Mineral Characterization in Human Body: A Dual Energy Approach
Abstract
1. Introduction
2. Materials and Methods
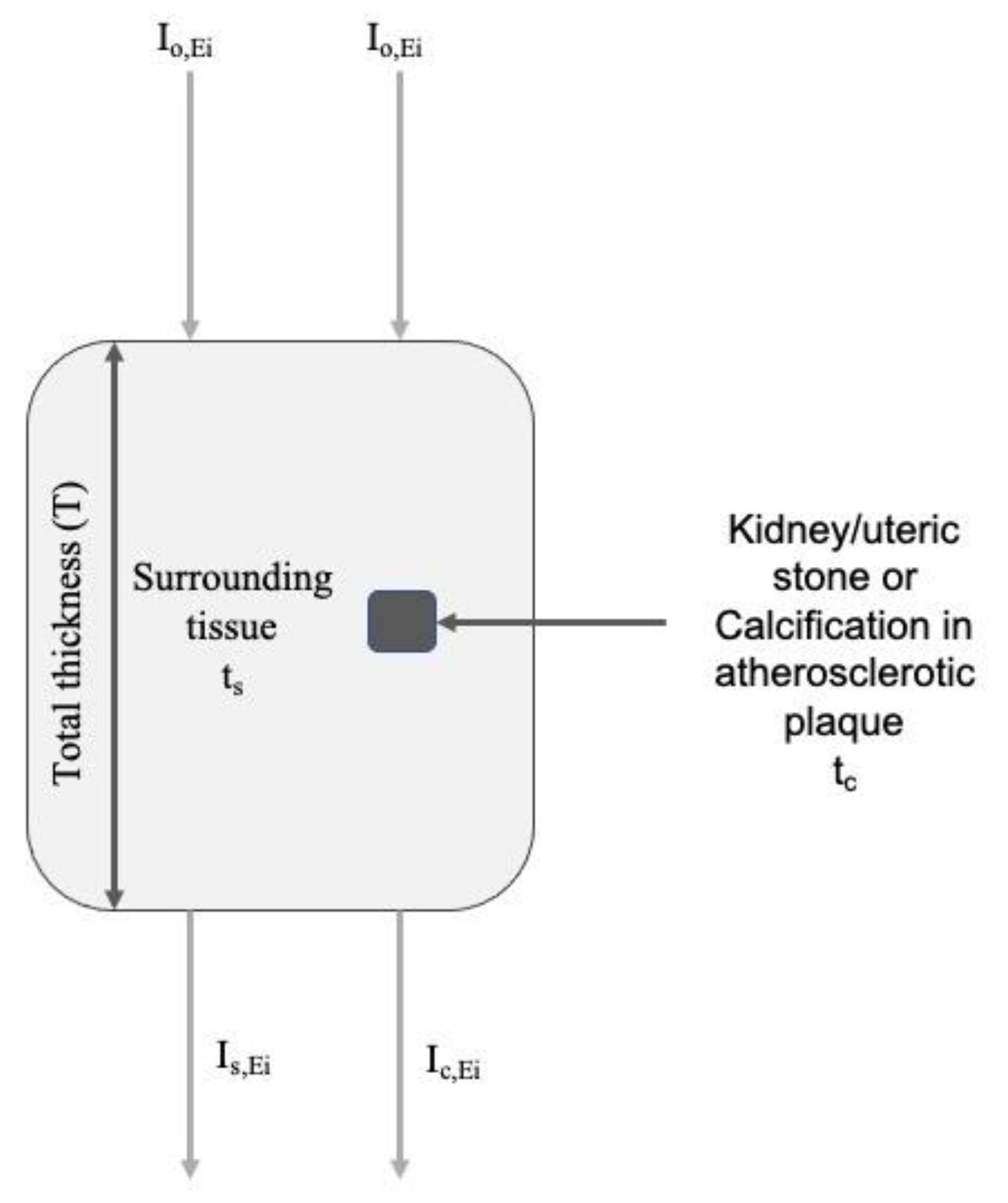
2.1. Simulation Study
2.1.1. Monoenergetic Beams
2.1.2. Polyenergetic X-rays
2.2. Experimental Verification
2.2.1. Irradiation Process
2.2.2. Phantoms
3. Results and Discussion
3.1. Simulation Study
3.1.1. Monoenergetic Beams
3.1.2. Polyenergetic X-rays
3.2. Experimental Verification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scales, C.D.; Saigal, C.S.; Hanley, J.M.; Dick, A.W.; Setodji, C.M.; Litwin, M.S.; NIDDK Urologic Diseases in America Project. The impact of unplanned post procedure visits in the management of patients with urinary stones. Surgery 2014, 155, 769–775. [Google Scholar] [CrossRef]
- Saigal, C.S.; Joyce, G.; Timilsina, A.R.; Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? Kidney Int. 2005, 68, 1808–1814. [Google Scholar] [CrossRef]
- Jendeberg, J.; Geijer, H.; Alshamari, M.; Cierzniak, B.; Lidén, M. Size matters: The width and location of a ureteral stone accurately predict the chance of spontaneous passage. Eur. Radiol. 2017, 27, 4775–4785. [Google Scholar] [CrossRef]
- Parmar, M.S. Kidney Stones. BMJ 2004, 328, 1420–1424. [Google Scholar] [CrossRef]
- Sandegard, E. Prognosis of stone in the ureter. Acta Chir. Scand. Suppl. 1956, 219, 1–67. [Google Scholar] [PubMed]
- Ueno, A.; Kawamura, T.; Ogawa, A.; Takayasu, H. Relation of spontaneous passage of ureteral calculi to size. Urology 1977, 10, 544–546. [Google Scholar] [CrossRef]
- Coll, D.M.; Varanelli, M.J.; Smith, R.C. Relationship of Spontaneous Passage of Ureteral Calculi to Stone Size and Location as Revealed by Unenhanced Helical CT. Am. J. Roentgenol. 2002, 178, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Maxwell, A.D.; Bailey, M.R. Some Work on the Diagnosis and Management of Kidney Stones with Ultra-sound. Acoust. Today 2017, 13, 52–59. [Google Scholar]
- Eliahou, R.; Hidas, G.; Duvdevani, M.; Sosna, J. Determination of Renal Stone Composition with Dual-Energy Computed Tomography: An Emerging Application. Semin. Ultrasound CT MRI 2010, 31, 315–320. [Google Scholar] [CrossRef]
- Qu, M.; Ramirez-Giraldo, J.C.; Leng, S.; Williams, J.C.; Vrtiska, T.J.; Lieske, J.C.; McCollough, C.H. Dual-Energy Dual-Source CT With Additional Spectral Filtration Can Improve the Differentiation of Non–Uric Acid Renal Stones: An Ex Vivo Phantom Study. Am. J. Roentgenol. 2011, 196, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Kourambas, J.; Aslan, P.; Teh, C.L.; Mathias, B.J.; Preminger, G.M. Role of Stone Analysis in Metabolic Evaluation and Medical Treatment of Nephrolithiasis. J. Endourol. 2001, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Poindexter, J.R.; Adams-Huet, B.; Pearle, M.S. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am. J. Med. 2003, 115, 26–32. [Google Scholar] [CrossRef]
- Zilberman, D.; Ferrandino, M.; Preminger, G.; Paulson, E.; Lipkin, M.; Boll, D. In Vivo Determination of Urinary Stone Composition Using Dual Energy Computerized Tomography With Advanced Post-Acquisition Processing. J. Urol. 2010, 184, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Zhao, R.; Liu, B.; Yu, Y.-Q. Determination of urinary stone composition using dual-energy spectral CT: Initial in vitro analysis. Clin. Radiol. 2013, 68, e370–e377. [Google Scholar] [CrossRef] [PubMed]
- Wisenbaugh, E.S.; Paden, R.G.; Silva, A.C.; Humphreys, M.R. Dual-energy vs Conventional Computed Tomography in Determining Stone Composition. Urology 2014, 83, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.L. Effectiveness of Treatment Modalities on Kidney Stone Recurrence. Clin. J. Am. Soc. Nephrol. 2017, 12, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.G.; Urban, M.W. Detecting Kidney Stones Using Twinkling Artifacts: Survey of Kidney Stones with Varying Composition and Size. Ultrasound Med. Biol. 2020, 46, 156–166. [Google Scholar] [CrossRef]
- Macfarlane, M.T. Urology, 5th ed.; Lippincott William & Wilkins: Baltimore, MD, USA, 2015; pp. 132–133. [Google Scholar]
- Primak, A.N.; Fletcher, J.G.; Vrtiska, T.J.; Dzyubak, O.P.; Lieske, J.C.; Jackson, M.E.; Williams, J.C.; McCollough, C.H. Noninvasive Differentiation of Uric Acid versus Non–Uric Acid Kidney Stones Using Dual-Energy CT. Acad. Radiol. 2007, 14, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Taheri, M.; Taheri, F. What is the state of the stone analysis techniques in urolithiasis? Urol. J. 2012, 9, 445–454. [Google Scholar]
- Evan, A.P.; Worcester, E.M.; Coe, F.L.; Williams, J.; Lingeman, J.E. Mechanisms of human kidney stone formation. Urolithiasis 2014, 43, 19–32. [Google Scholar] [CrossRef]
- Sethmann, I.; Grohé, B.; Kleebe, H.-J. Replacement of hydroxylapatite by whewellite: Implications for kidney-stone formation. Miner. Mag. 2014, 78, 91–100. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Ashayeri, N.Y.; Moore, F.; Irani, D.; Asadi, S.; Zarasvandi, A.; Salari, M. Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals 2016, 6, 131. [Google Scholar] [CrossRef]
- Vásquez-Quitral, P.; Arana, J.T.; Miras, M.C.; Acevedo, D.F.; Barbero, C.A.; Neira-Carrillo, A. Effect of Diazotated Sulphonated Polystyrene Films on the Calcium Oxalate Crystallization. Crystals 2017, 7, 70. [Google Scholar] [CrossRef]
- Grant, C.M.; Guzman, G.; Stainback, R.P.; Amdur, R.L.; Mufarrij, P.W.; Guzman, G. Variation in Kidney Stone Composition Within the United States. J. Endourol. 2018, 32, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Letavernier, E.; Bouderlique, E.; Zaworski, J.; Martin, L.; Daudon, M. Pseudoxanthoma Elasticum, Kidney Stones and Pyrophosphate: From a Rare Disease to Urolithiasis and Vascular Calcifications. Int. J. Mol. Sci. 2019, 20, 6353. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Chiu, K.-Y.; Chen, H.-Y.; Lin, W.-Y.; Chen, Y.-H.; Chen, W.-C. Animal Models for Studying Stone Disease. Diagnostics 2020, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, M.; Tio, M.C.; Waikar, S.S.; Ferraro, P.M. Dietary Oxalate Intake and Kidney Outcomes. Nutrients 2020, 12, 2673. [Google Scholar] [CrossRef]
- Daudon, M.; Hennequin, C.; Lacour, B.; Le Moël, G.; Donsimoni, R.; Fellahi, S.; Paris, M.; Troupel, S. Sex- and age-related composition of 10,617 calculi analyzed by infrared spectroscopy. Urol. Res. 1995, 23, 319–326. [Google Scholar] [CrossRef]
- Frochot, V.; Castiglione, V.; Lucas, I.T.; Haymann, J.-P.; Letavernier, E.; Bazin, D.; Fogazzi, G.B.; Daudon, M. Advances in the identification of calcium carbonate urinary crystals. Clin. Chim. Acta 2021, 515, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zarse, C.A.; McAteer, J.A.; Tann, M.; Sommer, A.J.; Kim, S.C.; Paterson, R.F.; Hatt, E.K.; Lingeman, J.E.; Evan, A.P.; Williams, J.C. Helical computed tomography accurately reports urinary stone composition using attenuation values: In vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology 2004, 63, 828–833. [Google Scholar] [CrossRef]
- Mitcheson, H.D.; Zamenhof, R.G.; Bankoff, M.S.; Prien, E.L. Determination of the Chemical Composition of Urinary Calculi by Computerized Tomography. J. Urol. 1983, 130, 814–819. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients With Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Yahagi, K.; Davis, H.R.; Arbustini, E.; Virmani, R. Sex differences in coronary artery disease: Pathological observations. Atherosclerosis 2015, 239, 260–267. [Google Scholar] [CrossRef]
- Spagnoli, L.G.; Mauriello, A.; Sangiorgi, G.; Fratoni, S.; Bonanno, E.; Schwartz, R.S.; Piepgras, D.G.; Pistolese, R.; Ippoliti, A.; Holmes, D.R. Extracranial Thrombotically Active Carotid Plaque as a Risk Factor for Ischemic Stroke. JAMA 2004, 292, 1845–1852. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.E.; Van der Heiden, K.; Farrell, E.; Gijsen, F.J.; Akyildiz, A.C. Calcifications in atherosclerotic plaques and impact on plaque biomechanics. J. Biomech. 2019, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.D.; Vesey, A.T.; Joshi, N.V.; Newby, D.E.; Dweck, M.R. Salt in the wound: 18F-fluoride positron emission tomography for identification of vulnerable coronary plaques. Cardiovasc. Diagn. Ther. 2015, 5, 150–155. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.D.; Kuusisto, J.; Reichenbach, D.D.; Ferguson, M.; Giachelli, C.; Alpers, C.E.; Otto, C.M. Osteopontin Is Expressed in Human Aortic Valvular Lesions. Circulation 1995, 92, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dilsizian, V. Targeted PET/CT Imaging of Vulnerable Atherosclerotic Plaques: Microcalcification with Sodium Fluoride and Inflammation with Fluorodeoxyglucose. Curr. Cardiol. Rep. 2013, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.P.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-Derived Matrix Vesicles: An Alternative Novel Mechanism for Microcalcification in Athero-sclerotic Plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.D.; Hohl, T.; Hutcheson, J.D.; Moss, A.J.; Schlotter, F.; Blaser, M.C.; Park, M.-A.; Lee, L.H.; Singh, S.A.; Alcaide-Corral, C.J.; et al. 18F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ. Cardiovasc. Imaging 2019, 12, e007835. [Google Scholar] [CrossRef]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front. Physiol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Hasegawa, T.; Ehara, S.; Matsumoto, K.; Mizutani, K.; Iguchi, T.; Ishii, H.; Nakagawa, M.; Shimada, K.; Yoshiyama, M. New insights into spotty calcification and plaque rupture in acute coronary syndrome: An optical coherence tomography study. Heart Vessel. 2016, 31, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Jia, Y.; Fan, J.; Wang, H.; Fan, C.; Wu, L.; Si, X.; Hao, X.; Wu, P.; et al. Sodium-fluoride PET-CT for the non-invasive evaluation of coronary plaques in symptomatic patients with coronary artery disease: A cross-correlation study with intravascular ultrasound. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2181–2189. [Google Scholar] [CrossRef]
- Becker, A.; Epple, M.; Müller, K.; Schmitz, I. A comparative study of clinically well-characterized human atherosclerotic plaques with histological, chemical, and ultrastructural methods. J. Inorg. Biochem. 2004, 98, 2032–2038. [Google Scholar] [CrossRef]
- Kim, K.M. Calcification of matrix vesicles in human aortic valve and aortic media. Fed. Proc. 1976, 35, 156–162. [Google Scholar]
- Schmid, K.; McSharry, W.O.; Pameijer, C.H.; Binette, J. Chemical and physicochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis 1980, 37, 199–210. [Google Scholar] [CrossRef]
- Ewence, A.E.; Bootman, M.; Roderick, H.L.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, C.M.; Proudfoot, D. Calcium Phosphate Crystals Induce Cell Death in Human Vascular Smooth Muscle Cells: A Potential Mechanism in Atherosclerotic Plaque Destabilization. Circ. Res. 2008, 103, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, I.; Perri, E. Ultrastructural, Elemental and Mineralogical Analysis of Vascular Calcification in Atherosclerosis. Microsc. Microanal. 2017, 23, 1030–1039. [Google Scholar] [CrossRef]
- Viegas, C.; Araújo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): Challenging old concepts with new facts. Aging 2019, 11, 4274–4299. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sato, Y.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawakami, R.; Gadhoke, N.V.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis 2020, 306, 85–95. [Google Scholar] [CrossRef]
- Florea, A.; Sigl, J.; Morgenroth, A.; Vogg, A.; Sahnoun, S.; Winz, O.; Bucerius, J.; Schurgers, L.; Mottaghy, F. Sodium [18F]Fluoride PET Can Efficiently Monitor In Vivo Atherosclerotic Plaque Calcification Progression and Treatment. Cells 2021, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Babic, M.; Tölle, M.; Van Der Giet, M.; Schuchardt, M. Research Models for Studying Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 2204. [Google Scholar] [CrossRef]
- Myung, S.-K.; Kim, H.-B.; Lee, Y.-J.; Choi, Y.-J.; Oh, S.-W. Calcium Supplements and Risk of Cardiovascular Disease: A Meta-Analysis of Clinical Trials. Nutrients 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, G.A.; Micheletti, R.G.; Currier, J.S.; Singer, E.; Fishbein, M.C. Atherosclerotic oxalosis in coronary arteries. Cardiovasc. Pathol. 2008, 17, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bischetti, S.; Scimeca, M.; Bonanno, E.; Federici, M.; Anemona, L.; Menghini, R.; Casella, S.; Cardellini, M.; Ippoliti, A.; Mauriello, A. Carotid plaque instability is not related to quantity but to elemental composition of calcification. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice Computed Tomographic Characteristics of Coronary Lesions in Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef]
- Pflederer, T.; Marwan, M.; Schepis, T.; Ropers, D.; Seltmann, M.; Muschiol, G.; Daniel, W.G.; Achenbach, S. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis 2010, 211, 437–444. [Google Scholar] [CrossRef]
- Feuchtner, G.M.; Barbieri, F.; Langer, C.; Beyer, C.; Widmann, G.; Friedrich, G.J.; Cartes-Zumelzu, F.; Plank, F. Non obstructive high-risk plaque but not calcified by coronary CTA, and the G-score predict ischemia. J. Cardiovasc. Comput. Tomogr. 2019, 13, 305–314. [Google Scholar] [CrossRef]
- Kataoka, Y.; Wolski, K.; Uno, K.; Puri, R.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Spotty Calcification as a Marker of Accelerated Progression of Coronary Atherosclerosis: Insights From Serial Intravascular Ultrasound. J. Am. Coll. Cardiol. 2012, 59, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Antoniou, C.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Hear. Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Higuchi, C.; Nakaoka, T.; Omori, H.; Ogawa, T.; Sakura, H.; Nitta, K. Compositional Analysis of Coronary Artery Calcification in Dialysis Patients in vivo by Dual-Energy Computed Tomography Angiography. Ther. Apher. Dial. 2018, 22, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, M.; Kubo, T.; Takarada, S.; Kitabata, H.; Ino, Y.; Tanimoto, T.; Komukai, K.; Tanaka, A.; Imanishi, T.; Akasaka, T. Coronary Superficial and Spotty Calcium Deposits in Culprit Coronary Lesions of Acute Coronary Syndrome as Determined by Optical Coherence Tomography. Am. J. Cardiol. 2013, 112, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.; Lee, J.S.; Soeda, T.; Higuma, T.; Minami, Y.; Wang, Z.; Lee, H.; Yokoyama, H.; Yokota, T.; Okumura, K.; et al. Coronary Calcification and Plaque Vulnerability. Circ. Cardiovasc. Imaging 2016, 9, 003929. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does It Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Barreto, M.; Schoenhagen, P.; Nair, A.; Amatangelo, S.; Milite, M.; Obuchowski, N.A.; Lieber, M.L.; Halliburton, S.S. Potential of dual-energy computed tomography to characterize atherosclerotic plaque: Ex vivo assessment of human coronary arteries in comparison to histology. J. Cardiovasc. Comput. Tomogr. 2008, 2, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Boll, D.T.; Merkle, E.M.; Paulson, E.K.; Mirza, R.A.; Fleiter, T.R. Calcified Vascular Plaque Specimens: Assessment with Cardiac Dual-Energy Multidetector CT in Anthropomorphically Moving Heart Phantom. Radiology 2008, 249, 119–126. [Google Scholar] [CrossRef]
- Henzler, T.; Porubsky, S.; Kayed, H.; Harder, N.; Krissak, U.R.; Meyer, M.; Sueselbeck, T.; Marx, A.; Michaely, H.; Schoepf, U.J.; et al. Attenuation-based characterization of coronary atherosclerotic plaque: Comparison of dual source and dual energy CT with single-source CT and histopathology. Eur. J. Radiol. 2011, 80, 54–59. [Google Scholar] [CrossRef]
- Nakajima, S.; Ito, H.; Mitsuhashi, T.; Kubo, Y.; Matsui, K.; Tanaka, I.; Fukui, R.; Omori, H.; Nakaoka, T.; Sakura, H.; et al. Clinical application of effective atomic number for classifying non-calcified coronary plaques by dual-energy computed tomography. Atherosclerosis 2017, 261, 138–143. [Google Scholar] [CrossRef]
- Ding, H.; Wang, C.; Malkasian, S.; Johnson, T.; Molloi, S. Characterization of arterial plaque composition with dual energy computed tomography: A simulation study. Int. J. Cardiovasc. Imaging 2021, 37, 331–341. [Google Scholar] [CrossRef]
- Alexander, R.T.; Hemmelgarn, B.R.; Wiebe, N.; Bello, A.; Samuel, S.; Klarenbach, S.W.; Curhan, G.C.; Tonelli, M. Kidney Stones and Cardiovascular Events: A Cohort Study. Clin. J. Am. Soc. Nephrol. 2013, 9, 506–512. [Google Scholar] [CrossRef]
- Devarajan, A. Cross-talk between renal lithogenesis and atherosclerosis: An unveiled link between kidney stone formation and cardiovascular diseases. Clin. Sci. 2018, 132, 615–626. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Eisner, B.H.; Gambaro, G.; Rimm, E.B.; Mukamal, K.J.; Curhan, G.C. History of Kidney Stones and the Risk of Coronary Heart Disease. JAMA 2013, 310, 408–415. [Google Scholar] [CrossRef]
- Aydin, H.; Yencilek, F.; Erihan, I.B.; Okan, B.; Sarica, K. Increased 10-year cardiovascular disease and mortality risk scores in asymptomatic patients with calcium oxalate urolithiasis. Urol. Res. 2011, 39, 451–458. [Google Scholar] [CrossRef]
- Huang, H.S.; Liao, P.C.; Liu, C.J. Calcium Kidney Stones are Associated with Increased Risk of Carotid Atherosclerosis: The Link between Urinary Stone Risks, Carotid Intima-Media Thickness, and Oxidative Stress Markers. J. Clin. Med. 2020, 9, 729. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, Y.; Gao, C.; Yan, P.; Xu, L. Urolithiasis, Independent of Uric Acid, Increased Risk of Coronary Artery and Carotid Atherosclerosis: A Meta-Analysis of Observational Studies. BioMed Res. Int. 2020, 2020, 1–11. Available online: https://www.hindawi.com/journals/bmri/2020/1026240/ (accessed on 25 February 2021). [CrossRef]
- Arafa, A.; Eshak, E.S.; Iso, H. Oxalates, urinary stones and risk of cardiovascular diseases. Med. Hypotheses 2020, 137, 109570. [Google Scholar] [CrossRef]
- Martini, N.; Koukou, V.; Fountos, G.; Michail, C.; Bakas, A.; Kandarakis, I.; Speller, R.; Nikiforidis, G. Characterization of breast calcification types using dual energy x-ray method. Phys. Med. Biol. 2017, 62, 7741–7764. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Koukou, V.; Michail, C.; Fountos, G. Dual Energy X-ray Methods for the Characterization, Quantification and Imaging of Calcification Minerals and Masses in Breast. Crystals 2020, 10, 198. [Google Scholar] [CrossRef]
- Keppler, U.; Menges, V. Differences in microcalcification in breast tumors. Virchows Arch. A Path. Anat. Histol. 1981, 393, 307–313. [Google Scholar] [CrossRef]
- Frappart, L.; Rémy, I.; Lin, H.C.; Bremond, A.; Raudrant, D.; Grousson, B.; Vauzelle, J.L. Different types of microcalcifications observed in breast pathology. Vichows Arch. A Pathol. Anat. 1987, 410, 179–187. [Google Scholar] [CrossRef]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Identifying Microcalcifications in Benign and Malignant Breast Lesions by Probing Differences in Their Chemical Composition Using Raman Spectros-copy. Cancer Res. 2002, 62, 5375–5380. [Google Scholar]
- Fandos-Morera, A.; Prats-Esteve, M.; Tura-Soteras, J.M.; Traveria-Cros, A. Breast tumors: Composition of microcalcifications. Radiology 1988, 169, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Matousek, P.; Ronayne, K.L.; Parker, A.W.; Rogers, K.; Stone, N. Depth profiling of calcifications in breast tissue using picosecond Kerr-gated Raman spectroscopy. Analyst 2007, 132, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Koukou, V.; Martini, N.; Fountos, G.; Michail, C.; Sotiropoulou, P.; Bakas, A.; Kalyvas, N.; Kandarakis, I.; Speller, R.; Nikiforidis, G. Dual energy subtraction method for breast calcification imaging. Nucl. Instrum. Methods Phys. Res. Sect. A 2017, 848, 31–38. [Google Scholar] [CrossRef]
- Linardatos, D.; Koukou, V.; Martini, N.; Konstantinidis, A.; Bakas, A.; Fountos, G.; Valais, I.; Michail, C. On the Response of a Micro Non-Destructive Testing X-ray Detector. Materials 2021, 14, 888. [Google Scholar] [CrossRef]
- Gong, J.K.; Arnold, J.S.; Cohn, S.H. The density of organic and volatile and non-volatile inorganic components of bone. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1964, 149, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lemacks, M.R.; Kappadath, S.C.; Shaw, C.C.; Liu, X.; Whitman, G.J. A dual-energy subtraction technique for microcalcification imaging in digital mammography—A signal-to-noise analysis. Med. Phys. 2002, 29, 1739–1751. [Google Scholar] [CrossRef]
- Brandan, M.-E.; Ramírez, R.V. Evaluation of dual-energy subtraction of digital mammography images under conditions found in a commercial unit. Phys. Med. Biol. 2006, 51, 2307–2320. [Google Scholar] [CrossRef]
- Resoltech WWA Range. Clear Casting Epoxy Systems. Available online: https://www.resoltech.com/en/markets/wwa-range-detail.html (accessed on 25 February 2021).
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 KeV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest. 1995. Available online: http://physics.nist.gov/PhysRefData/XrayMassCoef/cover.html (accessed on 25 February 2021).
- Available online: http://www.cirsinc.com/products/radiation-therapy/imrt-thorax-phantom/ (accessed on 25 February 2021).
- Available online: http://www.cirsinc.com/products/ultrasound/zerdine-hydrogel/triple-modality-3d-abdominal-phantom/ (accessed on 25 February 2021).
- Available online: http://www.cirsinc.com/products/ultrasound/zerdine-hydrogel/image-guided-abdominal-biopsy-phantom/ (accessed on 25 February 2021).
- Available online: http://www.cirsinc.com/products/radiation-therapy/3-dimensional-torso-phantom/ (accessed on 25 February 2021).
- Fountos, G.; Yasumura, S.; Glaros, D. The skeletal calcium/phosphorus ratio: A new in vivo method of determination. Med. Phys. 1997, 24, 1303–1310. [Google Scholar] [CrossRef]
- Boone, J.M.; Seibert, J.A. An accurate method for computer-generating tungsten anode X-ray spectra from 30 to 140 kV. Med. Phys. 1997, 24, 1661–1670. [Google Scholar] [CrossRef]
- Available online: https://radcal.com/ (accessed on 25 February 2021).
- Available online: https://delmedical.com/ (accessed on 25 February 2021).
- Koukou, V.; Martini, N.; Michail, C.; Sotiropoulou, P.; Fountzoula, C.; Kalyvas, N.; Kandarakis, I.; Nikiforidis, G.; Fountos, G. Dual Energy Method for Breast Imaging: A Simulation Study. Comput. Math. Methods Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Martini, N.; Koukou, V.; Michail, C.; Sotiropoulou, P.; Kalyvas, N.; Kandarakis, I.; Nikiforidis, G.; Fountos, G. Pencil Beam Spectral Measurements of Ce, Ho, Yb, and Ba Powders for Potential Use in Medical Applications. J. Spectrosc. 2015, 2015, 1–8. Available online: https://www.hindawi.com/journals/jspec/2015/563763/ (accessed on 25 February 2021). [CrossRef]
- Michail, C.M.; Spyropoulou, V.A.; Fountos, G.P.; Kalyvas, N.I.; Valais, I.G.; Kandarakis, I.S.; Panayiotakis, G.S. Experimental and Theoretical Evaluation of a High Resolution CMOS Based Detector Under X-Ray Imaging Conditions. IEEE Trans. Nucl. Sci. 2010, 58, 314–322. [Google Scholar] [CrossRef]
- Milton, R.C. An Extended Table of Critical Values for the Mann-Whitney (Wilcoxon) Two-Sample Statistic. J. Am. Stat. Assoc. 1964, 59, 925. [Google Scholar] [CrossRef]
- Brisbane, W.; Bailey, M.R.; Sorensen, M.D. An overview of kidney stone imaging techniques. Nat. Rev. Urol. 2016, 13, 654–662. [Google Scholar] [CrossRef]
- Pai, R.; Modh, R.; Lamoureux, R.H.; Deitte, L.; Wymer, D.C.; Mench, A.; Lipnharski, I.; Henriksen, C.; Arreola, M.; Canales, B.K. Image Quality and Patient-Specific Organ Doses in Stone Protocol CT: A Comparison of Traditional CT to Low Dose CT with Iterative Reconstruction. BioMed Res. Int. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hokamp, N.G.; Salem, J.; Hesse, A.; Holz, J.A.; Ritter, M.; Heidenreich, A.; Maintz, D.; Haneder, S. Low-Dose Characterization of Kidney Stones Using Spectral Detector Computed Tomography: An Ex Vivo Study. Investig. Radiol. 2018, 53, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hokamp, N.G.; Lennartz, S.; Salem, J.; Dos Santos, D.P.; Heidenreich, A.; Maintz, D.; Haneder, S. Dose independent characterization of renal stones by means of dual energy computed tomography and machine learning: An ex-vivo study. Eur. Radiol. 2019, 30, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Raskin, D.; Winkler, H.; Kleinmann, N.; Schor-Bardach, R.; Guranda, L.; Muzikansky, G.; Portnoy, O. Very low-dose computerized tomography for confirmation of urinary stone presence. World J. Urol. 2021, 39, 233–238. [Google Scholar] [CrossRef]
- Morsbach, F.; Wurnig, M.C.; Müller, D.; Krauss, B.; Korporaal, J.G.; Alkadhi, H. Feasibility of Single-Source Dual-Energy Computed Tomography for Urinary Stone Characterization and Value of Iterative Reconstructions. Investig. Radiol. 2014, 49, 125–130. [Google Scholar] [CrossRef][Green Version]
- Ascenti, G.; Siragusa, C.; Racchiusa, S.; Ielo, I.; Privitera, G.; Midili, F.; Mazziotti, S. Stone-Targeted Dual-Energy CT: A New Diagnostic Approach to Urinary Calculosis. Am. J. Roentgenol. 2010, 195, 953–958. [Google Scholar] [CrossRef]
- Eiber, M.; Holzapfel, K.; Frimberger, M.; Straub, M.; Schneider, H.; Rummeny, E.J.; Dobritz, M.; Huber, A. Targeted dual-energy single-source CT for characterisation of urinary calculi: Experimental and clinical experience. Eur. Radiol. 2012, 22, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Yu, L.; Cardona, D.G.; Liu, Y.; Duan, X.; Ai, S.; Leng, S.; Shiung, M.; McCollough, C.H. Radiation Dose Reduction in Dual-Energy CT: Does It Affect the Accuracy of Urinary Stone Characterization? Am. J. Roentgenol. 2015, 205, W172–W176. [Google Scholar] [CrossRef]
- Cheng, R.Z.; Shkolyar, E.; Chang, T.C.; Spradling, K.; Ganesan, C.; Song, S.; Pao, A.C.; Leppert, J.T.; Elliott, C.S.; To’O, K.; et al. Ultra-Low-Dose CT: An Effective Follow-Up Imaging Modality for Ureterolithiasis. J. Endourol. 2020, 34, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Yamamoto, H.; Nakamoto, Y.; Sasaki, K.; Toshimitsu, S.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. Predictive Value of 18 F-Sodium Fluoride Positron Emission Tomography in Detecting High-Risk Coronary Artery Disease in Combination With Computed Tomography. J. Am. Hear. Assoc. 2018, 7, e010224. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Nakamoto, Y.; Fujii, Y.; Sasaki, K.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. Relationship between coronary arterial 18F-sodium fluoride uptake and epicardial adipose tissue analyzed using computed tomography. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.A.; Nicol, E.D.; Bull, R.K.; Roobottom, C.A.; Williams, M.C.; Harden, S.P. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J. Cardiovasc. Comput. Tomogr. 2017, 11, 268–273. [Google Scholar] [CrossRef]
- Takagi, H.; Tanaka, R.; Nagata, K.; Ninomiya, R.; Arakita, K.; Schuijf, J.D.; Yoshioka, K. Diagnostic performance of coronary CT angiography with ultra-high-resolution CT: Comparison with invasive coronary angiography. Eur. J. Radiol. 2018, 101, 30–37. [Google Scholar] [CrossRef]
- Jo, B.-D.; Lee, Y.-J.; Kim, D.-H.; Kim, H.-J. Scatter correction using a primary modulator for dual energy digital radiography: A Monte Carlo simulation study. J. Korean Phys. Soc. 2014, 65, 541–552. [Google Scholar] [CrossRef]
- Ai, K.; Gao, Y.; Yu, G. A scatter correction method for dual-energy digital mammography: Monte Carlo simulation. J. X-ray Sci. Technol. 2014, 22, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.G.; Zink, F.; Peppler, W.W.; Ergun, D.L.; Mistretta, C.A. A regional convolution kernel algorithm for scatter correction in dual-energy images: Comparison to single-kernel algorithms. Med. Phys. 1994, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]







| HAp | CaCO3 | CaC2O4 | HAp | CaCO3 | CaC2O4 |
|---|---|---|---|---|---|
| 0.50 | 0.61 | 0.99 | 1.80 | 2.23 | 3.75 |
| 0.60 | 0.74 | 1.21 | 1.90 | 2.35 | 3.96 |
| 0.70 | 0.86 | 1.43 | 2.00 | 2.47 | 4.17 |
| 0.80 | 0.99 | 1.65 | 2.10 | 2.60 | 4.38 |
| 0.90 | 1.12 | 1.86 | 2.20 | 2.72 | 4.59 |
| 1.00 | 1.24 | 2.08 | 2.30 | 2.85 | 4.80 |
| 1.10 | 1.36 | 2.29 | 2.40 | 2.97 | 5.00 |
| 1.20 | 1.48 | 2.50 | 2.50 | 3.10 | 5.21 |
| 1.30 | 1.61 | 2.71 | 2.60 | 3.22 | 5.42 |
| 1.40 | 1.73 | 2.92 | 2.70 | 3.34 | 5.63 |
| 1.50 | 1.86 | 3.13 | 2.80 | 3.46 | 5.84 |
| 1.60 | 1.98 | 3.34 | 2.90 | 3.59 | 6.05 |
| 1.70 | 2.11 | 3.55 | 3.00 | 3.71 | 6.25 |
| Low Energy Filters | High Energy Filters | ||
|---|---|---|---|
| Filter Material | K-Edge (keV) | Filter Material | Density (g cm−3) |
| Cerium (Ce) | 40.44 | Gallium (Ga) | 5.90 |
| Praseodymium (Pr) | 41.99 | Vanadium (V) | 6.00 |
| Neodymium (Nd) | 43.57 | Antimony (Sb) | 6.69 |
| Promethium (Pr) | 45.18 | Chromium (Cr) | 7.18 |
| Samarium (Sm) | 46.83 | Tin (Sn) | 7.31 |
| Europium (Eu) | 48.52 | Copper (Cu) | 8.96 |
| Aluminum (Al) | 1.56 | Bismuth (Bi) | 9.75 |
| Case | HAp Thickness (mm) | FN (%) | FP (%) | OA (%) |
|---|---|---|---|---|
| HAp; CaCO3 | 0.50 | 51.86 | 35.51 | 87.37 |
| 1.00 | 31.57 | 29.83 | 61.40 | |
| 1.50 | 24.83 | 22.94 | 47.77 | |
| 2.00 | 19.39 | 18.35 | 37.73 | |
| 2.50 | 15.62 | 14.77 | 30.40 | |
| 3.00 | 13.50 | 12.52 | 26.02 | |
| HAp; CaC2O4 | 0.50 | 50.46 | 32.58 | 83.04 |
| 1.00 | 28.01 | 26.43 | 54.44 | |
| 1.50 | 20.45 | 19.30 | 39.75 | |
| 2.00 | 15.11 | 14.11 | 29.21 | |
| 2.50 | 11.40 | 10.87 | 22.27 | |
| 3.00 | 8.99 | 9.04 | 18.03 | |
| CaCO3; CaC2O4 | 0.50 | 55.35 | 34.82 | 90.17 |
| 1.00 | 45.10 | 46.43 | 91.53 | |
| 1.50 | 45.23 | 43.36 | 88.59 | |
| 2.00 | 43.48 | 42.51 | 85.99 | |
| 2.50 | 41.88 | 41.53 | 83.40 | |
| 3.00 | 42.04 | 40.16 | 82.20 |
| HAp Thickness (mm) | Averaged Effective | Mann–Whitney U Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| U Value | ||||||||||
| HAp | CaCO3 | CaC2O4 | HAp | CaCO3 | CaC2O4 | HAp; CaCO3 | HAp; CaC2O4 | CaCO3; CaC2O4 | Critical Value | |
| 0.70 | 2.12 | 1.70 | 1.59 | 72.46 | 53.89 | 50.02 | 75 | 62 | 142 | 127 |
| 1.00 | 2.13 | 1.73 | 1.62 | 51.76 | 36.91 | 32.44 | 38 | 23 | 131 | 127 |
| 1.50 | 2.13 | 1.73 | 1.62 | 32.35 | 25.28 | 26.03 | 0 | 0 | 120 | 127 |
| 3.00 | 2.29 | 1.73 | 1.64 | 19.79 | 17.32 | 18.22 | 0 | 0 | 115 | 127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, N.; Koukou, V.; Michail, C.; Fountos, G. Mineral Characterization in Human Body: A Dual Energy Approach. Crystals 2021, 11, 345. https://doi.org/10.3390/cryst11040345
Martini N, Koukou V, Michail C, Fountos G. Mineral Characterization in Human Body: A Dual Energy Approach. Crystals. 2021; 11(4):345. https://doi.org/10.3390/cryst11040345
Chicago/Turabian StyleMartini, Niki, Vaia Koukou, Christos Michail, and George Fountos. 2021. "Mineral Characterization in Human Body: A Dual Energy Approach" Crystals 11, no. 4: 345. https://doi.org/10.3390/cryst11040345
APA StyleMartini, N., Koukou, V., Michail, C., & Fountos, G. (2021). Mineral Characterization in Human Body: A Dual Energy Approach. Crystals, 11(4), 345. https://doi.org/10.3390/cryst11040345








