reaction. ACS Energy Lett. 2017, 3, 7–13. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-Scale Aqueous Synthesis of Stable Few-Layered 1T-MoS2: Applications for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Lee, L.Y.S.; Wong, K.-y.; Lo, T.W.; Ho, K.H.; Lei, D.Y.; Zhao, H. 2H/1T Phase Transition of Multilayer MoS2 by Electrochemical Incorporation of S Vacancies. ACS Appl. Energy Mater. 2018, 1, 4754–4765. [Google Scholar] [CrossRef]
- Xue, J.Y.; Li, F.L.; Zhao, Z.Y.; Li, C.; Ni, C.Y.; Gu, H.W.; Young, D.J.; Lang, J.P. In Situ Generation of Bifunctional Fe-Doped MoS2 Nanocanopies for Efficient Electrocatalytic Water Splitting. Inorg. Chem. 2019, 58, 11202–11209. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Luo, J.; Zhou, C.; Zhang, T.; Arbiol, J.; Lu, X.; Mao, B.W.; Zhang, X.; Fransaer, J. Hierarchical Porous Ni3S4 with Enriched High-Valence Ni Sites as a Robust Electrocatalyst for Efficient Oxygen Evolution Reaction. Adv. Funct. Mater. 2019, 29, 1900315. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Wei, J.; Qin, G.; Ni, Y. Carbon cloth supported hierarchical core-shell NiCo2S4@CoNi-LDH nanoarrays as catalysts for efficient oxygen evolution reaction in alkaline solution. J. Alloys Compd. 2020, 830, 154658. [Google Scholar] [CrossRef]
- An, L.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.-C.; Cheng, F.; Xi, P. Epitaxial Heterogeneous Interfaces on N-NiMoO4/NiS2 Nanowires/Nanosheets to Boost Hydrogen and Oxygen Production for Overall Water Splitting. Adv. Funct. Mater. 2019, 29, 1805298. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, Z.; Pang, Q.; Zhang, J.Z. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Lin, H.; Li, X.; Chan, H.C.; Yang, L.; Gao, Q. MoS2–Ni3S2 Heteronanorods as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface Engineering of MoS2/Ni3 S2 Heterostructures for Highly Enhanced Electrochemical Overall-Water-Splitting Activity. Angew. Chem. Int. Ed. Engl. 2016, 55, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhang, T.; Wei, J.; Feng, P.; Yan, X.; Tang, Y. Facile synthesis of Co and Ce dual-doped Ni3S2 nanosheets on Ni foam for enhanced oxygen evolution reaction. Nano Res. 2020, 13, 2130–2135. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Xia, W.; Chen, T.; Bando, Y.; Kang, Y.M.; Hossain, M.S.A.; Sugahara, Y.; et al. Mesoporous Iron-doped MoS2/CoMo2S4 Heterostructures through Organic-Metal Cooperative Interactions on Spherical Micelles for Electrochemical Water Splitting. ACS Nano 2020, 14, 4141–4152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, M.; Li, Y.; Dong, H.; Sun, D.; Liu, X.; Xu, L.; Tian, Z.; Tang, Y. Regulating the Electronic Structure of CoP Nanosheets by O Incorporation for High-Efficiency Electrochemical Overall Water Splitting. Adv. Funct. Mater. 2019, 30, 1905252. [Google Scholar] [CrossRef]
- Liu, G.; Thummavichai, K.; Lv, X.; Chen, W.; Lin, T.; Tan, S.; Zeng, M.; Chen, Y.; Wang, N.; Zhu, Y. Defect-Rich Heterogeneous MoS2/rGO/NiS Nanocomposite for Efficient pH-Universal Hydrogen Evolution. Nanomaterials 2021, 11, 662. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xia, W.; Akita, T.; Zou, R.; Xu, Q. Metal-Organic Framework-Derived Honeycomb-Like Open Porous Nanostructures as Precious-Metal-Free Catalysts for Highly Efficient Oxygen Electroreduction. Adv. Mater. 2016, 28, 6391–6398. [Google Scholar] [CrossRef]
- Wang, L.; Duan, X.; Liu, X.; Gu, J.; Si, R.; Qiu, Y.; Qiu, Y.; Shi, D.; Chen, F.; Sun, X.; et al. Atomically Dispersed Mo Supported on Metallic Co9S8 Nanoflakes as an Advanced Noble-Metal-Free Bifunctional Water Splitting Catalyst Working in Universal pH Conditions. Adv. Energy Mater. 2019, 10, 1903137. [Google Scholar] [CrossRef]
- Li, B.; Zeng, H.C. Architecture and Preparation of Hollow Catalytic Devices. Adv. Mater. 2019, 31, e1801104. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, K.; Liu, Q.; Li, Y.; Li, M.; Su, C.-Y.; Ogiwara, N.; Kobayashi, H.; Kitagawa, H.; Liu, M.; et al. Missing-linker metal-organic frameworks for oxygen evolution reaction. Nat. Commun. 2019, 10, 5048. [Google Scholar] [CrossRef] [PubMed]
- Thangasamy, P.; Oh, S.; Nam, S.; Randriamahazaka, H.; Oh, I.K. Ferrocene-Incorporated Cobalt Sulfide Nanoarchitecture for Superior Oxygen Evolution Reaction. Small 2020, 16, e2001665. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kong, L.; Ji, Y.; White, J.; Li, Y.; Zhang, J.; An, P.; Liu, S.; Lee, S.-T.; Ma, T. Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation. Nat. Commun. 2019, 10, 2149. [Google Scholar] [CrossRef] [Green Version]
- Sha, L.; Liu, T.; Ye, K.; Zhu, K.; Yan, J.; Yin, J.; Wang, G.; Cao, D. A heterogeneous interface on NiS@Ni3S2/NiMoO4 heterostructures for efficient urea electrolysis. J. Mater. Chem. A 2020, 8, 18055–18063. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Shen, Y.; Wu, Z. Ni-doped MoS2 nanoparticles as highly active hydrogen evolution electrocatalysts. RSC Adv. 2016, 6, 16656–16661. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Liu, G.; Liu, S.; Chen, W.; Cao, D.; Song, T.; Wang, N.; Zhu, Y. Three-Dimensional Flower-like Fe, C-Doped-MoS2/Ni3S2 Heterostructures Spheres for Accelerating Electrocatalytic Oxygen and Hydrogen Evolution. Crystals 2021, 11, 340. https://doi.org/10.3390/cryst11040340
Lv X, Liu G, Liu S, Chen W, Cao D, Song T, Wang N, Zhu Y. Three-Dimensional Flower-like Fe, C-Doped-MoS2/Ni3S2 Heterostructures Spheres for Accelerating Electrocatalytic Oxygen and Hydrogen Evolution. Crystals. 2021; 11(4):340. https://doi.org/10.3390/cryst11040340
Chicago/Turabian StyleLv, Xuefeng, Guangsheng Liu, Song Liu, Wenting Chen, Dehua Cao, Taize Song, Nannan Wang, and Yanqiu Zhu. 2021. "Three-Dimensional Flower-like Fe, C-Doped-MoS2/Ni3S2 Heterostructures Spheres for Accelerating Electrocatalytic Oxygen and Hydrogen Evolution" Crystals 11, no. 4: 340. https://doi.org/10.3390/cryst11040340
APA StyleLv, X., Liu, G., Liu, S., Chen, W., Cao, D., Song, T., Wang, N., & Zhu, Y. (2021). Three-Dimensional Flower-like Fe, C-Doped-MoS2/Ni3S2 Heterostructures Spheres for Accelerating Electrocatalytic Oxygen and Hydrogen Evolution. Crystals, 11(4), 340. https://doi.org/10.3390/cryst11040340





