3.1. Study of LiNbO3:B Crystals Defective Structure by Raman Spectroscopy
Raman spectra of LiNbO
3:B (0.55–1.24 mol%) crystals were previously studied in papers [
21,
22,
41,
42]. The papers focus on an influence of a disorder in cation sublattice on A
1(TO) symmetry type phonons in Y(ZZ)Ȳ scattering geometry and on photorefractive effect manifestation in Raman spectra.
Figure 4 demonstrates Raman spectra of SLN, CLN and LiNbO
3:B (0.55–1.24 mol%) in Y(ZX)Ȳ and Y(ZZ)Ȳ scattering geometries. These geometries correspond to fundamental vibration of the lattice of E(TO) and A
1(TO) symmetry types. Spectra fragments with the most articulated changes at a crystal composition variation are given on
Figure 5.
Table 1 demonstrates main parameters (ν—frequency, S—band width, I—band intensity) of bands appearing in studied crystals.
Figure 6 demonstrates changes in half-widths and intensities of 576 and 630 cm
−1 Raman bands and in PILS speckle-structure indicatrix opening angle θ. SLN Raman spectrum contains all nine bands corresponding to E(TO) symmetry type phonons permitted by selection rules in Y(ZX)Ȳ scattering geometry,
Figure 4,
Table 1. Low-intense bands 179 and 611 cm
−1 are clearly observed in SLN Raman spectrum. However, they dissolve by disordering effects in spectra of CLN and LiNbO
3:B (0.55–1.24 mol%). Frequencies of all bands stay the same within error,
Figure 4 and
Figure 5,
Table 1. This indicates that secondary structure changes (they appear at changes in Li/Nb ratio and B
3+ cation concentration) affect very little quasi-elastic lattice constants in studied LN crystals. At the same time,
Figure 4,
Figure 5 and
Figure 6 and
Table 1 reveal that width and intensities of LiNbO
3:B Raman bands change significantly in all Raman spectra areas: two-particle states of acoustic phonons (100–150 cm
−1); vibration of cations located in oxygen octahedra BO
6 (B: Nb, Li, dopant) (200–300 cm
−1); vibrations of oxygen octahedra atoms (500–900 cm
−1).
Table 1 shows that LiNbO
3:B Raman bands in the area 150–300 cm
−1 are narrower than those of CLN; 152 and 240 cm
−1 widths of LiNbO
3:B Raman bands coincide with those of SLN. The 578 cm
−1 band corresponds to doubly degenerate E(TO) vibrations of oxygen atoms in O
6 octahedra. It is much wider in spectra of LiNbO
3:B (0.55–1.24 mol%) than in SLN and CLN,
Table 1,
Figure 6. Obtained data show that even very a faint change in boron concentration in the charge and melt (0.55–1.24 mol% B
2O
3) leads to a significant ordering of cation sublattice along the polar axis at both doping methods. At the same time, oxygen octahedra O
6 are distorted. New bands in the area of Raman spectra corresponding to oxygen octahedra vibrations would mean a change in their geometry. However, the area contains no new bands.
Due to selection rules [
2,
43], fundamental vibrations of E(TO) symmetry type should manifest in Y(ZX)Ȳ scattering geometry, A
1(TO) symmetry type—in Y(ZZ)Ȳ. A
1(TO) symmetry type vibrations are forbidden in Y(ZX)Ȳ scattering geometry [
2]. However, due to photorefractive effect, they manifest in this scattering geometry. The bands corresponding to A
1(TO) symmetry type vibrations have intensities proportional to photorefractive effect value. Papers [
2,
44] have shown that the 630 cm
−1 band is the most suitable for photorefractive effect evaluation. The band corresponds to A
1(TO) vibrations of oxygen atoms in O
6 octahedra.
The E(TO) 580 cm
−1 band does not change with changes in photorefractive effect value, at least in crystals with a low photorefractive effect. Thus, photorefractive effect can be evaluated due to the formula I
rel = (I
630/I
580) × 100% [
11,
13,
44,
45]. Structure disorder contributes the most to LN Raman bands widening during dopant concentration increase at a constant temperature [
2,
43]. Structure disorder and photorefractive effect contribute the most to LN Raman bands intensity changes [
2,
43].
Figure 6b shows that the least relative intensity of the 630 cm
−1 band is observed in CLN and SLN crystals spectra, the greatest—in LiNbO
3:B (0.55–0.83 mol%) crystals. In LiNbO
3:B spectra the 630 cm
−1 band’s intensity first increases, than decreases with an increase in B
3+ concentration,
Figure 6b. In addition, concentration behavior of 630 cm
−1 band intensity and widths of 630 cm
−1 (A
1(TO)) and 576 cm
−1 (E(TO)) bands in the Y(ZX)Ȳ Raman spectra of LiNbO
3:B well coincide with the behavior of PILS opening angle θ,
Figure 6. Thus, oxygen octahedra distortions that occur due to a change in cation sublattice order and photorefractive effect depend on boron concentration in the charge in studied LiNbO
3:B crystals.
Obtained data allow us to conclude that boron structures the melt, which actually means that a congruent LN crystal is growth from a boron-doped charge. Such a crystal has noticeable differences in fine structural features and physical characteristics from a nominally pure CLN crystal, grown from a congruent charge. Obtained results can have the following explanation.
We believe that boron structures the melt so that crystals grown from it have a decreased amount of Nb
Li. Raman spectra confirm this conclusion. Raman spectra of both nominally pure and doped CLN crystals contain a low-intense band 120 cm
−1 (A
1(TO)),
Figure 5. The band corresponds to two-particle states of acoustic phonons with a total wave vector equal to zero [
2]. Due to [
46], it cannot correspond to a pseudoscalar mode A
2 forbidden in a point group C
3v by the selection rules. This band is split in two, 105 and 118 cm
−1 bands, in a spectrum of a CLN crystal,
Figure 5. This happens due to a refinement of the selection rules in the wave vector of two-particle states of A
1(TO) acoustic phonons [
2,
47,
48]. It is a well-known fact [
2,
47,
48], that spectrum of a perfect SLN crystal is free of the 120 cm
−1 band,
Figure 5. In addition, SLN crystals are characterized by the perfect order in a cation sublattice, it is free of Nb
Li defects. Intensity of this band first decreases, than increases in LiNbO
3:B with an increase in a dopant concentration,
Figure 5. The band is split in two components 112 and 123 cm
−1 in a spectrum of a crystal LiNbO
3:B (1.24 mol%),
Figure 5. This indicates a high structure perfection of the crystal. A decrease in the 120 cm
−1 band intensity also indicates a decrease in Nb
Li defects amount at its doping by B
3+ ions. Cation sublattice orders (Raman bands in the area 150–300 cm
−1 narrow,
Table 1) simultaneously with a decrease in resonant anharmonic interaction between the lowest-frequency fundamental A
1(TO) vibrations 254, 274 cm
−1 and two-particle A
1(TO) acoustic excitations. The latter is discussed in detail in [
49]. A degree of mixing between A
1(TO) single-phonon and multi-phonon states depends on the value of this interaction, which also influences the spectrum in the 120 cm
−1 area. Thus, LN crystals doping can effectively govern interaction between single-phonon and two-phonon states. Paper [
50] has revealed that 120 cm
−1 band intensity is connected with an acoustic Q-factor of LN crystal. The higher Q-factor is, the smaller the band intensity is, due to a decrease in the amount of Nb
Li defects. Thus, our data prove a higher structure perfection of nominally pure LN crystals grown from boron doped charge compared to that of a CLN crystal. The crystal LiNbO
3:B (1.24 mol%) is the most structurally perfect one.
3.2. Study of LiNbO3:B Crystal Structure by IR Spectroscopy in the Area of OH-Groups Vibration
LN single crystals obtained at air atmosphere always contain OH groups incorporated into the structure during growth [
14]. Localization of hydrogen atoms in LN structure greatly influences charge distribution and polarizability of oxygen octahedra clusters MeO
6 (Me: Li, Nb, dopant). Hydrogen atoms bond with oxygen of LN structure by hydrogen bonds. OH groups increase LN crystal conductivity and decrease optical damage and coercive field value [
10,
29]. Doping of LN crystal leads to a total restructure of the whole hydrogen bonds system.
Stretching and deformation vibrations of OH groups are supposed to manifest in Raman and IR absorption spectra of LN crystals in the areas 1600–1800 and 3450–3550 cm
−1, respectively. The amount of bands in these areas and their basic parameters depend on localization of hydrogen atoms in the crystal structure. The localization in its turn depends on stoichiometry, doping particularities and growth details of a single crystal [
14]. A perfect strictly stoichiometric LN crystal has no site for a hydrogen atom [
51]. IR spectrum of a real NSLN crystal contains only one narrow (S = 3 cm
−1) 3466 cm
−1 band [
26,
52]. A slight shift of stoichiometry leads to widening and splitting of this band in IR absorption spectrum into 3466 and 3480 cm
−1 bands. This is caused by a formation of complex defects Nb
Li–OH, V
Li, etc., in a structure of a non-stoichiometric LN crystal [
51]. CLN crystal IR spectrum contains three components of the band: and intense 3466 cm
−1 band, an average intense 3481 cm
-1 band and a low-intense 3489 cm
−1 band [
53]. Papers [
29,
52] have demonstrated that as LN structure approaches stoichiometric composition, width of the 3466 cm
−1 band and intensity of 3481 and 3489 cm
−1 bands decrease. Note that SLN crystal has a ratio Li/Nb = 1, point defects, such as the same cations in the neighbor sites are absent. Thus, studies of parameters of IR absorption bands in the OH-groups vibrations area contains important information about the nature of defects and their localization in the structure; the structural rearrangements that occur in LN crystals with a change in composition; and the peculiarities of a single crystal growth. Such information is also important at refinements of technologies of growing optically and structurally perfect LN single crystals.
Figure 7 demonstrates IR absorption spectra of studied LN crystals in the area 3420–3550 cm
−1. This area contains stretching vibrations of OH groups.
Table 2 demonstrates parameters of spectral bands. It is obvious that spectra of all studied crystals are different. An SLN crystal spectrum contains three bands with frequencies 3465, 3480, and 3488 cm
−1,
Figure 7. The bands in an SLN spectrum are much narrower than in other crystals,
Table 2. It is believed that a band in the frequency range 3465–3466 cm
−1 is characteristic of a highly perfects SLN crystals with only one hydrogen atom position, [
29,
52].
Figure 7 clearly shows that a CLN spectrum is different from the one of an SLN crystal. IR spectra of a CLN is a blurred wide absorption band consisting of several components with the same polarization with frequencies 3467 (ν
1), 3483 (ν
2), and 3486 (ν
3) cm
−1 and a weak band with a frequency ~3490 cm
−1 (ν
4). A 3480–3485 cm
−1 band is considered characteristic of a CLN crystal and is attributed to stretching vibrations of a V
Li–OH complex [
54]. V
Li defects are absent from SLN crystal structure. This means that IR spectrum should not contain bands with frequencies 3480–3485 cm
−1, which is confirmed experimentally [
29,
52]. IR spectra of LiNbO
3:B (0.55–0.83 mol%) is similar to those of CLN: an OH-groups absorption band is split in three components of the same polarization ~3470, ~3483, and ~3486 cm
−1. Thus, hydrogen bonds in CLN and LiNbO
3:B crystals structure should be similar. Three components of CLN crystal are considered to be connected with stretching vibrations of OH groups located near Nb
Li4+–V
Li− defects [
53,
55]. However, intensities of all three components are higher in LiNbO
3:B than in CLN spectrum,
Table 2. At the same time widths of two bands are narrower than in CLN spectrum, widths of the band 3485 cm
−1 is not narrower,
Table 2. This indicates ordering of OH groups distribution in LiNbO
3:B crystals structure.
A computer simulation has been carried out in a paper [
56]. The paper considers possible sites of hydrogen atom in SLN crystals. The paper has shown that all hypothetical hydrogen sites on O–O bonds located near V
Li defects are unstable and should be excluded from further consideration. Moreover, the hydrogen atom site on C axis has not been confirmed. Analysis of possible hydrogen atoms trajectories in LN crystal structure shows that it can be associated in SLN structure only with one oxygen ion in the upper oxygen triangle of NbO
6 octahedron. Dipole moment orientation of OH-group is slightly different from a direction of a short O–O bond. Thus, creation of a hydrogen bond leads not only to a cardinal change in the wave functions of the outer electron orbitals of the oxygen ion and the parameters of its electronic polarizability, but also to a strong distortion of the entire NbO
6 octahedron [
56]. Such changes in a crystal structure should manifest in 850–900 cm
−1 area of Raman spectra. Bonds in this area correspond to stretching bridge vibrations B–O–B (B: Nb or dopant) of oxygen in NbO
6 octahedra along the polar axis [
2].
Table 1 shows that spectra of LiNbO
3:B and nominally pure SLN, CLN crystals contain only one band in the area 850–900 cm
−1. The width of this band depends on the Li/Nb value. This band width is minimal in SLN and maximal in CLN crystal spectrum,
Table 1. An 850 cm
−1 Raman band width has an intermediate value in LiNbO
3:B (0.55–0.83 mol%) crystals,
Table 1. The character of the Me
1-O-Me
2 bridge bond is determined not only by the Me-O bridges strength in octahedra. Widening of bands in the 850–900 cm
−1 area is determined by the fact that Nb cations are located in different sites in octahedra, their concentration is quite high and they interact weakly between each other [
2]. Changes in LN crystals Raman spectra in the area of stretching bridge vibrations correlate with changes observed in IR spectra,
Figure 7. This result confirms conclusions of a theoretical work [
56] and experimental data [
29,
52].
Papers [
57,
58] have clearly indicated that the ratio I
rel = I
3480/I
3465 between intensities of 3480 and 3465 cm
−1 IR bands almost linearly decreases with an increase in R = Li/Nb ratio. Note that I
rel = 0 if the 3480 cm
−1 band is absent from the spectrum. Evaluation of I
rel allows one to determine R with higher precision (0.01 mol% Li
2O) than other methods [
57,
58]. For example, determination of Li
2O concentration due to Curie temperature has an error 0.1 mol% Li
2O. A ratio I
rel = I
3480/I
3465 = 0.78 for an SLN crystal studied in our research. This means that R = Li/Nb = 0.98. This result is different from data obtained by Raman spectroscopy: at R = 1 a low-intense 120 cm
−1 band should be absent from SLN spectrum,
Figure 5. Changes can be explained by the following: 120 cm
−1 Raman band is sensitive only towards the R = Li/Nb ratio (which automatically include all point and complex defects); 3466 cm
−1 IR band is sensitive only towards Nb
Li–OH complex defects. This is why R determined due to IR spectra is smaller, than that determined due to Raman spectra.
A Li/Nb ratio can also be calculated with a high precision (up to 0.01 mol%) from a fundamental absorption edge [
59]. However, this kind of experiment should be carried out at fine plates (0.5–1.0 mm) of LN crystals. Moreover, studied samples must not have additional absorption bands in the fundamental absorption edge, as it is in crystals doped by multiply charged cations Fe, Cu, etc. Empirical formulae for Li/Nb evaluation in nominally pure LN crystals were suggested in [
59]. The formulae apply data on fundamental absorption edge:
where
—fundamental optical absorption edge corresponding to a wavelength at an absorption coefficient α = 20 cm
−1.
Knowing Li/Nb value, one can calculate concentration of point defects in LN crystal. Due to a Li-vacancies compensation model [
2], concentration of Nb
Li4+ and V
Li− point defects in mol% in a crystal lattice of nominally pure LN crystals can be calculated due to a formula [
59]:
Paper [
59] offers a model for determination of Li/Nb ratio in crystals that have absorption bands directly in the region of the fundamental absorption edge. Ratio of the integrated intensity of the band with a maximum at 3466 cm
−1 (
) to the total integrated intensity of the IR absorption spectrum (
) (
) was determined for each crystal’s spectrum. In the formula α(ν)—absorption coefficient in dependence on wavelength in cm
−1.
A Li/Nb ratio can be determined in crystals doped by photochrome or photorefractive cations absorbing in visible or UV spectral area due to the obtained
value and Li/Nb ratio [
59]. This method can obviously be applied for other cases; for example, for LN crystal samples with a width >1 mm, when fundamental optical absorption edge
is impossible to determine. In this paper we have used samples with a width Z = 4 mm. Our calculations concerning Li/Nb and Nb
Li4+ and V
Li− point defects concentration obtained due to the abovementioned formulae are demonstrated in
Table 2.
A Klauer [
60] method can be applied to calculate concentration of OH-groups:
where
C(
OH)—concentration of OH defects in a sample, (cm
−3),
—a total integral intensity of IR spectrum in the OH stretching vibrations area (
,
—absorption coefficient in dependence on wavelength in cm
−1),
—intensity if ion absorption = (9.125 ± 1.369) × 10
−18 cm [
60,
61].
Calculation results are demonstrated in
Table 2. Concentration of OH-groups is minimal in SLN and maximal in LiNbO
3:B (0.55 and 0.83 mol%) crystal,
Table 2. CLN and LiNbO
3:B(0.69 mol%) crystals have an intermediate value of OH groups,
Table 2. A decrease in the amount of bonded OH groups apparently leads to an increase in free protons in LN crystals, which can influence electrical conductivity of the crystals and decrease photorefractive effect.
3.3. Study of LiNbO3:B Crystals Optical Uniformity by PILS and Laser Conoscopy
We have studied optical uniformity of crystals by laser conoscopy at laser radiation (λ
0 = 532 nm) power
p = 1 and 90 mW,
Figure 8. When conoscopic patterns of LN crystals at low power differ from pattern of a perfect crystal, it means that changes are caused by defects, mechanical stresses, or compositional heterogeneity. Conoscopic pattern distortions at high-power radiation are caused by laser-induced static and fluctuating defects. Laser conoscopy and PILS method are unable to provide direct information about crystals intrinsic structure and defects type. However, they detect information on the optical homogeneity and photorefractive properties of crystals.
Figure 8 shows that conoscopic patterns of studied LiNbO
3:B crystals at both exciting radiation powers correspond to a pattern of a perfect single axis LN. Thus, conoscopic patterns confirm optical single axis in LiNbO
3:B (0.55–1.24 mol%) crystals,
Figure 8. Slight signs of anomalous optical biaxiality are observed only on a 90 mW conoscopic pattern of a LiNbO
3:B (0.83 mol%) crystal,
Figure 8. A “Maltese cross” is deformed in the center, fragments are shifted from its center; angle between branches is not 90°; isochromes are slightly oval, however their geometry is not distorted. Appearance of anomalous biaxiality can be caused by the existence of local micro-regions and clusters with birefringence in the crystal. In addition, observed distortion in LiNbO
3:B (0.83 mol%) crystal conoscopic patterns can be associated with an increase in the effect of photorefraction,
Figure 8.
PILS is a direct consequence of the photorefractive effect. It arises in a ferroelectric crystal on defects induced by exciting radiation with fluctuating refractive index, dielectric constant, and other physical parameters [
62,
63]. Laser radiation scattered by such defects interferes with pumping, forming a complex pattern of minima and maxima of the scattered light intensity (speckle structure),
Figure 8. It causes strong laser beam destruction in the crystal; it is an interfering factor for the radiation generation and conversion [
4]. The shape and features of the PILS indicatrix speckle structure depend on the crystal structure, the state of its defectiveness, as well as on the radiation polarization and the experiment geometry [
62,
64]. The time and opening angle of the PILS indicatrix determine the response speed of the electro-optical modulators and gates. PILS parameters are determined by the depth of traps, the mobility of electrons responsible for the magnitude of the photorefractive effect, as well as the interaction of laser radiation with a defective crystal [
62].
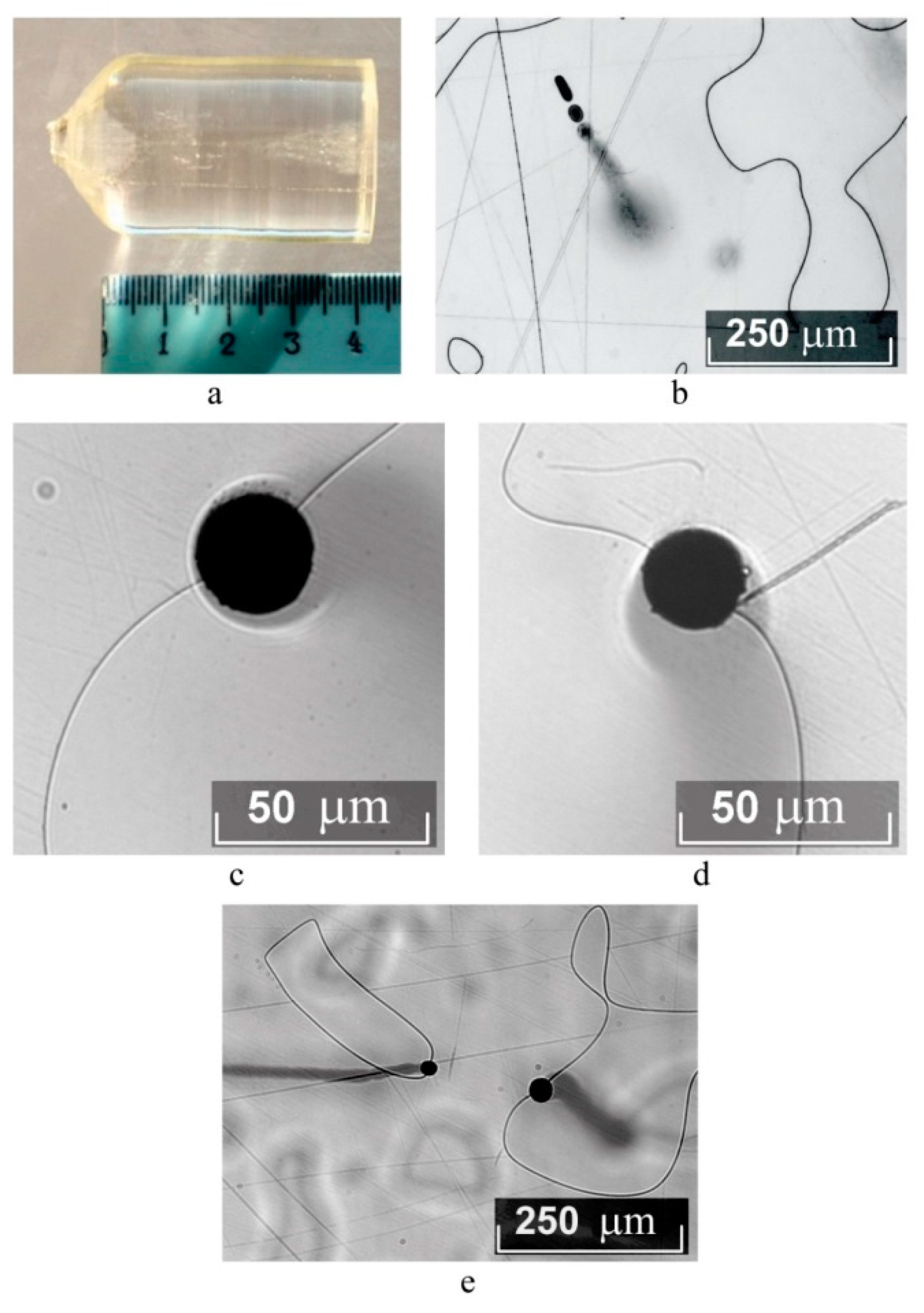
Figure 8 demonstrates PILS patterns of studied crystals. The time of full PILS indicatrix speckle structure opening for the above crystals is 60 s. According to PILS data, a crystal LiNbO
3:B (1.24 mol%) possesses the highest optical damage resistance. PILS speckle structure indicatrix of this crystal does not open even at a high excitation radiation power 160 mW, despite other crystals,
Figure 8. Conoscopic and PILS patterns show that structure uniformity of LiNbO
3:B is similar to that of a CLN crystal and is much higher than that in a SLN crystal,
Figure 8. Note that photorefractive effect is much higher in SLN than in CLN and LiNbO
3:B (0.55–1.24 mol%) crystals. An SLN crystal PILS opening angle θ is 56°; it does not exceed 22° in LiNbO
3:B (0.55–1.24 mol%) crystals,
Figure 6,
Table 3.
It is a known fact that SLN crystals have non-uniform refractive index along the growth axis, while in CLN crystals it is stable. Photorefractive effect manifests itself much stronger in SLN crystals, than in CLN crystals [
2,
65]. A change in the structure and properties of a melt, as well as crystal doping, adds levels in the band gap and thereby changes the photorefractive properties and electrical conductivity of the crystal. The crystal LiNbO
3:B (0.83 mol%, direct doping) is photorefractive, has a pronounced PILS and is characterized by a uniform refractive index along the growth axis, as evidenced by a clear conoscopic pattern,
Figure 8. At the same time, the crystal LiNbO
3:B (1.24 mol%, homogeneous doping) has a reduced effect of photorefraction; a more blurred conoscopic pattern indicates the presence of fluctuations in the refractive index along the polar axis. A similar, but much more diffuse conoscopic pattern is observed for SLN crystal,
Figure 8. At the same time, a discontinuous structure of the laser beam is observed when it propagates along the polar axis in SLN, (Figure 21 in [
66]). This indicates a much higher inhomogeneity of the refractive index in SLN in comparison with the LiNbO
3:B (1.24 mol%, homogeneous doping) crystal. For other crystals under study, a discontinuous structure of the laser beam has not been observed yet.
A decrease in the photorefractive effect can be due to a change in the band structure and electro-optical properties of the crystal due to the structuring of the melt with boron. A low photorefractive effect value in the LiNbO
3:B (1.24 mol%, homogeneous doping) crystal can be explained by a change in the electro-optical properties of the crystal. Due to works [
67,
68], a change in electro-optical coefficients in nominally pure LN crystals at a change in Li/Nb ratio leads to a change in ionic contribution to the magnitude of the electro-optical effect. We assume that boron influence leads to such a structuring of a melt that crystals grown from the melt have a more strict crystal structure compared to CLN crystals, in which MeO
6 octahedra have less ability to deform.
3.4. Investigation of Photovoltaic Fields and Bandgap in LiNbO3:B Crystals by PILS and Optical Spectroscopy
Figure 9 demonstrates optical absorption spectra of SLN, CLN, and LiNbO
3:B (0.55, 0.83 mol%) crystals. The absorption edge of LiNbO
3:B crystals is shifted to longer wavelengths compared to the absorption edge of SLN, CLN crystals. At this, absorption spectra of LiNbO
3:B crystals has a steeper rise in comparison with SLN. This indicates a higher optical uniformity of crystals grown from a boron-doped charge. It is believed that Nb
Li defects bring the main contribution to the change in the position of the fundamental absorption edge in nominally pure CLN crystals [
2,
59]. Thus, we can draw conclusions about the mechanism of non-photorefractive impurities incorporation into the crystal lattice.
Coincidence of the concentration dependences of Nb
Li defects and the Urbach parameter on the Li/Nb ratio in the crystal has been spotted in a paper [
59]. This agrees with the conclusions of [
69] that the Urbach absorption occurs in the LN crystal as a result of the transition of electrons from the filled states “2p” of oxygen to the empty states “4d” of niobium. A decrease in the concentration of Nb
Li defects as the Li/Nb ratio approaches unity leads to a fundamental absorption edge shift toward longer wavelengths as a result of a decrease in the density of local states associated with Nb
Li defects near the bottom of the conduction band. The transition of electrons from the valence band to these states is possible in LN under the light.
Figure 9 demonstrates that CLN absorption edge is maximally shifted to short-wavelength region.
Table 2 shows that for CLN Li/Nb = 0.942, C(V
Li) = 3.90 mol% and C(Nb
Li) = 0.98 mol%. It is a known fact that CLN crystals have Li deficiency near 6 mol% (Li/Nb = 0.946) [
2]. According to the compensation of Li-vacancies model, the crystal lattice of a CLN crystal contains ~1 mol% Nb
Li4+ point defects and ~4 mol% V
Li− point defects [
54,
70]. At the same time, Nb
Li4+ is completely absent from a perfect SLN crystal structure. LiNbO
3:B (0.55 and 0.83 mol%) crystals have ordering of structural units of the cation sublattice and the content of Nb
Li and V
Li point defects between SLN and CLN [
24]. Approximation to stoichiometry in LiNbO
3:B crystals is possible by binding excess niobium in a congruent melt due to the complexing ability of boron compounds [
24]. Obviously, the position of the fundamental absorption edge is determined not only by the concentration of Nb
Li and V
Li point defects. The number of shallow electron traps depends significantly on the Li/Nb ratio [
2,
7,
12,
71]. Under laser radiation a spatial charge separation occurs and an internal electric field arises, leading to a photoinduced change in refractive indices in LN crystal as a result of photoexcitation processes (photovoltaic and diffusion current) [
7,
12,
63,
71]. Photovoltaic
Epv and diffusion
ED fields presented in
Table 3 were calculated due to formulae [
31]:
where
Epv—photovoltaic field, E
D—diffusion field,
—wavelength,
—scattered radiation angle,
and
—amplification factors (indices “−” and “+” indicate the direction of the scattered radiation against and along the direction of the crystal polar axis, respectively),
and
—refractive indices of the extraordinary and ordinary rays, respectively,
and
—LN electro-optical coefficients.
An amplification factor
can be calculated in dependence on PILS opening angle due to a formula [
31]:
where
—scattered radiation intensity,
—primary scattering intensity (incident beam),
—The effective interaction interval, which is calculated depending on the scattering angle, according to the following formulas [
31]:
where
—crystal thickness,
—laser beam diameter.
Figure 10 and
Figure 11 demonstrate angular distribution of the scattered radiation intensity at different wavelengths and dependences
ED (a) and
Epv (b) on the wavelengths in studied LN crystals. The band gap for the studied crystals is in the range 3.37–3.78 eV,
Table 3. The least band gap was observed in LiNbO
3:B and SLN,
Table 3, the greatest—in CLN (3.78 eV). Due to [
72], a pure CLN crystal band gap is 3.72 eV, which is close to the value characteristic of wide-gap semiconductors.
A predominant photorefraction mechanism in a LN crystal is photovoltaic, which means that the value of the photovoltaic field (
Epv) is much greater than that of the diffusion field (E
D) [
71].
Table 3,
Figure 10 and
Figure 11 show: an increase in photovoltaic field in a LN crystal is accompanied with an increase in PILS scattering angle θ and an asymmetry of its indicatrix. The photovoltaic field
Epv determines the magnitude of the induced birefringence (the effect of photorefraction). SLN has the least
Epv at 476.5, 488.0, 514.5, and 530.9 nm excitation. Thus, a diffusion charge transport mechanism is manifested in SLN stronger than in other crystals. This picture is characteristic of crystals with a large number of shallow electron traps.
A dependence of E
pv has a maximum at excitation by 514.5 nm for all studied crystals, but LiNbO
3:B (0.55 mol%),
Figure 11. At the same time, E
pv values of LiNbO
3:B (0.55 and 0.83 mol%) crystals are close: 5458 and 5554 V/cm, respectively. Curious that
ED of a LiNbO
3:B (0.83 mol%) crystal is close to that of a CLN. This may indicate an almost equal number of shallow electron traps in these crystals.
ED value of a LiNbO
3:B (0.55 mol%) crystal is larger than that of CLN and LiNbO
3:B (0.83 mol%) crystals; at the same time, its PILS indicatrix opening angle is minimal,
Table 3.
3.5. Chemical Interactions in Systems Li2O–Nb2O5, Li2O–B2O3–Nb2O5; Particularities of LN Crystallization from Melts with Non-Metal Dopants
Solid-phase chemical reactions with the formation of various borates occur in the process of high-temperature synthesis of the charge in the Li
2O–B
2O
3–Nb
2O
5 system. To evaluate the melt–crystal system, it is advisable to use the distribution coefficients K
D and the ∆C parameter. K
D is the ratio of the impurity concentration in the crystal at the initial moment of growth and the impurity concentration in the melt, ∆C characterizes the compositional uniformity of the crystal; it is defined as the difference between the dopant concentration at the cone and the end of the crystal. A crucial particularity of boron is its ability to change LiNbO
3:B melt and crystal structure without incorporation to the crystal. Its effective distribution coefficient (K
D) is only ~3 × 10
−4 [
19,
20,
25]. Such small values of the boron distribution coefficient in the Li
2O–Nb
2O
5–B
2O
3 system are due to the fact [
73] that boron should not incorporate into the LiNbO
3:B crystal, since the LN phase has no region of boron solubility in the solid state,
Figure 12. The small amount of boron that does incorporate into the LN structure can be either be mechanically captured or incorporate into the tetrahedral voids of the crystal structure. Note that boron B
3+ has a small ion radius. Quasi-double diagrams are polythermal sections of the general quasi-ternary diagram of the state of systems Li
2O–Nb
2O
5–B
2O
3: LiNbO
3–LiBO
2, and LiNbO
3–Li
3B
2O
4.5. These cross sections have a simple form, since they contain only one two-phase eutectic transformation each and have no solubility regions,
Figure 12, [
73]. This type of phase diagram is generally favorable for the quality of the growing LiNbO
3:B crystal since crystallization proceeds from the two-phase region and the only crystallizing phase is LN. If we disregard the radical change in the structure of the melt upon doping with boron, crystal grown from such a melt should chemically and structurally correspond to a CLN grown from an undoped melt. Wherein studied LiNbO
3:B crystals have Nb
Li defects amount close to that of an SLN crystal. Moreover, the LiNbO
3:B crystal has a more ordered structure than CLN [
25]. Note that Nb
Li are the deepest electron traps responsible for the photorefractive effect. The reason for this is apparently an electronic structure of boron: it is a strong complexing element due to 1 electron at a p–orbital. The electron provides high ionization energies and electronegativity values at a small ionic radius, which leads to a significant change in the structure of the boron-containing melt.
Table 4 demonstrates ∆C and K
D for exact B, Mg, Zn, and Ce dopants concentrations in the melt. The necessity to use K
D and ∆C is substantiated in detail in the works [
27,
74]. The distribution coefficient K
D for doped (B, Zn, Mg, and Ce) LN crystals is clearly a function of the electronic structure of the dopant; the ∆C case is not so obvious,
Table 4. The ∆C parameter value is quite small for all studied dopants except Zn. In fact, the value is comparable to the error of the method for determining the dopant concentration,
Table 4.
Table 4 data reveal high concentration uniformity in dopant distribution along the polar axis at a K
D coefficient strongly different from the unity. This is especially characteristic of LiNbO
3:B crystals, in which K
D << 1 and ∆C ~ 10
−6 wt%. The explanation of this fact can be the following. The initial LN charge composition ceases to be congruent with the addition of dopant,
Figure 12. This, in particular, means that the spectrum of variations of ionic complexes in the melt in terms of structure and components greatly increases. The melt is trapped in a limited zone near the crystallization front, which has a constant temperature. Consequently, those ionic complexes for which this temperature is solidus (T
c1) will crystallize, naturally, taking into account some supercooling. As part of the volume of the melt is consumed, the concentration of the dopant (in our case, boron) in the remaining melt increases and the ratio of the concentrations of various ionic complexes changes. Accordingly, the fraction of ionic complexes for which the temperature T
c1 is the solidus temperature will also change, it will become noticeably less. This will continue until the system reaches a certain critical state, at which the concentration of complexes with solidus temperature T
c1 is insufficient for the growth of a crystal with a constant dopant concentration. This circumstance limits the fraction of the melt that can be crystallized to obtain a compositionally uniform LiNbO
3:B crystal. Upon reaching the described critical state, the further behavior of the system can be different: from a significant change in the dopant concentration distribution along the growth axis to defects such as cellular growth and crystallization of a different composition phase,
Figure 3 [
19,
27,
74]. Many factors affect the conditions for reaching such a critical state, and hence the possible dimensions of a LiNbO
3:B crystal of high optical quality with a constant dopant concentration distribution in the crystal boule volume. This is the composition of the melt, the thermodynamics of the initial components, the graphical expression of which is the phase diagram, the structure of the melt consisting of ionic complexes with different thermodynamic and kinetic characteristics, and even the technical capabilities (sensitivity and reaction time constant) of the control and monitoring system of the growth process.
An important conclusion follows from the above reasoning for the technology of doped lithium niobate crystals: LN crystals with a uniform distribution of dopant can be grown by Czochralski at K
D that is noticeably different from unity during crystallization of only a well-defined part of the melt. Consequently, the length and diameter of such a LN crystal boule is limited. In different systems, the limiting sizes of such structurally and compositionally uniform crystals will differ markedly. Moreover, for different systems, there may be different physical and chemical reasons for this, expressed in the difference in the thermodynamic parameters of the systems, that is, in the form of phase diagrams. LN has no regions of homogeneity with either boron or its compounds in the boron-containing system Li
2O–Nb
2O
5–B
2O
3. As the crystal grows, only the pure LN phase crystallizes,
Figure 12. However, at the same time, there will be a significant increase in the boron content in the melt and, as a consequence, a decrease in the crystallization temperature and a rather radical increase in the melt viscosity, which limits convective flows,
Figure 12. C
0, C
1 and C
2—the composition of the melt or the concentration of boron in the melt, T
1 and T
2 are the crystallization temperature corresponding to the composition of the C
1 and C
2. All this, most likely, limits the maximum possible boron concentration in the melt to 0.12 wt%, since an increase in the boron concentration above this critical concentration in the melt leads to cellular growth and other irreparable defects (for example, defects in the form of “channels”) in LiNbO
3:B crystals,
Figure 1,
Figure 2 and
Figure 3, [
19,
25]. Our studies have shown that the fraction of the melt, the crystallization of which leads to the production of an optically uniform LiNbO
3:B crystal, does not exceed 17–18% [
19,
25].
The above reasoning is valid for evaluating the technological growth parameters when doping lithium niobate with any dopants. For example, the phase diagram of the Li
2O–Nb
2O
5–MgO system is less complex than that of the system Li
2O–Nb
2O
5–ZnO [
75,
76]. The Zn diagram has a greater number of phases and they are less stable; the concentration regions of existence of different phases and mixtures of phases are less extended [
75]. These physical and chemical particularities of a Zn system explain a greater number of concentration thresholds at LN doping with Zn rather than Mg. The particularities also explain LiNbO
3:Zn crystals tend to cracking and separation of impurity phases during growth [
27]. Phase stresses in LiNbO
3:Zn crystals are also a consequence of Zn system particularities. The stresses are the reason why these crystals claim special modes of after-growth treatment and turning them to a single domain state [
27]. Thus, a volume of a structurally and chemically uniform boule and the fraction of the crystallized melt for LiNbO
3:Zn will be less than for LiNbO
3:Mg crystals. The fraction of crystallized melt in the case of Li
2O–Nb
2O
5–ZnO system is ≤~20%, Li
2O–Nb
2O
5–MgO system ≤~30% [
16,
27,
74]. The fraction in the case of Li
2O–Nb
2O
5–CeO
2 system strongly depends on the initial dopant concentration in the melt; the crystallized melt fraction is significantly lower than in systems with magnesium and zinc. These facts are caused by significantly lower distribution coefficients K
D,
Table 4. This leads to a fast increase in the dopant concentration in the melt and achieving of the critical state at which the complexes concentration with solidus temperature T
c1 is insufficient for crystal growth with a constant dopant concentration. This is why only ≤~12% fraction of the melt should crystallize to turn into a structurally and chemically uniform LiNbO
3:Ce crystal [
76].
Results obtained in [
77] based on the analysis of the dopant electronic structure are important for predicting the technological conditions of growth and the quality control of doped LN crystals. At this, p-elements (non-metals, in our case, boron), which have a higher chemical activity due to the greater number of valence electrons than that of metals, make the melt more homogeneous at the level of ionic complexes. It is exactly this fact that allows us to grow LN crystals from a congruent melt with a defective structure close to one in SLN. Metals (s and d elements, Zn and Mg) have a similar effect on the LN melt and crystals properties. Nevertheless, Zn-doped crystals have an increased ununiformity of dopant incorporation to the crystals, tend to cracking due to phase stresses; they also are characterized by additional claims towards growth conditions and after-growth electrothermal treatment of boules. The f-element metals, due to their electronic structure, form the structure of the melt in such a way that only a relatively small fraction of the melt can be crystallized to obtain a compositionally uniform crystals, for example, LiNbO
3:Ce.
LN is characterized by a high melting temperature (~1526 K) and a high chemical activity of the melt, which requires platinum crucibles. This is the reason for the extremely small number of works devoted to the study of the structure of even nominally pure LN melts. In recent years, the need has arisen to obtain optically highly perfect LN single crystals (nominally pure and doped). The existence in the melt of strongly bound groups of atoms or ions (clusters) of a certain structure was considered as the main concept in the literature [
78]. In [
79,
80,
81], high-temperature Raman spectroscopy was effectively used to study the crystallization from a melt. In the pre-crystallization temperature of a LN melt, the Raman spectra revealed a noticeable change in the structure of the melt and a mismatch between the structure of anionic motifs in the melt and crystal. The mismatch effect near the melting point creates serious obstacles to the nucleation of equilibrium structures [
79,
80,
81]. Metastable phases have an advantage in crystallization under these conditions. The phases crystal lattice is very far from the structure of the short-range order of the melt. The mismatch in the anionic structure of the melt and crystal can also affect the growth of crystal faces or the formation of structural defects [
79,
80].
LN structure is a framework of oxygen octahedra O
6, articulated by vertices and faces, with the densest hexagonal packing. Inside the octahedra there are intrinsic (Li
+ and Nb
5+) or doping cations. The bonds in the octahedra occupied by niobium are predominantly covalent [
1]. At the same time, the lithium ion is bound to oxygen atoms only by electrostatic interaction. Due to the predominance of the covalent bond type, the niobium cation tends to form in the melt anionic motifs consisting of NbO
4 tetrahedra. That is, when LN melts, anionic structure rearranges significantly: the coordination of niobium atoms changes from octahedral to tetrahedral, which is confirmed by Raman spectroscopy data [
79,
80,
81]. Strong covalent bond implies the ability to preserve the structure of oxyanions in the molten state. Thus, the melt can contain not only isolated tetrahedral groups, but also complexes with a stable structure [
79]. Raman studies of Li
2O–Nb
2O
5 system melts have revealed not only bands corresponding to vibrations of terminal NbO
3− and middle NbO
2− groups (815–870 cm
−1) but also bands corresponding to symmetric stretching bridge vibrations of Nb–O–Nb bonds (670–690 cm
−1). Bridge bands are located at the junction of the tetrahedra [
79,
80]. Probably, octahedra NbO
6 exist in the melt in the pre-crystallization temperature range along with NbO
4 tetrahedra. Earlier paper [
82] studied Raman spectra of Nb
2O
5, intended for charge synthesis and LN crystal growth. The study has revealed five bands 814, 845, 902, 965, and 995 cm
−1 in the region of stretching bridge vibrations of oxygen atoms along the polar axis (800–1100 cm
−1). This indicates a variety of island structures consisting of fragments of chains of octahedra and tetrahedra in niobium pentoxide.
Boron is a highly chemically active dopant. Its fundamental properties are change in crystallization temperature, viscosity, and surface tension of melts [
83]. We [
19] have qualitatively revealed an increase in melt viscosity, in the melting temperature by ~10 K, and the Curie temperature by ~50 K compared to nominally pure CLN crystal during growth of studied LiNbO
3:B (0.55–1.24 mol%) crystals. The crystal chemistry of boron oxide compounds is extremely diverse: it is determined by the possibility of double hybridization of the boron atom, sp
2– and sp
3–, with the formation of [BO
3]
3− triangles and [BO
4]
5− tetrahedra, respectively [
84]. Boron neutral atom radius is 0.88 Å; B
3+–0.15 Å for B(III), 0.25 Å for B(IV). In the ground state, boron atoms have the 2s
22p
1 configuration with one unpaired electron and two vacant p-orbitals. Due to the presence of a free orbital in a small boron atom, boron is one of the strongest acceptors of unshared electron pairs. The use of this orbital in the donor-acceptor interaction allows the coordination number of the boron atom to increase to four. The atoms and atomic groups surrounding the boron atom are located at three corners of the tetrahedron, the fourth corner of which remains free, and a positive electric field of significant intensity is created in this corner. This circumstance explains the pronounced ability of boron compounds to provide stable molecular complexes. Boron triangles and tetrahedra can be present in isolation or polymerize among themselves through a common oxygen atom [
84]. The tendency towards formation of bulky polyanions explains the high viscosity of melts [
85]. It is well known that alkali metal oxides are modifiers of the boron-oxygen network of the melt and transfer the boron atom to a four-coordinated state, i.e., [BO
4] tetrahedra are formed [
85]. When boron-oxygen triangles and tetrahedra are combined, the absolute values of specific negative charges for complex anions decrease monotonically in the series of ortho-, pyro-, meta-, and polyborates. This ensures sufficient stability of numerous condensed compounds with monovalent as well as large divalent elements. In [
80], temperature changes in lithium metaborate melts were investigated from the Raman spectra. The work has shown the presence of a chain boron-oxygen anion in the melt. All these crystal-chemical laws predetermine the reaction potential of melts containing borates.
The triple system Li
2O–B
2O
3–Nb
2O
5 has also been studied in [
73,
86]. The authors analyzed over 30 different molar ratios of the components. Paper [
73] has revealed in the system six different lithium borates (LiB
3O
5, Li
2B
4O
7, LiBO
2, Li
6B
4O
9, Li
4B
2O
5 and Li
3BO
3), one niobium borate (Nb
3BO
9) and three different lithium niobates (LiNb
3O
8, LiNbO
3 and Li
3NbO
4). According to this work, lithium borates exhibit remarkable solubility in LN at temperatures below 1100 °C, and thus are suitable for crystal growth as a flux. In addition, the concentration range of LN crystallization, determined by the spontaneous nucleation method, is rather wide in the Li
2O–B
2O
3–Nb
2O
5 ternary system.
There is a known HTTSSG method of growing SLN single crystals with a reduced photorefractive effect from a congruent melt in the presence of K
2O flux [
36,
87,
88]. The absence of potassium in the crystal structure becomes evident from a comparison of the ionic radii of Li
+ and K
+ (0.68 Å and 1.38 Å, respectively). Isomorphic substitution of related elements of the alkaline group (lithium with potassium) in the cationic sublattice of the crystal seems unlikely with such a significant difference in ionic radii. Thus, potassium, like boron, does not incorporate in the crystal structure [
89,
90,
91].
We assume that boron-containing polyanions, forming stable covalent bonds in the melt with niobium-containing polyanions, thereby bind the excess of niobium and increase the Li/Nb ratio in the melt. As a result, the grown crystal approaches a stoichiometric composition in terms of the cation sublattice ordering degree, in the same way as when using a K
2O flux. Such a crystal is characterized by a reduced content of Nb
Li defects and lithium vacancies (V
Li) in comparison with a CLN crystal,
Table 2. This is confirmed by an increase in Curie temperature (T
C) by ~47 K in comparison with a CLN crystal [
19]. For a comparable in magnitude change in the T
C of a LN crystal doped with metallic impurities (Mg, Zn, etc.), significantly higher impurity concentrations (~2 to 3 wt%) are required.
Paper [
92] has shown that melts of inorganic polymers that form chain and ring structures with the inclusion of ions of a solute (B
2O
3, Na
2B
4O
7, Li
6B
4O
9, etc.), which tend to form glasses, are good solvents. For example, in [
93], the formation of a high-temperature poorly soluble borate Al
5BO
9 is reported; a fair amount of Al
2O
3 is removed from the melt. It can be assumed that metal cations in the melt that incorporate into the crystal structure as uncontrolled impurities [
87] will also be removed from the melt, as a result of which the LN crystal will be more perfect.
Due to Raman data (
Table 1) oxygen octahedra are less deformed in the structure of a LiNbO
3:B (homogeneous doping) than in LiNbO
3:B (direct doping) crystal. This obviously happens due to an increase in the ordering of Li
+ and Nb
5+ cations and vacancies along the polar axis. We have concluded this because widths of 432 and 875 cm
−1 bands in a LiNbO
3:B (direct doping) crystal increase in the Raman spectrum. The bands correspond to deformation and stretching vibrations of oxygen atoms in the bridge Me–O–Me (Me: Li
+, Nb
5+). It is important to stress that 875 cm
−1 band parameters are sensitive towards the magnitude of the dipole moment and, thus, the spontaneous polarization of the crystal [
2].
LiNbO
3:Mg crystals were studied in a paper [
32]: the crystals were obtained by direct and homogeneous doping. Raman spectroscopy, optical study of macro- and microstructure have revealed higher ordering of homogeneously doped crystals compared to directly doped crystals. Magnesium incorporates directly to niobium pentoxide structure at a homogeneous doping. It has inter-polyhedra coordination with a uniform distribution in the melt volume. Clusters formed in a melt with different doping methods have different structures and sizes; therefore, melts, other things being equal, should crystallize in different ways [
50,
79,
94]. Crystallization occurs through the attachment of the clusters to a growing crystal.
[BO
4]
5− tetrahedra can be both of correct and distorted form. Boron forms three covalent bonds with oxygen atoms, and the fourth bond in the tetrahedron is formed by the donor–acceptor mechanism. For this reason, the lengths of B–O bonds in the tetrahedron vary from 1.462 to 1.512 Å, the spread of angles <O–B–O>— 104–115° [
84]. During homogeneous LN crystal doping, the boron-containing reagent is introduced directly into the niobium stripping solution. Only one type of borates (Nb
3BO
9) forms initially. Thus, B–O and Nb–O are less different in the length than at direct doping, when seven different borates form [
73]. Obviously, due to this, tetrahedra, and then the octahedra formed in the precrystallization region, will be less distorted during homogeneous doping. Therefore, our Raman data confirmed that oxygen octahedra are less deformed in the structure of the LiNbO
3:B (homogeneous doping) than in LiNbO
3:B (direct doping) crystals,
Table 1.
3.6. Computer Simulation of the B3+ Ion Localization in the LN Crystal Structure
Calculations of the total energy of the Coulomb interaction of point charges (U, eV) were carried out for the oxygen-octahedral lithium niobate cluster (Li
+, Nb
5+, O
2−) with the B
3+ ion considered in the sp
2–hybrid state as part of the [BO
3]
3– plane triangles. Calculations were performed using the formula:
where
and
—charges in fractions of an electron,
—distance between the centers of interacting charges [Å],
—constant expressed by the formula (eV·Å):
where
—charge of an electron,
—dielectric constant.
The system (cluster) consists of two Li
+ cations, two Nb
5+ cations, one B
3+ cation, and twenty O
2− oxygen anions; it is not electrically neutral. We consider only a cluster consisting of six O
6 oxygen octahedra, “torn out” from a large electrically neutral system, in order to study how the energy of the B
3+ interaction with the surrounding fragment of the LN crystal structure depends on the B
3+ position in tetrahedral voids. Taking a larger fragment of the structure is impractical, since the electrostatic interaction of point charges strongly decreases with distance. Li
+ and Nb
5+ cations sites correspond in our calculations to the structure of the ferroelectric LN phase: lithium is shifted to the lower oxygen plane, niobium—to the upper oxygen plane [
2]. The calculations were based on the structural data of the CLN crystal [
95]. The work simulates two processes. In the first case, the energy of the Coulomb interaction of the B
3+ ion with a fragment of the CLN crystal structure (6 O
6 octahedra) is calculated. In this case, we took coordinates of the cell basis with constant lattice parameters (a and c = 5.1489 and 13.8631 Å, respectively), though changing with increasing temperature [
2]. In the second case, we took the same the LN structure fragment, but with different lattice parameters a and c. For this, the cell parameters were normalized to a temperature of 297 K [
96]. Due to similar results obtained in the first and second cases, only experimental data with constant parameters a and c are discussed below.
A fragment of the structure (six oxygen octahedra) taken for modeling is shown on
Figure 13. We considered seven possible locations of the boron cation: at the centers of the tetrahedral faces of the first and second octahedral layers bordering the corresponding octahedra (lithium, niobium, and vacant), and also in the plane of the oxygen triplet separating the octahedral layers. In this case, the calculations did not take into account the replacement of lithium with niobium. The calculation results are shown on
Figure 14. According to
Figure 14, the maximum energy value for both cases corresponds to the presence of a boron cation in the [BO
3]
3– group bordering on niobium octahedra. For other possible arrangements of boron, the sum of the energy of the Coulomb interaction is much lower, which can be considered as a theoretically possible location of B
3+ cations in the crystal structure.
We did not take into account the influence of boron in the tetrahedral face on point charges outside the system under consideration, since this influence significantly decreases with distance. It should be noted that the B
3+ cation, embedding in the tetrahedral voids of the crystal structure during the growth process introduces an excess positive charge into the system. In this case, to achieve the minimum energy of the system, it will be advantageous for boron to occupy positions, first of all, in tetrahedral voids bordering lithium and vacant octahedra, or in the oxygen plane separating the oxygen-octahedral layers,
Figure 14. The formation of Nb
Li point defects when boron is in the tetrahedral face is unlikely, since in this case it leads to an increase in the total energy due to the high localization of positive charges.
Thus, according to calculations, the presence of the B
3+ ion in the crystal structure can prevent the formation of a deep electron trap Nb
Li, at least within the considered system. The results indicate that, in contrast to the metallic K
2O flux, when the potassium element does not incorporate into the crystal structure, the nonmetallic B
2O
3 flux has a complex effect on the LN single crystals structure. It structures the melt, changes the microscopic crystallization mechanisms, thereby, aligns and brings K
Li and K
Nb closer to unity (the distribution coefficients of lithium and niobium in the LiNbO
3:B crystal). It reduces the formation of Nb
Li point defects by at least the number of B
3+ cations incorporated into the tetrahedral voids of the structure, corresponding to the boron concentration in the LiNbO
3:B crystal (~4 × 10
−4 mol%). As shown above, in the Li
2O–Nb
2O
5–B
2O
3 system, there are no regions of homogeneity of boron and its compounds with lithium niobate, and CLN should be the only crystallizing phase. However, due to the binding of an excess of niobium in the melt with boron, the fraction of lithium in the structure of the growing crystal increases, which increases the Li/Nb ratio and brings the structure of LiNbO
3:B crystals closer to that of SLN. The increase in the lithium content is additionally confirmed by the calculation of the Li
2O concentration in the crystals from the increase in the Curie temperature for CLN and LiNbO
3:B (0.83 mol%) crystals—1145 and 1189 °C, respectively [
97].
Thus, due to Raman spectroscopy, structural units of the cation sublattice are more ordered in LiNbO
3:B crystals,
Table 1. LN crystals doped with certain concentrations of metal cations also have an increased cation sublattice order [
2,
98]. Metal dopants do incorporate into the crystal structure. In the case of boron, such ordering is impossible—boron incorporates into crystal tetrahedral voids and due to our data (see paragraph 3.5.) changes O–O bonds length. This changes the O
6 oxygen octahedra size and the distribution of Nb
5+ and Li
+ cations in octahedra. As a result, a more energetically favorable distribution of cations over octahedra is established in the crystal, the arrangement of cations along the polar axis orders. Indeed, the anionic sublattice of the LiNbO
3:B crystal turns out to be less distorted than of CLN crystal,
Table 1. At this, the polarizability of the oxygen-octahedral MeO
6 clusters, which determines the nonlinear optical properties of the crystal, changes. This confidently manifests in the LiNbO
3:B crystals Raman spectrum. 576 and 630 cm
−1 bands correspond to doubly degenerate E(TO) and totally symmetric A
1(TO) vibrations of oxygen atoms in O
6 oxygen octahedra, respectively. They substantially widen ~2 times in LiNbO
3:B spectrum in comparison with SLN and CLN,
Table 1. Changes in O–O bonds length caused by trace amounts of boron localizing in tetrahedral voids can be achieved only by a much higher concentration of metal elements Zn
2+, Mg
2+ [
2,
25,
26,
98]. Bands corresponding to vibrations of oxygen atoms in oxygen octahedra widen smoothly in LiNbO
3:Zn and LiNbO
3:Mg Raman spectra and sharply—in LiNbO
3:B spectrum. This can be explained by the following: tetrahedral voids of the LN crystal structure act as a kind of “buffer”, they compensate for the deformation of the oxygen framework of the structure [
99]. They compensate for various influences on the anionic sublattice, including the distortion of oxygen octahedra due to the presence of metal cations Zn, Mg, etc., in them. In the case of LiNbO
3:B crystals, some of the tetrahedra are already filled with B
3+ cations, which negatively affects their “buffer” ability, which explains the sharp increase in the 576 and 630 cm
−1 Raman bands widths.
Table 1 demonstrates that 880 cm
−1 Raman band width occupies an intermediate value between the widths of this band in the spectrum of SLN and CLN crystals—25(26), 20 and 30 cm
−1, respectively. The band corresponds to the oxygen atoms stretching bridge vibrations of the A
1(LO) symmetry type along the polar axis in the Me–O–Me bridge (Me: Li
+, Nb
5+, dopant). A decrease in the 880 cm
−1 band width in LiNbO
3:B spectrum may be associated with an increase in the cation sublattice order of these crystals. Aligning k
0 Li and Nb distribution coefficients during crystal growth explains the approach of the Li/Nb ratio to 1 in LiNbO
3:B; boron in tetrahedral voids limits the Nb
Li structural defect formation.
Thus, the ordering of the cation sublattice of the crystal manifests itself in the Raman spectrum not only in the range 200–300 cm
−1, corresponding to the vibrations of metal cations in oxygen octahedra along the polar axis, but is also indirectly traced by the parameters of the 880 cm
−1 band. Due to this data, even traces amounts of B
3+ cations incorporating to LN oxygen tetrahedra noticeably distort the anionic framework. This leads to a noticeable asymmetry of oxygen octahedra (compared with that in CLN crystals) and a change in their polarizability. The deformation and polarizability of oxygen-octahedral MeO
6 clusters, in turn, determine the LN crystal electro-optical properties [
1,
2]. At this, the cationic sublattice of LiNbO
3:B crystals, on the contrary, turns out to be more ordered in comparison with the that of the CLN crystal, which is also confirmed by Raman data,
Table 1.