Effect of Synthesis Method of Nickel–Samarium-Doped Ceria Anode on Distribution of Triple-Phase Boundary and Electrochemical Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Fabrication of the Samples
2.2. Characterization
3. Results
3.1. Powder Analysis
3.2. Sintered Pellet Properties
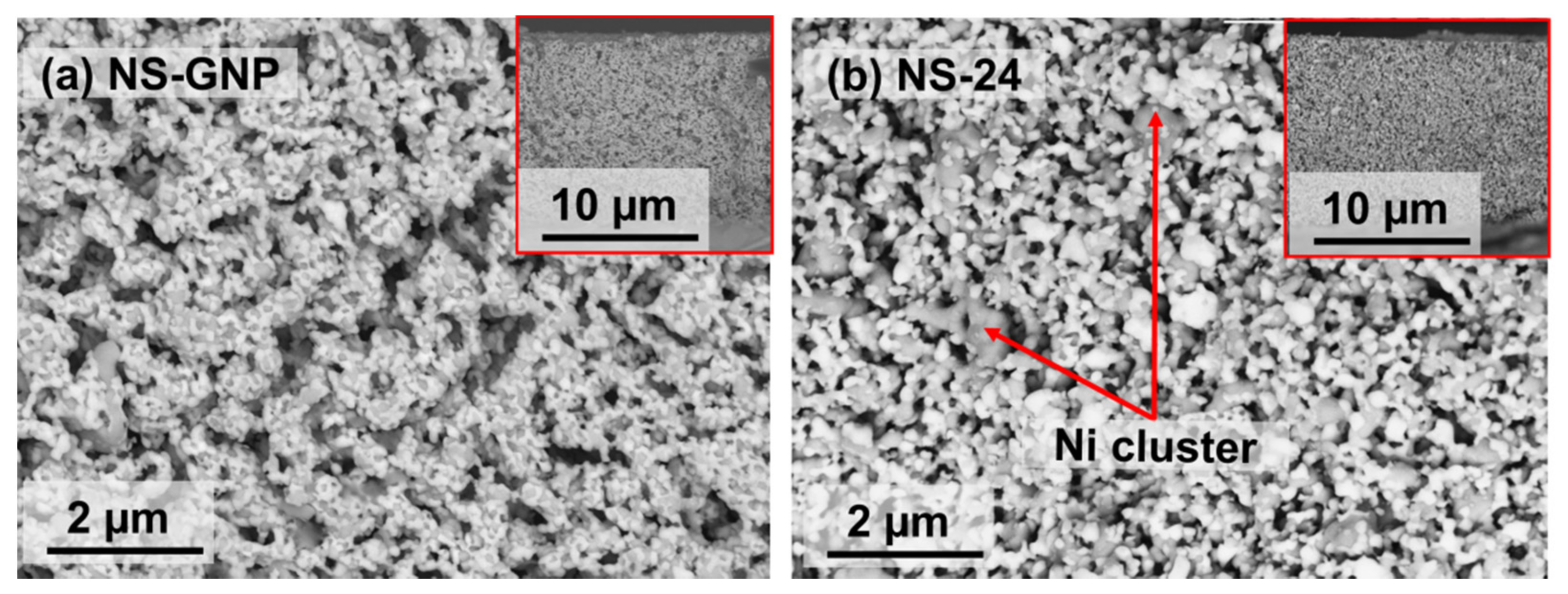
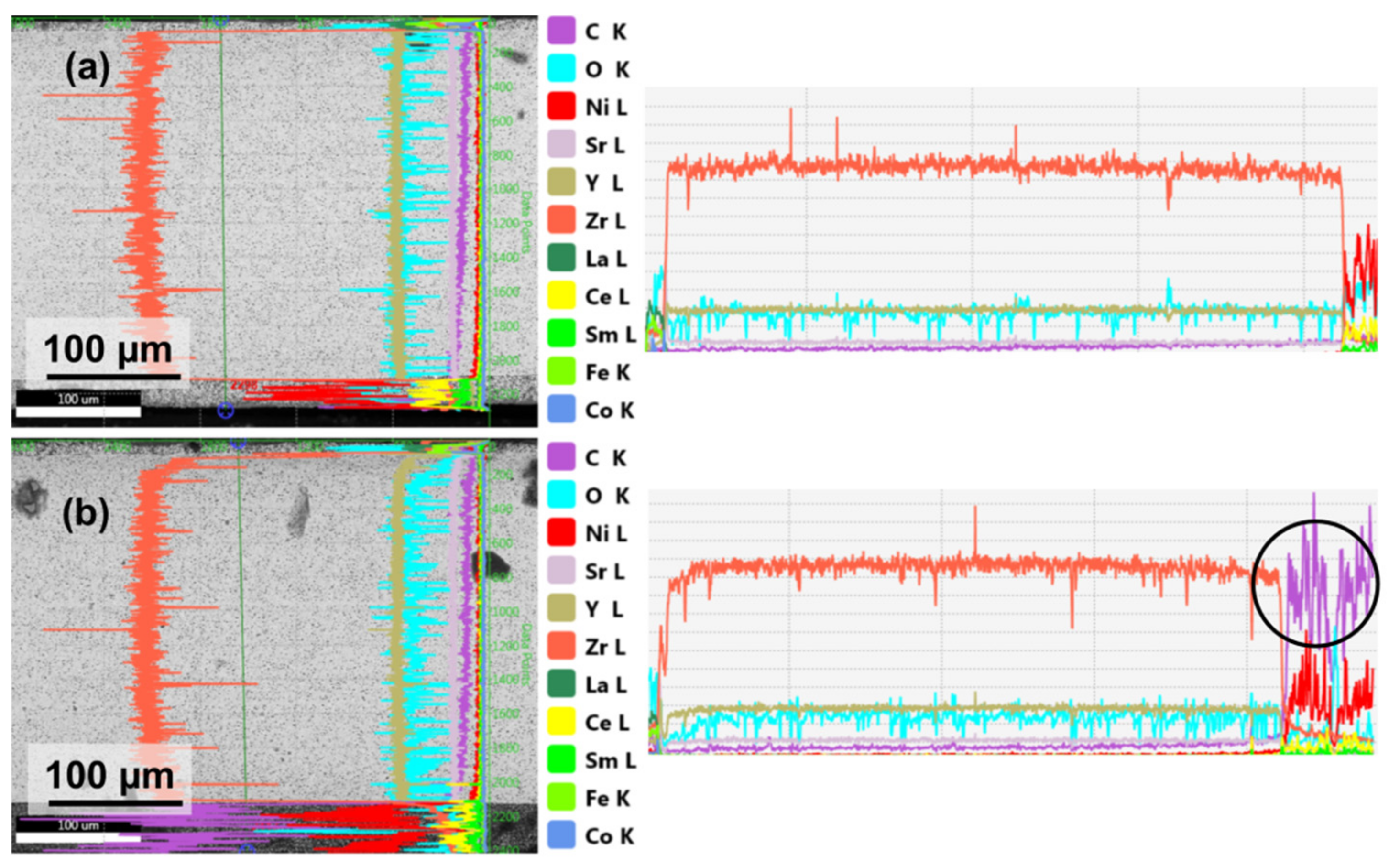
3.3. Microstructural Properties
3.4. Mechanical Properties
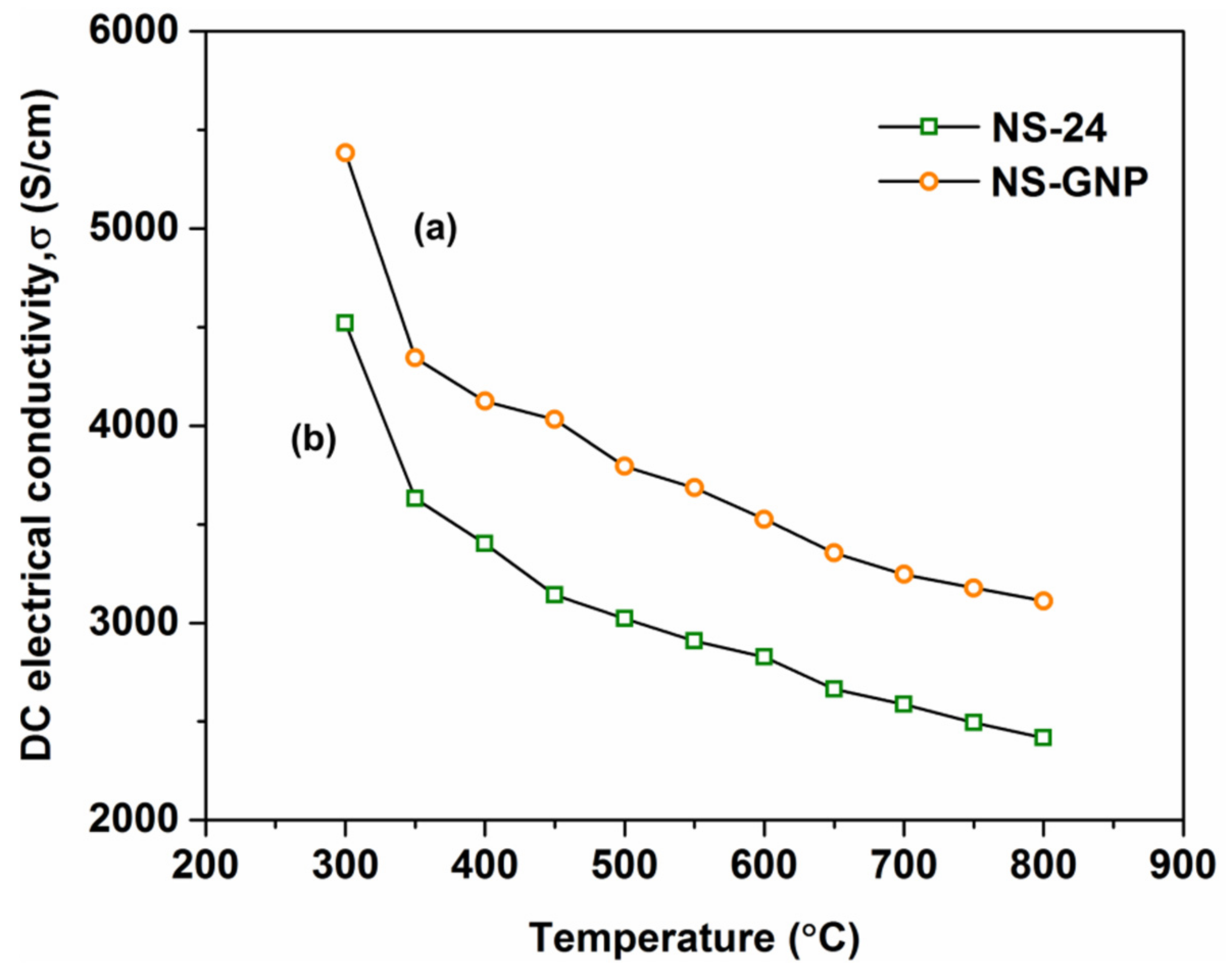
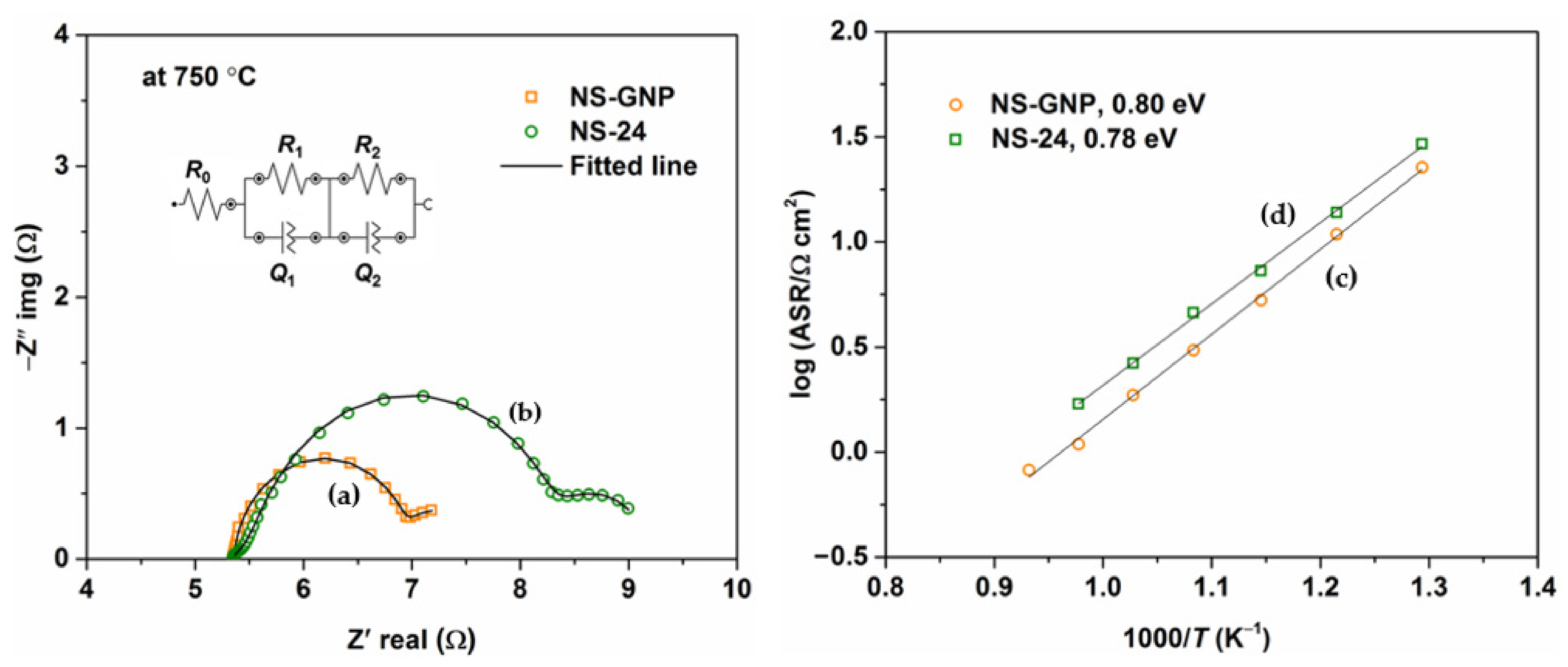
3.5. Electrical Properties
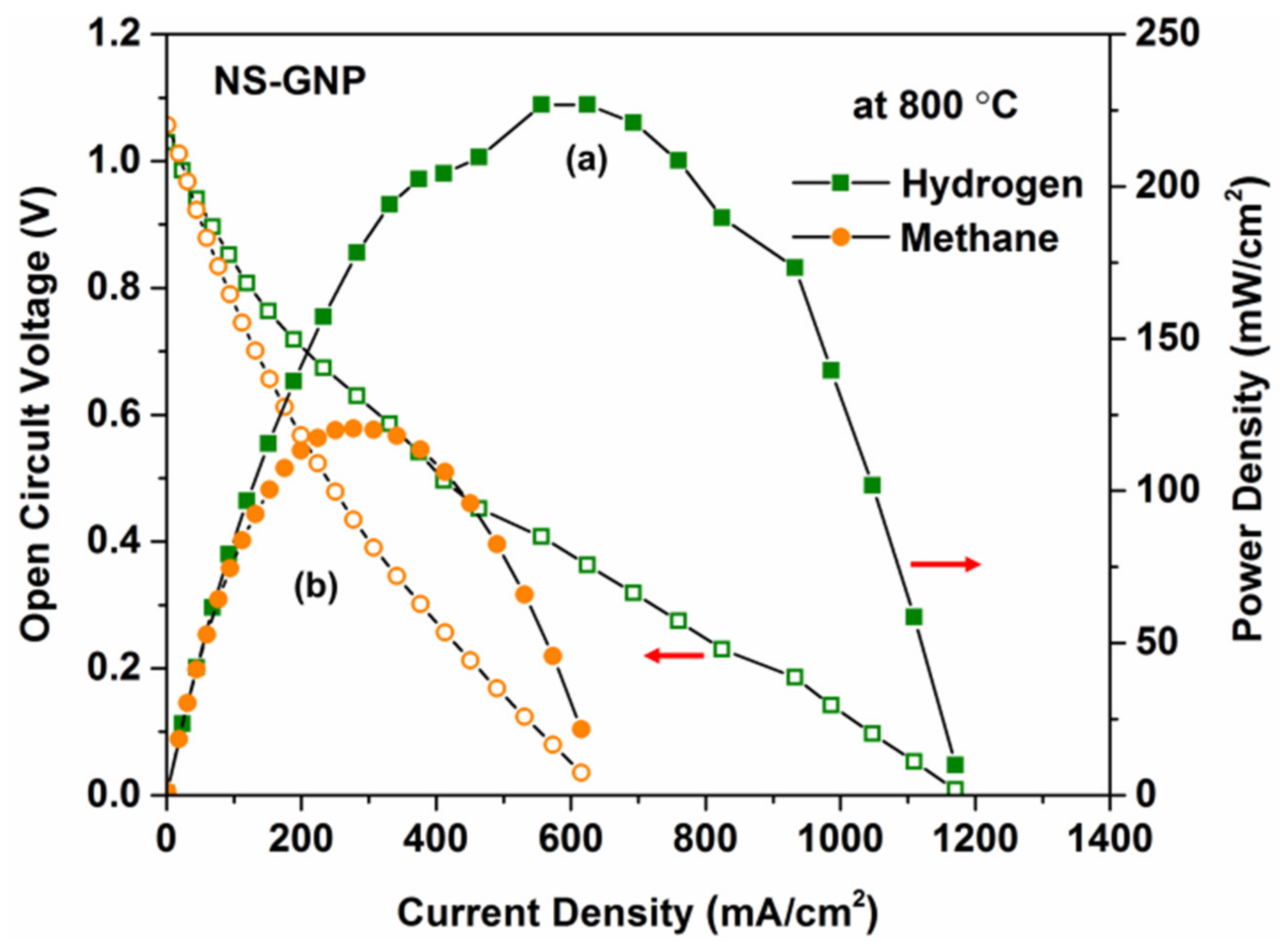
3.6. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceram. Int. 2020, 46, 23314–23325. [Google Scholar] [CrossRef]
- Wan Yusof, W.N.A.; Abdul Samat, A.; Norman, N.W.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A. Synthesis and Characterization of Zn-doped LiCoO2 Material Prepared via Glycinenitrate Combustion Method for Proton Conducting Solid Oxide Fuel Cell Application. J. Kejuruter. 2018, SI1, 11–15. [Google Scholar]
- Skalar, T.; Zupan, K.; Marinšek, M. Microstructure tailoring of combustion-derived Ni-GDC and Ni-SDC composites as anode materials for intermediate temperature solid oxide fuel cells. J. Aust. Ceram. Soc. 2019, 55, 123–133. [Google Scholar] [CrossRef]
- Mahmud, L.; Muchtar, A.; Rao, S.; Jais, A. Processing of composites based on NiO, samarium-doped ceria and carbonates (NiO-SDCC) as anode support for solid oxide fuel cells. Process. Appl. Ceram. 2017, 11, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Mishina, T.; Miya, K.; Kikuchi, R.; Sugawara, T.; Takagaki, A.; Oyama, S.T. Ni-SDC Based Cermets for Direct Dry Reforming of Methane on SOFC Anode. ECS Trans. 2017, 78, 1161–1167. [Google Scholar] [CrossRef]
- Chien, A.C.; Ye, N.J. Effect of preparation method and particle size of Ni/SDC catalyst on methane oxidation. Catal. Commun. 2021, 106312. [Google Scholar] [CrossRef]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef] [Green Version]
- Che Abdullah, S.S.; Ismail, S.S. Influence of NiO to SDC ratio on the properties of Ni-SDC cermet prepared via reduction process. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 1–8. [Google Scholar] [CrossRef]
- Yao, X.; Li, P.; Yu, B.; Yang, F.; Li, J.; Zhao, Y.; Li, Y. Hydrothermally synthesized NiO-samarium doped ceria nano-composite as an anode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 22192–22200. [Google Scholar] [CrossRef]
- Park, S.Y.; Na, C.W.; Ahn, J.H.; Song, R.H.; Lee, J.H. Preparation of highly porous NiO-gadolinium-doped ceria nano-composite powders by one-pot glycine nitrate process for anode-supported tubular solid oxide fuel cells. J. Asian Ceram. Soc. 2014, 2, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.P.S.; Muchtar, A.; Somalu, M.R. A review on the selection of anode materials for solid-oxide fuel cells. Renew. Sustain. Energy Rev. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Choolaei, M.; Bull, T.; Ramirez Reina, T.; Amini Horri, B. Synthesis and characterisation of nanocrystalline CuO–Fe2O3/GDC anode powders for solid oxide fuel cells. Ceram. Int. 2020, 46, 14776–14786. [Google Scholar] [CrossRef]
- Sarikaya, A.; Petrovsky, V.; Dogan, F. Effect of the anode microstructure on the enhanced performance of solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 11370–11377. [Google Scholar] [CrossRef]
- Ahsan, M.; Irshad, M.; Fu, P.F.; Siraj, K.; Raza, R.; Javed, F. The effect of calcination temperature on the properties of Ni-SDC cermet anode. Ceram. Int. 2020, 46, 2780–2785. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mukhopadhyay, J.; Basu, R.N. Functional Anode Materials for Solid Oxide Fuel Cell—A Review. Trans. Indian Ceram. Soc. 2013, 72, 145–168. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Bi, H.; Yu, S.; Han, F.; Sun, J.; Zhu, L.; Yu, H.; Pei, L. Effects of NiO on the conductivity of Ce0.85Sm0.15O1.925 and on electrochemical properties of the cathode/electrolyte interface. J. Power Sources 2016, 320, 86–93. [Google Scholar] [CrossRef]
- Bobrowski, P.; Faryna, M.; Pędzich, Z. Microstructural Characterization of Yttria-Stabilized Zirconia Sintered at Different Temperatures Using 3D EBSD, 2D EBSD and Stereological Calculations. J. Mater. Eng. Perform. 2017, 26, 4681–4688. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Zabaleta, S.; Laguna-Bercero, M.A.; Ortega-San-Martín, L.; Larrea, A. Orientation relationships and interfaces in directionally solidified eutectics for solid oxide fuel cell anodes. J. Eur. Ceram. Soc. 2014, 34, 2123–2132. [Google Scholar] [CrossRef] [Green Version]
- Muhammed Ali, S.A.; Anwar, M.; Ashikin, N.; Muchtar, A.; Somalu, M.R. Influence of oxygen ion enrichment on optical, mechanical, and electrical properties of LSCF perovskite nanocomposite. Ceram. Int. 2018, 44, 10433–10442. [Google Scholar] [CrossRef]
- Skalar, T.; Zupan, K.; Marinšek, M.; Novosel, B.; Maček, J. Microstructure evaluation of Ni-SDC synthesized with an innovative method and Ni-SDC/SDC bi-layer construction. J. Eur. Ceram. Soc. 2014, 34, 347–354. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Yang, C.; Ma, S.; Chen, F. Self-rising synthesis of Ni-SDC cermets as anodes for solid oxide fuel cells. J. Power Sources 2010, 195, 1543–1550. [Google Scholar] [CrossRef]
- Ding, C.; Hashida, T. Synthesis and evaluation of NiO-Ce0.8Sm0.2O 1.9 nanocomposite powders for low-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 5567–5573. [Google Scholar] [CrossRef]
- Drozdz, E.; Stachura, M.; Wyrwa, J.; Rękas, M. Effect of the addition of pore former: Graphite and ammonium bicarbonate on the properties of Ni/Al2O3–3YSZ composite composite materials. J. Therm. Anal. Calorim. 2015, 122, 157–166. [Google Scholar] [CrossRef]
- Kishimoto, M.; Miyawaki, K.; Iwai, H.; Saito, M.; Yoshida, H. Effect of composition ratio of Ni-YSZ anode on distribution of effective three-phase boundary and power generation performance. Fuel Cells 2013, 13, 476–486. [Google Scholar] [CrossRef]
- Xu, B.; Tian, D. Preparation and characterization of NiO-Sm0.2Ce0.8O1.9 composite nanoparticles for solid oxide fuel cell anodes. In Proceedings of the 3rd International Conference on Mechatronics, Robotics and Automation, Shenzhen, China, 20–21 April 2015; pp. 813–817. [Google Scholar]
- Hanifi, A.R.; Laguna-Bercero, M.A.; Sandhu, N.K.; Etsell, T.H.; Sarkar, P. Tailoring the microstructure of a solid oxide fuel cell anode support by calcination and milling of YSZ. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.G.; Park, J.Y.; Song, H.; Kim, H.R.; Son, J.W.; Lee, J.H.; Je, H.J.; Kim, B.K.; Lee, H.W.; Yoon, K.J. Microstructure-polarization relations in nickel/ gadolinia-doped ceria anode for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2013, 39, 4713–4718. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, N.; Mao, Y.; Sun, K. Facile one-step fabrication of dual-pore anode for planar solid oxide fuel cell by the phase inversion. Electrochem. Commun. 2012, 22, 41–44. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Schwank, J.W. Evaluation of Ni/SDC as anode material for dry CH4 fueled Solid Oxide Fuel Cells. J. Power Sources 2014, 248, 239–245. [Google Scholar] [CrossRef]
- Jais, A.A.; Ali, S.A.M.; Anwar, M.; Somalu, M.R.; Muchtar, A.; Isahak, W.N.R.W.; Baharudin, N.A.; Lim, K.L.; Brandon, N.P. Performance of Ni/10Sc1CeSZ anode synthesized by glycine nitrate process assisted by microwave heating in a solid oxide fuel cell fueled with hydrogen or methane. J. Solid State Electrochem. 2020, 24, 711–722. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, J.; He, H.; Gao, J. Ni/SDC materials for solid oxide fuel cell anode applications by the glycine-nitrate method. Key Eng. Mater. 2010, 434–435, 731–734. [Google Scholar] [CrossRef]
- Xu, D.; Liu, X.; Wang, D.; Yi, G.; Gao, Y.; Zhang, D.; Su, W. Fabrication and characterization of SDC-LSGM composite electrolytes material in IT-SOFCs. J. Alloys Compd. 2007, 429, 292–295. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Yao, X.; Hou, N.; Fan, L.; Gan, T.; Zhao, Y.; Li, Y.; Schwank, J.W. Effect of Sn addition on improving the stability of Ni-Ce0.8Sm0.2O1.9 anode material for solid oxide fuel cells fed with dry CH4. Catal. Today 2019, 30, 209–216. [Google Scholar] [CrossRef]
- Zha, S.; Rauch, W.; Liu, M. Ni-Ce0.9Gd0.1O1.95 anode for GDC electrolyte-based low-temperature SOFCs. Solid State Ionics 2004, 166, 241–250. [Google Scholar] [CrossRef]
- Ideris, A.; Croiset, E.; Pritzker, M.; Amin, A. Direct-methane solid oxide fuel cell (SOFC) with Ni-SDC anode-supported cell. Int. J. Hydrogen Energy 2017, 42, 23118–23129. [Google Scholar] [CrossRef]
- Ideris, A.; Croiset, E.; Pritzker, M. Ni-samaria-doped ceria (Ni-SDC) anode-supported solid oxide fuel cell (SOFC) operating with CO. Int. J. Hydrogen Energy 2017, 42, 9180–9187. [Google Scholar] [CrossRef]

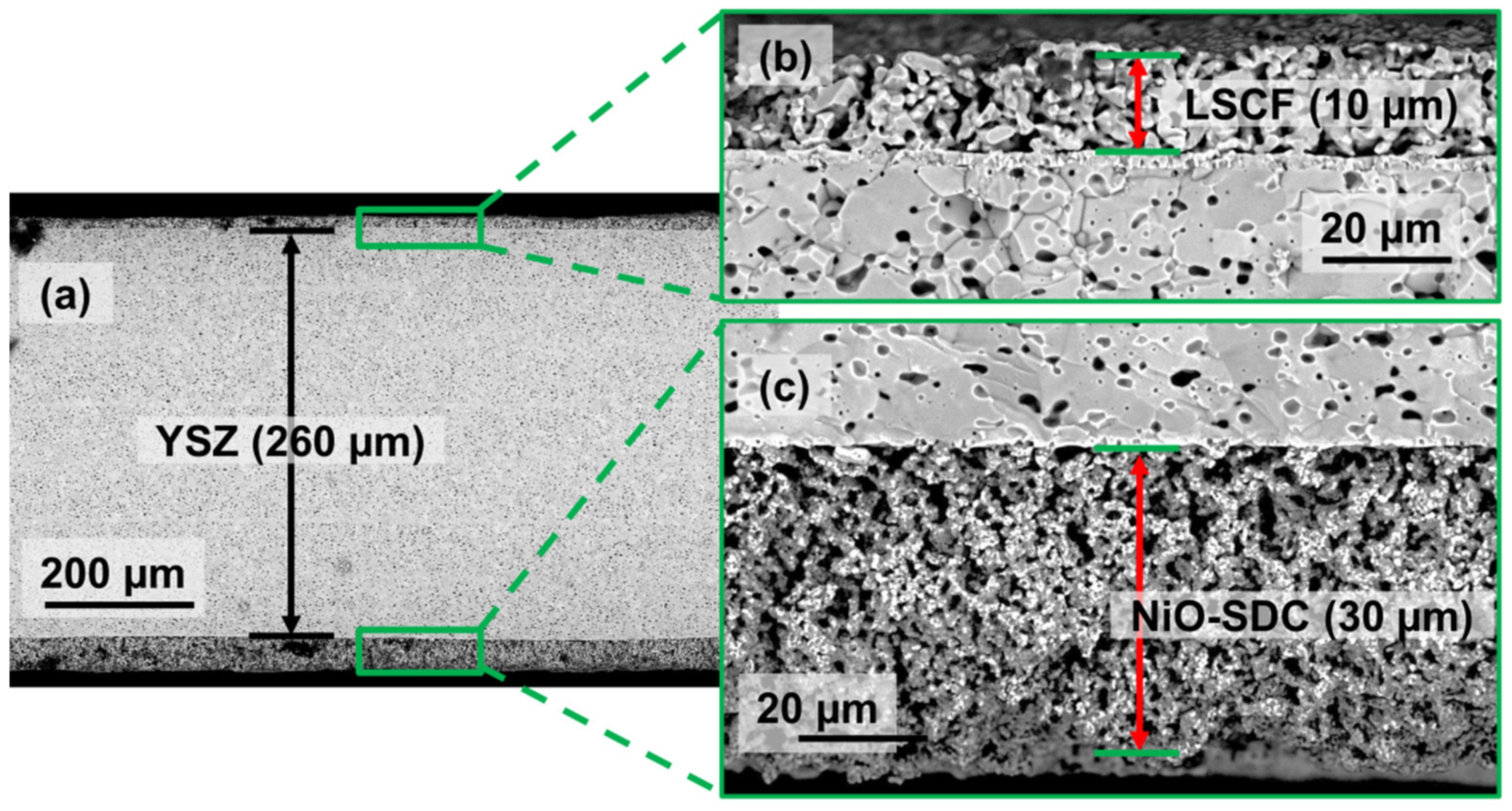

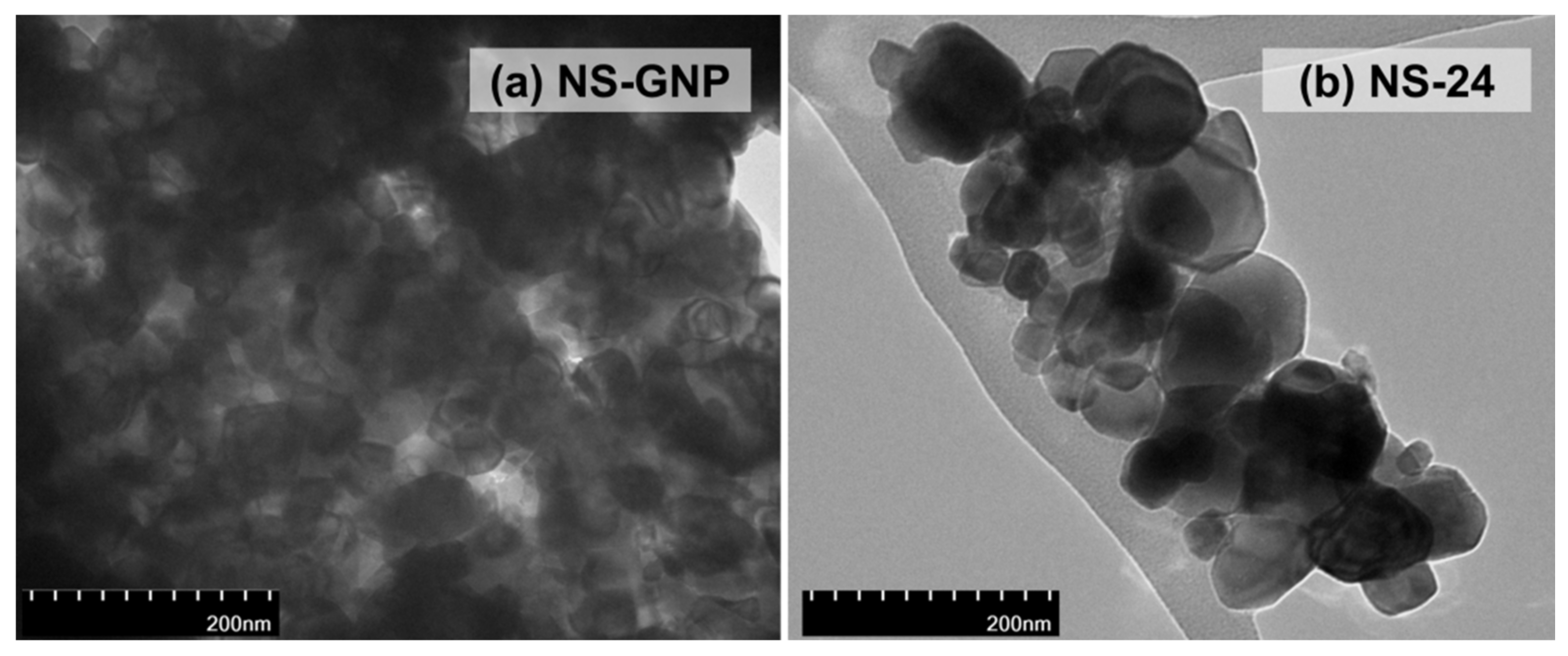

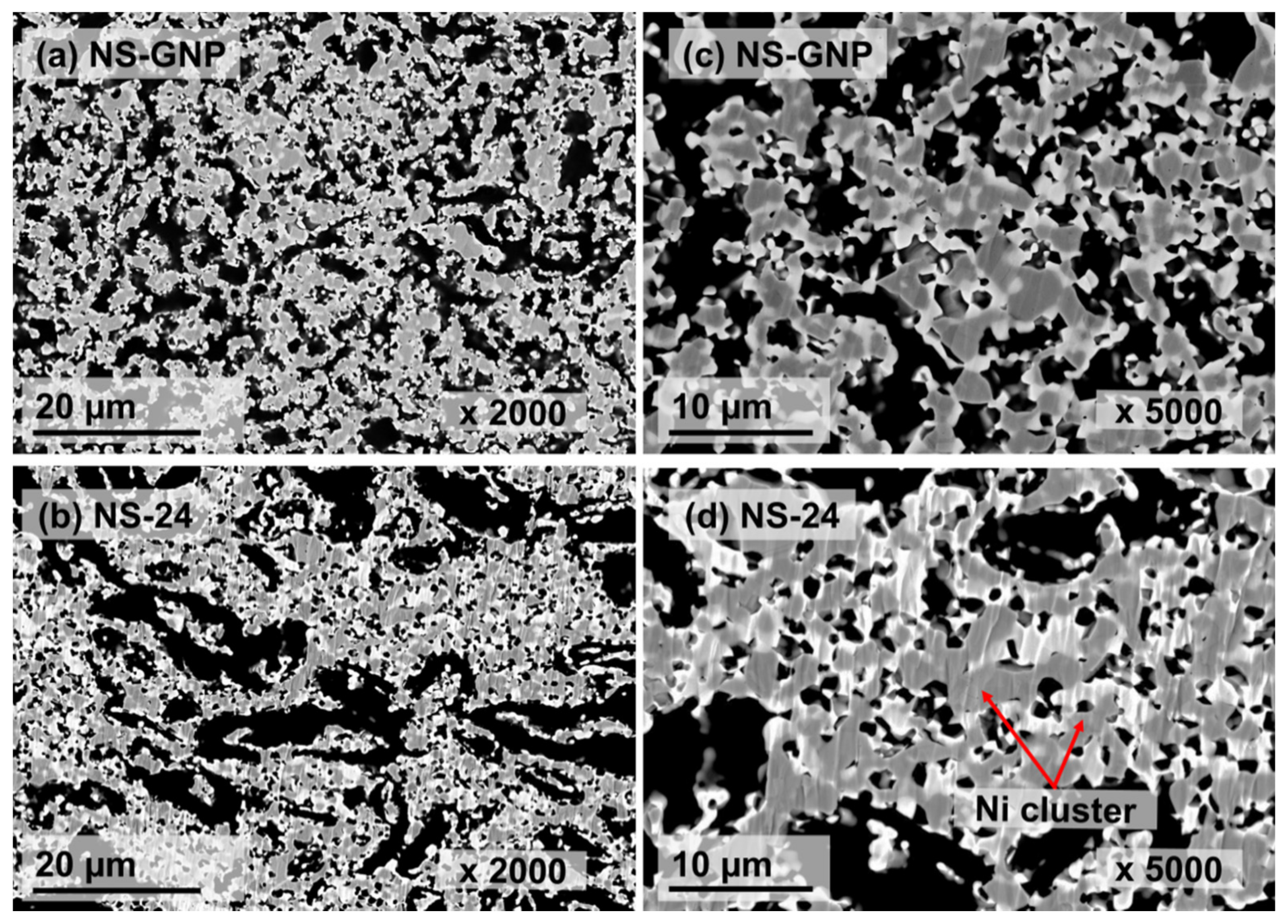
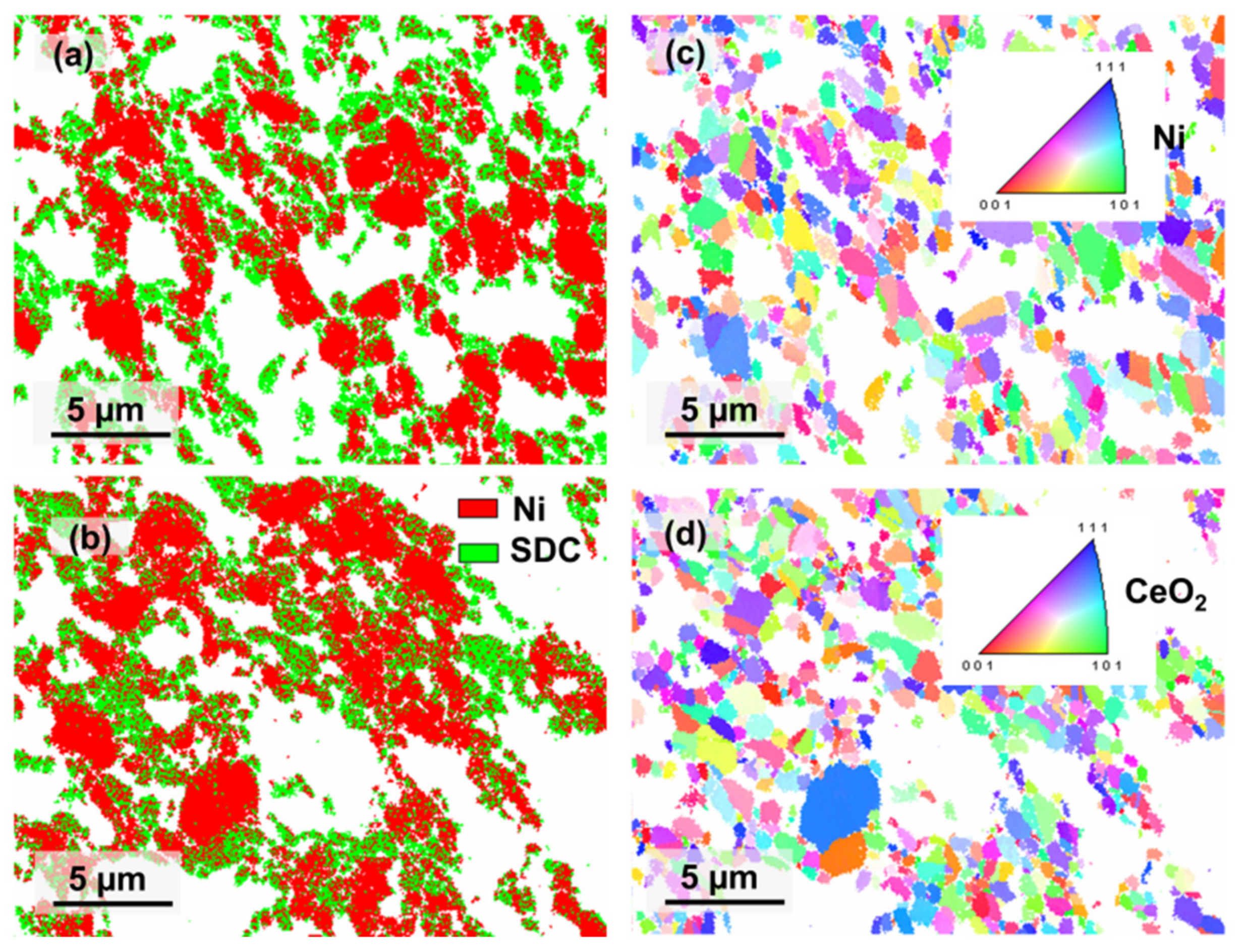
| Sample | Shrinkage (%) | Grain Size (µm) | Relative Density (%) | Porosity (%) | |
|---|---|---|---|---|---|
| Ni | SDC | ||||
| NS-24 (without pore former) | 23 | - | 85 ± 0.7 | 15 ± 0.7 | |
| NS-24 (with 15 wt.% pore former) | 20 | 1.88 ± 0.5 | 0.86 ± 0.2 | 59 ± 1 | 41 ± 1 |
| NS-GNP | 19 | 1.08 ± 0.1 | 0.56 ± 0.04 | 55 ± 0.5 | 45 ± 0.5 |
| Sample | Flexural Strength (MPa) | Vickers Hardness (MPa) |
|---|---|---|
| NS-24 (without pore former) | 169 ± 1.3 | 165 ± 1 |
| NS-24 (with 15 wt.% pore former) | 59 ± 1.1 | 33 ± 1.7 |
| NS-GNP | 78 ± 1.9 | 48 ± 1.1 |
| Single Cell Configuration | Anode Synthesis Method | Fuel | Operating Temperature (°C) | OCV (V) | Power Density (mW/cm2) | Reference |
|---|---|---|---|---|---|---|
| Ni−SDC/YSZ/LSCF | GNP | 20%H2/3%H2O | 800 | 1.03 | 227 | This work |
| (50 vol.% NiO) | ||||||
| Ni−SDC/YSZ/LSCF | GNP | 20%CH4/3%H2O | 800 | 1.05 | 121 | This work |
| (50 vol.% Ni) | ||||||
| Ni−SDC/SDC/LSCF | GNP | 97%H2/3%H2O | 800 | 0.8 | 394 | [31] |
| (65 wt.% NiO) | ||||||
| Ni−SDC/SDC/SSC | GNP | 100%H2/ | 750 | 0.8 | 100 | [32] |
| (65 wt.% NiO) | ||||||
| Ni−SDC/SDC−LSGM/SSC | GNP | 100%H2/ | 800 | 0.83 | 250 | [32] |
| (65 wt.% NiO) | ||||||
| Ni−YSZ/Ni/Ni−GDC/GDC/LSCF−GDC/GDC | GNP | 50%H2/3%H2O | 600 | 0.81 | 413 | [10] |
| (45 wt.% NiO) | ||||||
| Ni−SDC/SDC/BSCF | Ball milling | Dry 100%H2 | 700 | 0.83 | 625 | [33] |
| (50 wt.% NiO) | ||||||
| Ni−SDC/SDC/BSCF | Ball milling | Dry 100%CH4 | 700 | - | 670 | [33] |
| (50 wt.% NiO) | ||||||
| Ni−YSZ/Ni/Ni−GDC/GDC/LSCF−GDC/GDC | Ball milling | 50%H2/3%H2O | 600 | 0.80 | 301 | [10] |
| (45 wt.% NiO) | ||||||
| Ni−GDC/GDC/LSCF | Urea combustion | 97%H2/3%H2O | 600 | 0.83 | 225 | [21] |
| Ni−GDC/GDC/LSCF | Ball milling | 97%H2/3%H2O | 600 | 0.86 | 400 | [21] |
| Ni−GDC/GDC/SSC | Ball milling | 97%H2/3%H2O | 700 | - | 402 | [34] |
| (65 wt.% NiO) | ||||||
| Ni−SDC/SDC/SSC | Ball milling | 97%H2/3%H2O | 700 | 0.82 | 663 | [35] |
| (55 wt.% NiO) | ||||||
| Ni−SDC/SDC/SSC | Ball milling | Dry 100%CH4 | 700 | 0.83 | 697 | [35] |
| (55 wt.% NiO) | ||||||
| Ni−SDC/SDC/SSC | Ball milling | 40%CO/60%Co2 | 700 | 0.76 | 270 | [36] |
| (55 wt.% NiO) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh Abdul, M.A.; Yahaya, A.Z.; Anwar, M.; Soo, M.T.; Muchtar, A.; Kovrugin, V.M. Effect of Synthesis Method of Nickel–Samarium-Doped Ceria Anode on Distribution of Triple-Phase Boundary and Electrochemical Performance. Crystals 2021, 11, 513. https://doi.org/10.3390/cryst11050513
Shaikh Abdul MA, Yahaya AZ, Anwar M, Soo MT, Muchtar A, Kovrugin VM. Effect of Synthesis Method of Nickel–Samarium-Doped Ceria Anode on Distribution of Triple-Phase Boundary and Electrochemical Performance. Crystals. 2021; 11(5):513. https://doi.org/10.3390/cryst11050513
Chicago/Turabian StyleShaikh Abdul, Muhammed Ali, Ahmad Zubair Yahaya, Mustafa Anwar, Mun Teng Soo, Andanastuti Muchtar, and Vadim M. Kovrugin. 2021. "Effect of Synthesis Method of Nickel–Samarium-Doped Ceria Anode on Distribution of Triple-Phase Boundary and Electrochemical Performance" Crystals 11, no. 5: 513. https://doi.org/10.3390/cryst11050513








