The Amine Group as Halogen Bond Acceptor in Cocrystals of Aromatic Diamines and Perfluorinated Iodobenzenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution and Single-Crystal Synthesis of Cocrystals
2.2. Powder X-ray Diffraction Measurements
2.3. Single-Crystal X-ray Diffraction Measurements
2.4. Thermal Analysis
2.5. Calculations
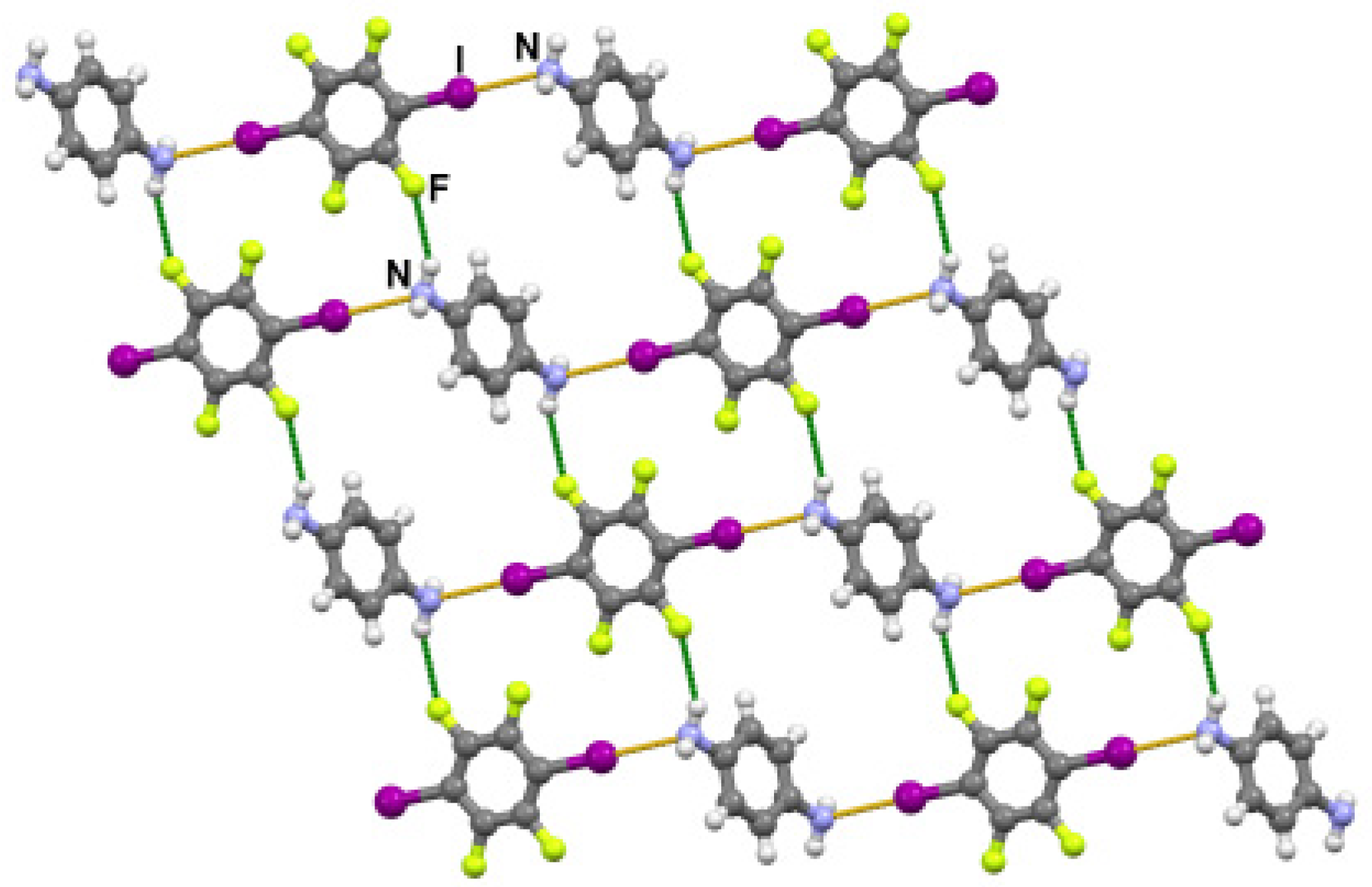
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Legon, A.C. π-Electron “Donor–Acceptor” Complexes B⋅⋅⋅ClF and the Existence of the “Chlorine Bond”. Chem. Eur. J. 1998, 4, 1890–1897. [Google Scholar] [CrossRef]
- Legon, A.C. Prereactive Complexes of Dihalogens XY with Lewis Bases B in the Gas Phase: A Systematic Case for the Halogen Analogue B∙∙∙XY of the Hydrogen Bond B∙∙∙HX. Angew. Chem. Int. Ed. 1999, 38, 2686–2714. [Google Scholar] [CrossRef]
- Corradi, E.; Meille, S.V.; Messina, M.T.; Metrangolo, P.; Resnati, G. Perfluorocarbon-hydrocarbon self-assembly. Part 6: α, ω-Diiodoperfluoroalkanes as pseudohalogens in supramolecular synthesis. Tetrahedron Lett. 1999, 40, 7519–7523. [Google Scholar] [CrossRef]
- Amico, V.; Meille, S.V.; Corradi, E.; Messina, M.T.; Resnati, G. Perfluorocarbon−Hydrocarbon Self-Assembling. 1D Infinite Chain Formation Driven by Nitrogen···Iodine Interactions. J. Am. Chem. Soc. 1998, 120, 8261–8262. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Halogen Bonding: A Paradigm in Supramolecular Chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Erdélyi, M. Halogen bonding in solution. Chem. Soc. Rev. 2012, 41, 3547–3557. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Pancholi, J.; Beer, P.D. Halogen bonding motifs for anion recognition. Coord. Chem. Rev. 2020, 416, 213281. [Google Scholar] [CrossRef]
- Resnati, G.; Penningtonb, W.T. The halogen bond: A new avenue in recognition and self-assembly. New J. Chem. 2018, 42, 10461–10462. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tuikka, M.; Haukka, M. Halogen Bonding in Crystal Engineering. In Recent Advances in Crystallography; Benedict, J.B., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Practical crystal engineering using halogen bonding: A hierarchy based on calculated molecular electrostatic potential surfaces. J. Mol. Struct. 2014, 1072, 20–27. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Structural Equivalence of Br and I Halogen Bonds: A Route to Isostructural Materials with Controllable Properties. Chem. Mater. 2008, 20, 6623–6626. [Google Scholar] [CrossRef]
- Chen, S.; Yin, H.; Wu, J.-J.; Lin, H.; Wang, X.D. Organic halogen-bonded co-crystals for optoelectronic applications. Sci. China Mater. 2020, 63, 1613–1630. [Google Scholar] [CrossRef]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen Bonding in Two-Dimensional Crystal Engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef]
- Brammer, L.; Espallargas, G.M.; Libri, S. Combining metals with halogen bonds. CrystEngComm 2008, 10, 1712–1727. [Google Scholar] [CrossRef]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.Q.; Wang, L.Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Nemec, V.; Lisac, K.; Bedeković, N.; Fotović, L.; Stilinović, V.; Cinčić, D. Crystal engineering strategies towards halogen bonded metal-organic multi-component solids: Salts, cocrystals and salt cocrystals. CrystEngComm 2021. [Google Scholar] [CrossRef]
- Jakupec, N.; Fotović, L.; Stilinović, V. The effect of halogen bonding on protonated hexacyanoferrate networks in hexacyanoferrates of halogenopyridines. CrystEngComm 2020, 22, 8142–8150. [Google Scholar] [CrossRef]
- Fotović, L.; Stilinovic, V. Halogenide anions as halogen and hydrogen bond acceptors in iodopyridinium halogenides. CrystEngComm 2020, 22, 4039–4046. [Google Scholar] [CrossRef]
- Derossi, S.; Brammer, L.; Hunter, C.A.; Ward, M.D. Halogen Bonded Supramolecular Assemblies of [Ru(bipy)(CN)4]2− Anions and N-Methyl-Halopyridinium Cations in the Solid State and in Solution. Inorg. Chem. 2009, 48, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Ormond-Prout, J.E.; Smart, P.; Brammer, L. Cyanometallates as Halogen Bond Acceptors. Cryst. Growth Des. 2011, 12, 205–216. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Zordan, F.; Marín, L.A.; Adams, H.; Shankland, K.; Van De Streek, J.; Brammer, L. Rational Modification of the Hierarchy of Intermolecular Interactions in Molecular Crystal Structures by Using Tunable Halogen Bonds. Chem. Eur. J. 2009, 15, 7554–7568. [Google Scholar] [CrossRef]
- Zordan, F.; Purver, S.L.; Adams, H.; Brammer, L. Halometallate and halide ions: Nucleophiles in competition for hydrogen bond and halogen bond formation in halopyridinium salts of mixed halide–halometallate anions. CrystEngComm 2005, 7, 350–354. [Google Scholar] [CrossRef]
- Lisac, K.; Topić, F.; Arhangelskis, M.; Cepić, S.; Julien, P.A.; Nickels, C.W.; Morris, A.J.; Friščić, T.; Cinčić, D. Halogen-bonded cocrystallization with phosphorus, arsenic and antimony acceptors. Nat. Commun. 2019, 10, 61. [Google Scholar] [CrossRef]
- Zbačnik, M.; Pajski, M.; Stilinović, V.; Vitković, M.; Cinčić, D. The halogen bonding proclivity of the ortho-methoxy–hydroxy group in cocrystals of o-vanillin imines and diiodotetrafluoro-benzenes. CrystEngComm 2017, 19, 5576–5582. [Google Scholar] [CrossRef]
- Carletta, A.; Zbačnik, M.; Vitković, M.; Tumanov, N.; Stilinović, V.; Wouters, J.; Cinčić, D. Halogen-bonded cocrystals of N-salicylidene Schiff bases and iodoperfluorinated benzenes: Hydroxyl oxygen as a halogen bond acceptor. CrystEngComm 2018, 20, 5332–5339. [Google Scholar] [CrossRef]
- Nemec, V.; Fotović, L.; Vitasović, T.; Cinčić, D. Halogen bonding of the aldehyde oxygen atom in cocrystals of aromatic aldehydes and 1,4-diiodotetrafluorobenzene. CrystEngComm 2019, 21, 3251–3255. [Google Scholar] [CrossRef]
- Stilinović, V.; Grgurić, T.; Piteša, T.; Nemec, V.; Cinčić, D. Bifurcated and Monocentric Halogen Bonds in Cocrystals of Metal(II) Acetylacetonates with p-Dihalotetrafluorobenzenes. Cryst. Growth Des. 2018, 19, 1245–1256. [Google Scholar] [CrossRef]
- Lisac, K.; Cinčić, D. Simple design for metal-based halogen-bonded cocrystals utilizing the M-Cl···I motif. CrystEngComm 2018, 20, 5955–5963. [Google Scholar] [CrossRef]
- Stilinović, V.; Horvat, G.; Hrenar, T.; Nemec, V.; Cinčić, D. Halogen and Hydrogen Bonding between (N-Halogeno)-succinimides and Pyridine Derivatives in Solution, the Solid State and In Silico. Chem. Eur. J. 2017, 23, 5244–5257. [Google Scholar] [CrossRef] [PubMed]
- Troff, R.W.; Mäkelä, T.; Topić, F.; Valkonen, A.; Raatikainen, K.; Rissanen, K. Alternative motifs for halogen bonding. Eur. J. Org. Chem. 2013, 9, 1617–1637. [Google Scholar] [CrossRef]
- Borley, W.; Watson, B.; Nizhnik, Y.P.; Zeller, M.; Rosokha, S.V. Complexes of Diiodine with Heteroaromatic N-Oxides: Effects of Halogen-Bond Acceptors in Halogen Bonding. J. Phys. Chem. A 2019, 123, 7113–7123. [Google Scholar] [CrossRef]
- Topić, F.; Rissanen, K. Systematic Construction of Ternary Cocrystals by Orthogonal and Robust Hydrogen and Halogen Bonds. J. Am. Chem. Soc. 2016, 138, 6610–6616. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.I.; Padgett, C.W.; Walsh, R.D.B.; Hanks, A.T.W.; Pennington, W.T. Noncovalent Interactions in 2-Mercapto-1-methylimidazole Complexes with Organic Iodides. Cryst. Growth Des. 2001, 1, 501–507. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. 4,4′-Bipyridine–2,4,5,6-tetrafluoro-1,3-diiodobenzene. Acta Cryst. 2007, E63, o4243. [Google Scholar] [CrossRef]
- Grebe, J.; Geiseler, G.; Harms, K.; Neumüller, B.; Dehnicke, K. Domino Effect in the Buildup of N-I-N-I Chains of the N-Iodine(triphenylphosphane)imine. Angew. Chem. Int. Ed. 1999, 38, 222–225. [Google Scholar] [CrossRef]
- Lucassen, A.C.B.; Karton, A.; Leitus, G.; Shimon, L.J.W.; Martin, J.M.L.; Van Der Boom, M.E.; Martin, G. Co-Crystallization of Sym-Triiodo-Trifluorobenzene with Bipyridyl Donors: Consistent Formation of Two Instead of Anticipated Three N···I Halogen Bonds. Cryst. Growth Des. 2007, 7, 386–392. [Google Scholar] [CrossRef]
- Bedeković, N.; Stilinović, V.; Friščić, T.D.; Cinčić, D. Comparison of isomeric meta- and para-diiodotetrafluorobenzene as halogen bond donors in crystal engineering. New J. Chem. 2018, 42, 10584–10591. [Google Scholar] [CrossRef]
- Ding, X.H.; Chang, Y.Z.; Ou, C.J.; Lin, J.Y.; Xie, L.H.; Huang, W. Halogen bonding in the co-crystallization of potentially ditopic diiodotetrafluorobenzene: A powerful tool for constructing multicomponent supramolecular assemblies. Natl. Sci. Rev. 2020, 7, 1906–1932. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Legon, C. The halogen bond: An interim perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. [Google Scholar] [CrossRef]
- Gamekkanda, J.C.; Sinha, A.S.; Desper, J.; Đaković, M.; Aakeröy, C.B. Competition between hydrogen bonds and halogen bonds: A structural study. New J. Chem. 2018, 42, 10539–10547. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. [Google Scholar] [CrossRef]
- Robertson, C.C.; Wright, J.S.; Carrington, E.J.; Perutz, R.N.; Hunter, C.A.; Brammer, L. Hydrogen bonding vs. halogen bonding: The solvent decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [CrossRef]
- Lombard, J.; Le Roex, T.; Haynes, D.A. Competition between Hydrogen and Halogen Bonds: The Effect of Solvent Volume. Cryst. Growth Des. 2020, 20, 7384–7391. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Nemec, V.; Cinčić, D. Uncommon halogen bond motifs in cocrystals of aromatic amines and 1,4-diiodotetrafluorobenzene. CrystEngComm 2016, 18, 7425–7429. [Google Scholar] [CrossRef]
- Rafilovich, M.; Bernstein, J. Serendipity and Four Polymorphic Forms of Benzidine, C12H12N2. J. Am. Chem. Soc. 2006, 128, 12185–12195. [Google Scholar] [CrossRef]
- Czapik, A.; Konowalska, H.; Gdaniec, M. p-Phenylenediamine and its dihydrate: Two-dimensional isomorphism and mechanism of the dehydration process, and N–H···N and N–H···π interactions. Acta Cryst. 2010, C66, 128–132. [Google Scholar] [CrossRef]
- Chawdhury, S.A.; Hargreaves, A.; Sullivan, R.A.L. The crystal and molecular structure of 4,4′-diamino-3,3′-dimethylbiphenyl (o-tolidine). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 1222–1228. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- CrysAlis PRO CCD; Agilent Technologies Ltd.: Yarnton, UK, 2014.
- CrysAlis PRO RED; Agilent Technologies Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.J.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- STARe Software, version 15.00; Mettler Toledo: Greifensee, Switzerland, 2016.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Peverati, R.; Truhlar, D.G. An improved and broadly accurate local approximation to the exchange–correlation density functional: The MN12-L functional for electronic structure calculations in chemistry and physics. Phys. Chem. Chem. Phys. 2012, 14, 13171–13174. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Karpfen, A. Struct. Bonding (Berl.) 2008, 126, 1. [Google Scholar]
- Rosokha, S.; Kochi, J. X-ray Structures and Electronic Spectra of the π-Halogen Complexes between Halogen Donors and Acceptors with π-Receptors. In Halogen Bonding. Fundamentals and Applications; Metrangolo, P., Resnati, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 126, pp. 137–160. [Google Scholar]
- Hunter, C.A.; Sanders, J.K.M. The nature of π–π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
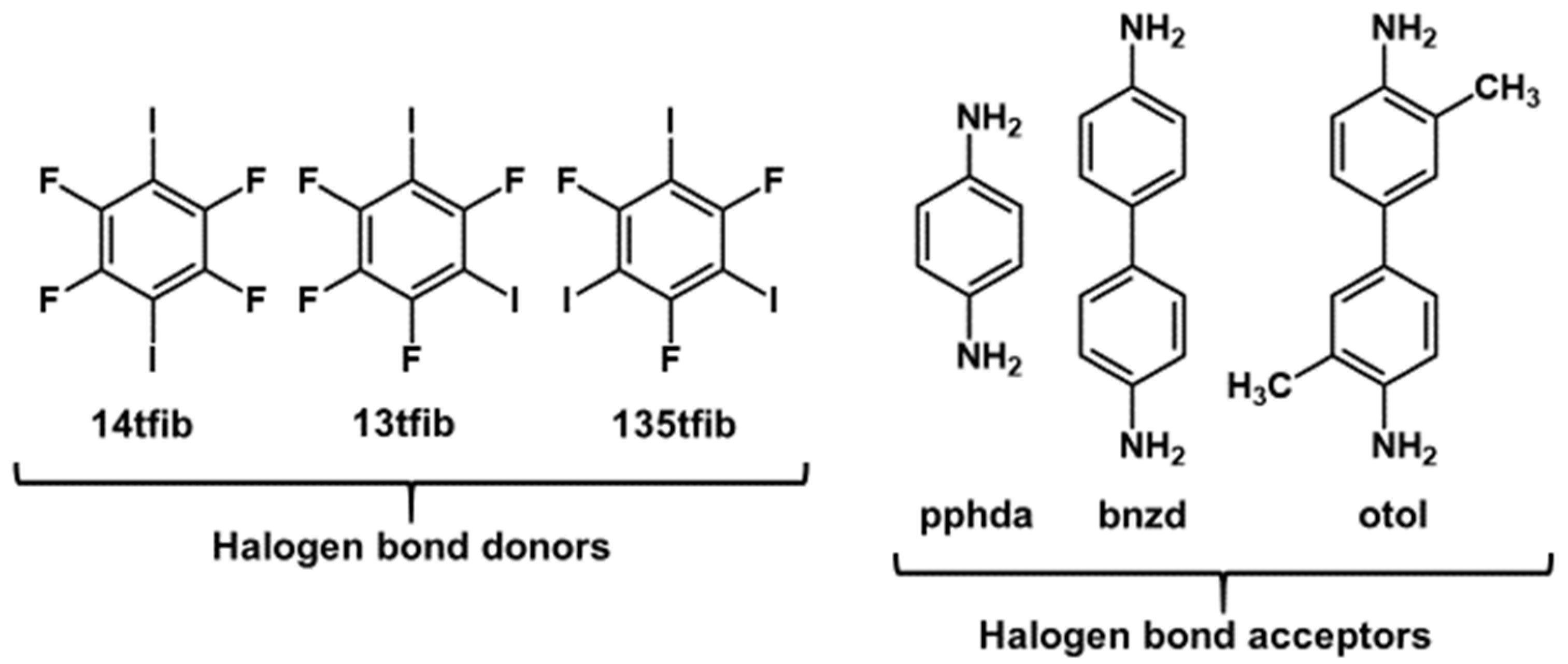





| pphda | bnzd | otol | |
|---|---|---|---|
| 14tfib | (pphda)(14tfib) | - | (otol)(14tfib) |
| 13tfib | - | (bnzd)(13tfib)2 | - |
| 135tfib | - | (bnzd)(135tfib)4 | (otol)(135tfib)2, |
| Complex | E/kJ mol−1 |
|---|---|
| (PhNH2)2 | −28.4 |
| (PhNH2)(ipfb) | −27.6 |
| (MeNH2)2 | −20.9 |
| (MeNH2)(ipfb) | −36.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uran, E.; Fotović, L.; Bedeković, N.; Stilinović, V.; Cinčić, D. The Amine Group as Halogen Bond Acceptor in Cocrystals of Aromatic Diamines and Perfluorinated Iodobenzenes. Crystals 2021, 11, 529. https://doi.org/10.3390/cryst11050529
Uran E, Fotović L, Bedeković N, Stilinović V, Cinčić D. The Amine Group as Halogen Bond Acceptor in Cocrystals of Aromatic Diamines and Perfluorinated Iodobenzenes. Crystals. 2021; 11(5):529. https://doi.org/10.3390/cryst11050529
Chicago/Turabian StyleUran, Erik, Luka Fotović, Nikola Bedeković, Vladimir Stilinović, and Dominik Cinčić. 2021. "The Amine Group as Halogen Bond Acceptor in Cocrystals of Aromatic Diamines and Perfluorinated Iodobenzenes" Crystals 11, no. 5: 529. https://doi.org/10.3390/cryst11050529
APA StyleUran, E., Fotović, L., Bedeković, N., Stilinović, V., & Cinčić, D. (2021). The Amine Group as Halogen Bond Acceptor in Cocrystals of Aromatic Diamines and Perfluorinated Iodobenzenes. Crystals, 11(5), 529. https://doi.org/10.3390/cryst11050529








