Hydrothermal Synthesis, Crystal Structure, and Spectroscopic Properties of Pure and Eu3+-Doped NaY[SO4]2 ∙ H2O and Its Anhydrate NaY[SO4]2
Abstract
1. Introduction
2. Materials and Methods
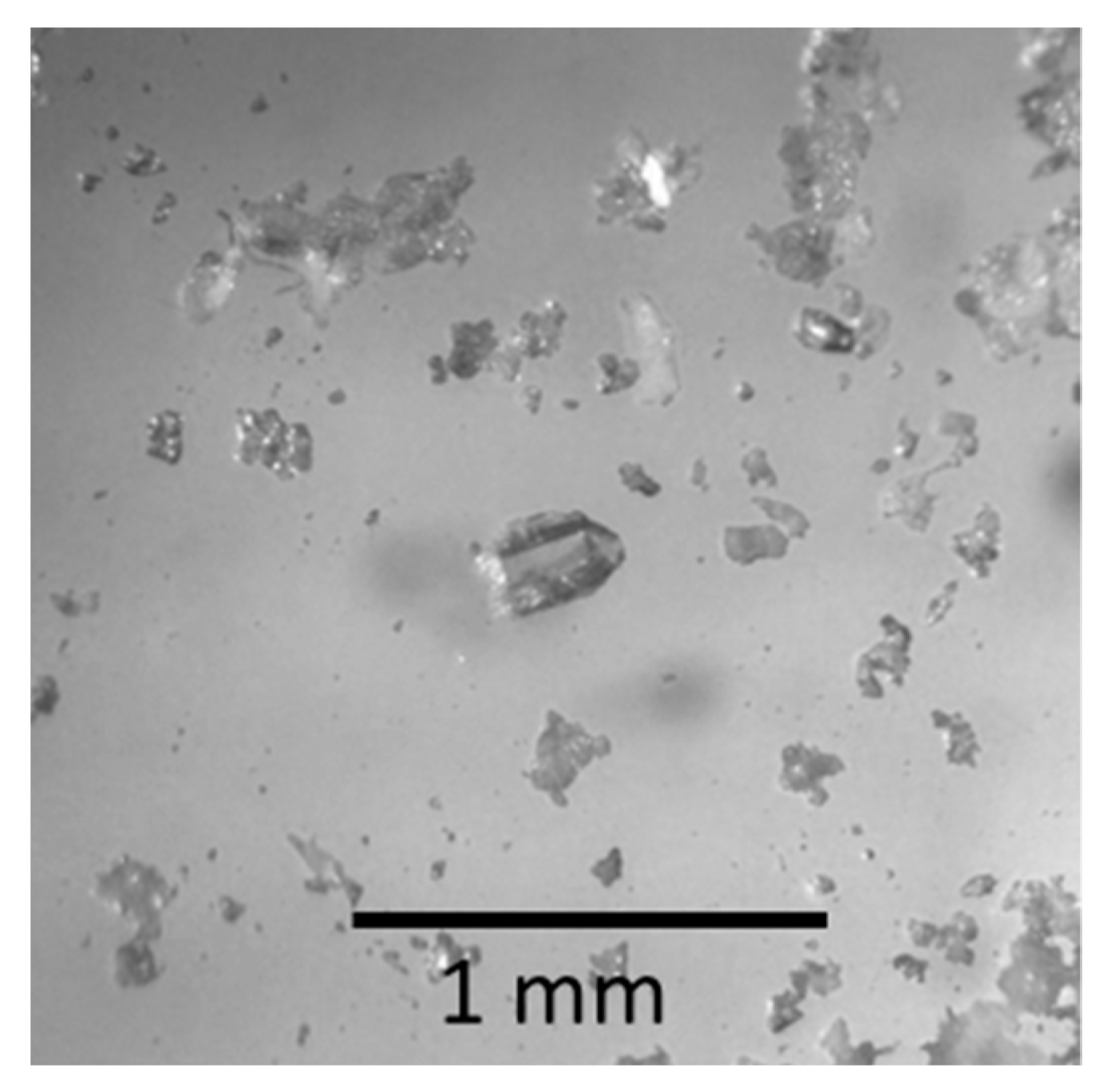
2.1. Synthesis
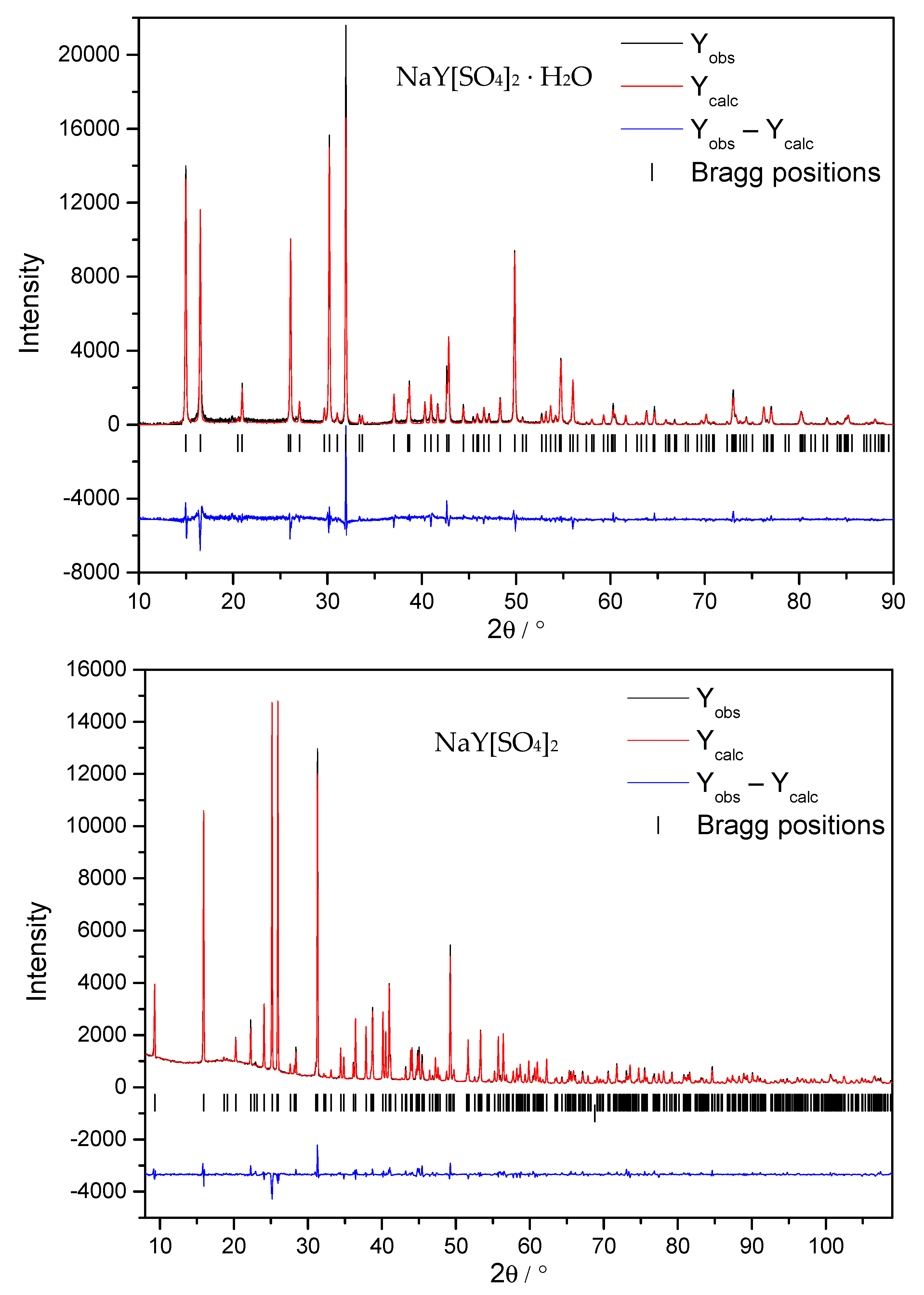
2.2. X-ray Experiments and Crystal-Structure Solution
2.3. Thermal Analysis
2.4. Luminescence Spectroscopy
2.5. IR and Raman Spectra
3. Results and Discussion
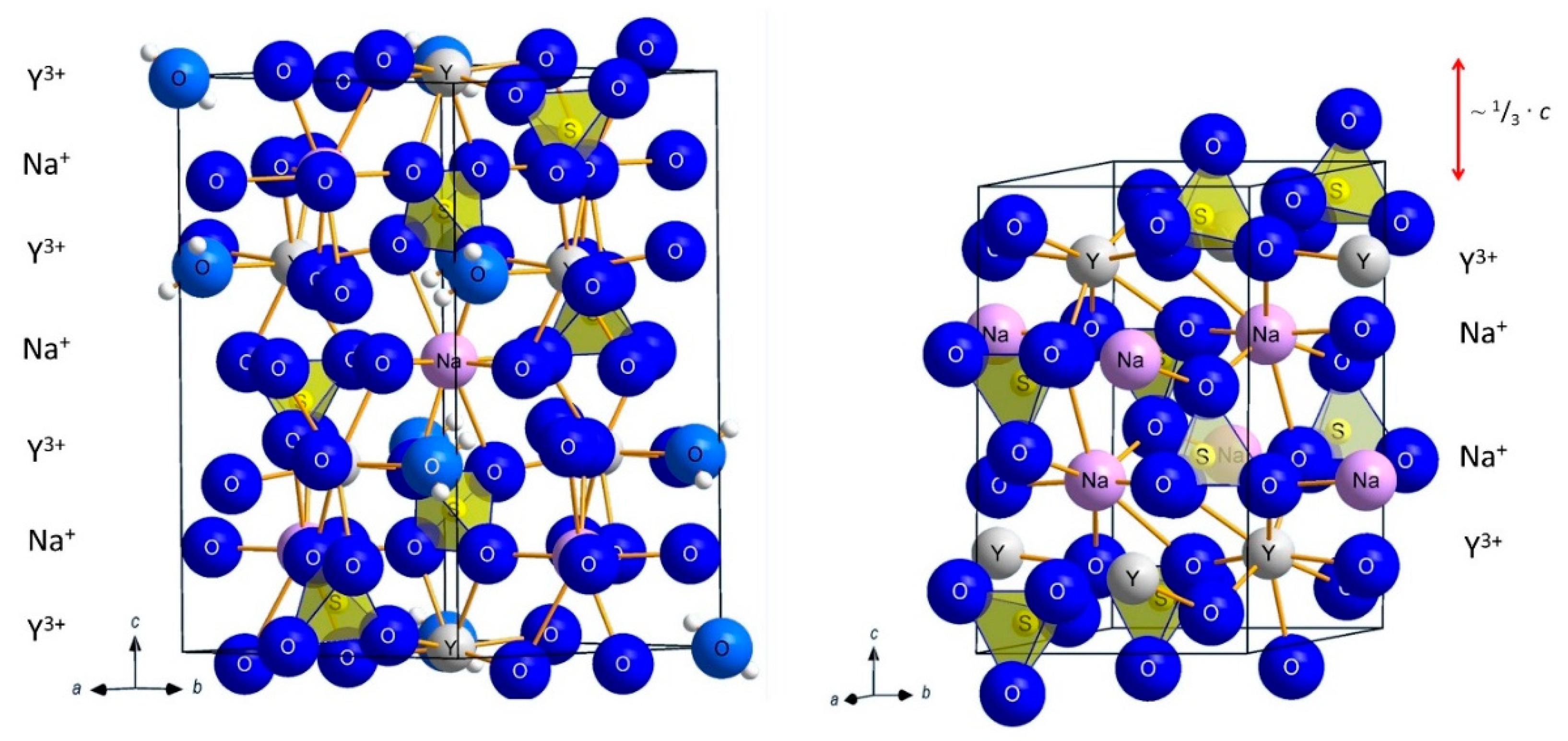
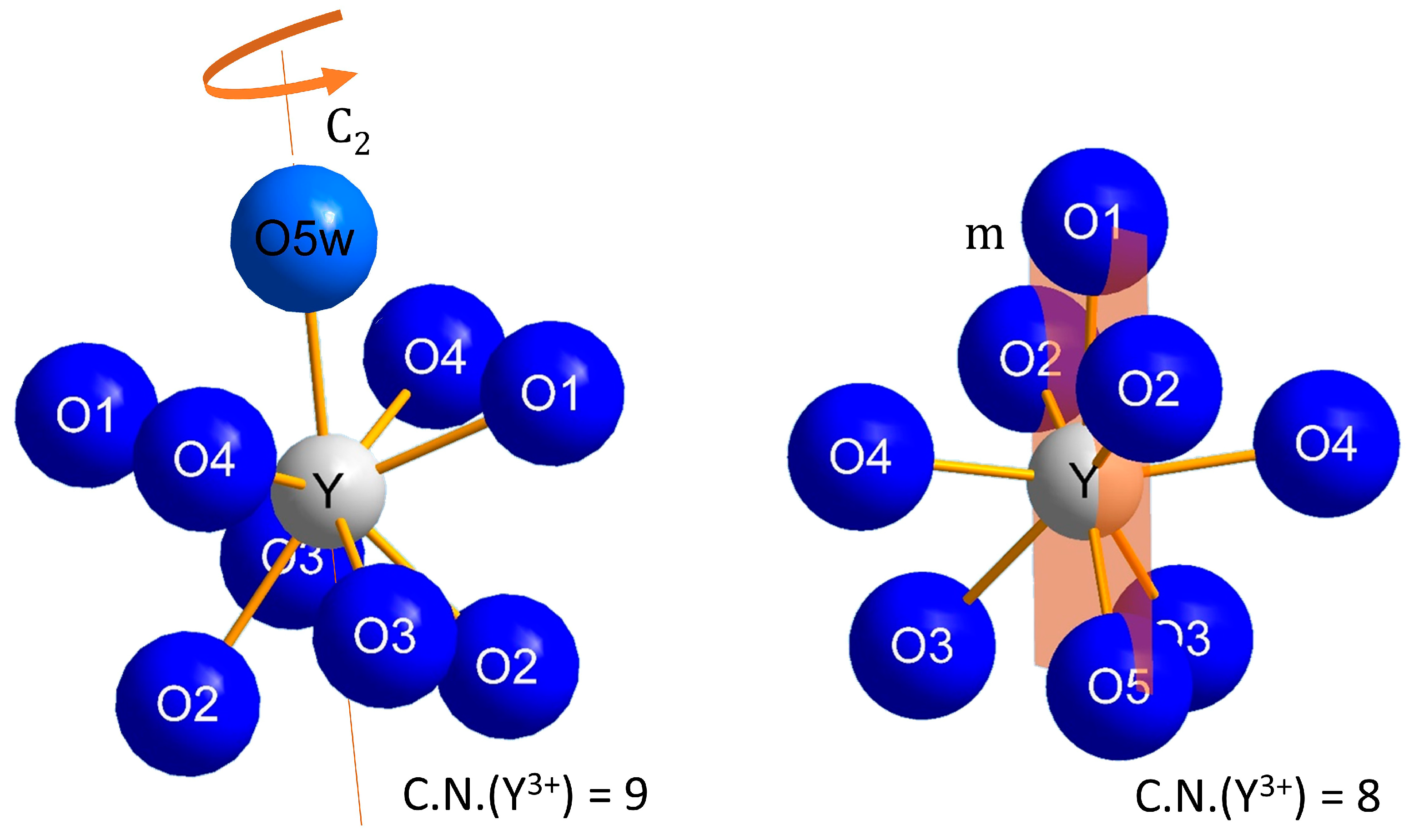
3.1. Structure Refinement and Description of NaY[SO4]2 ∙ H2O and NaY[SO4]2
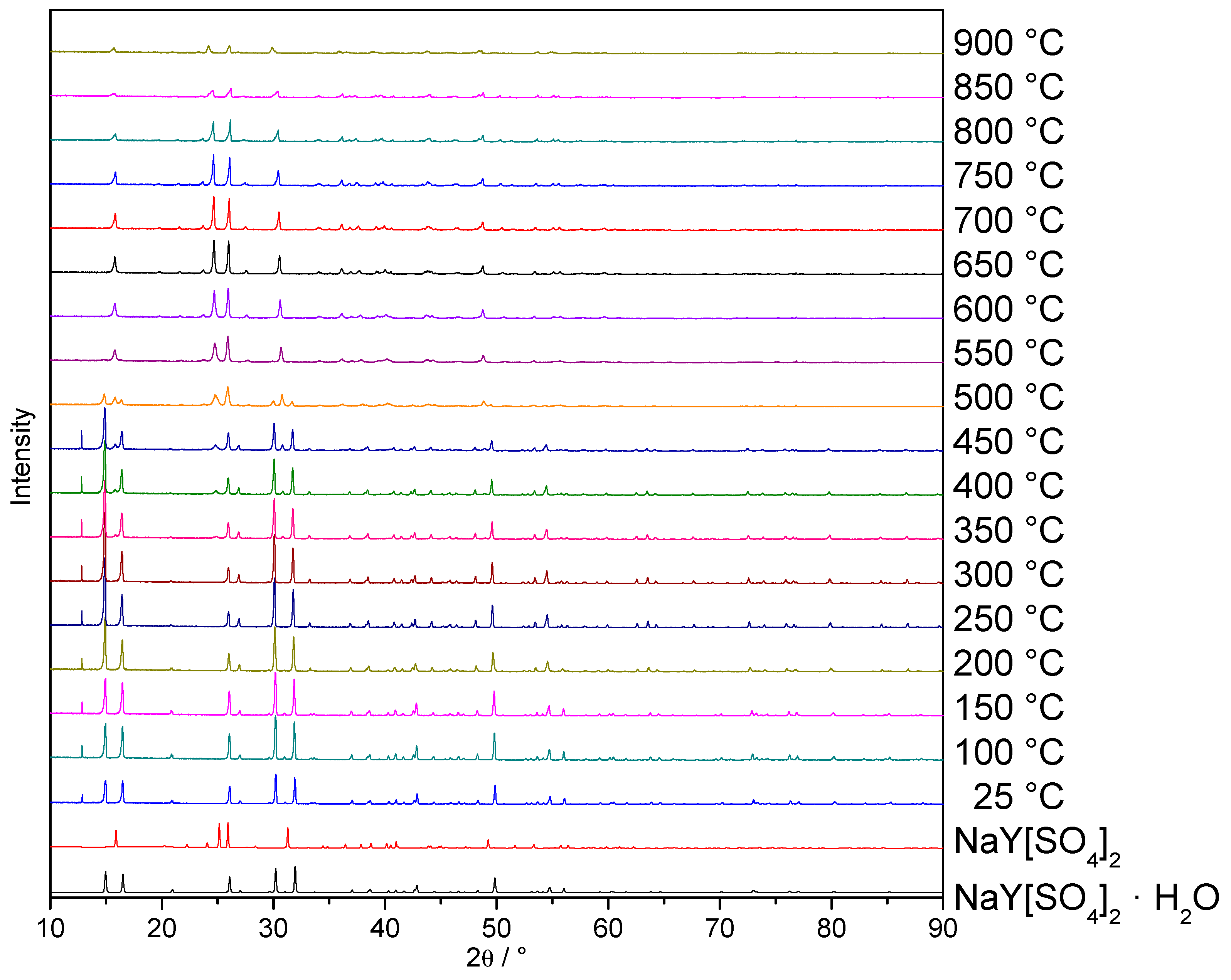
3.2. Thermal Analysis
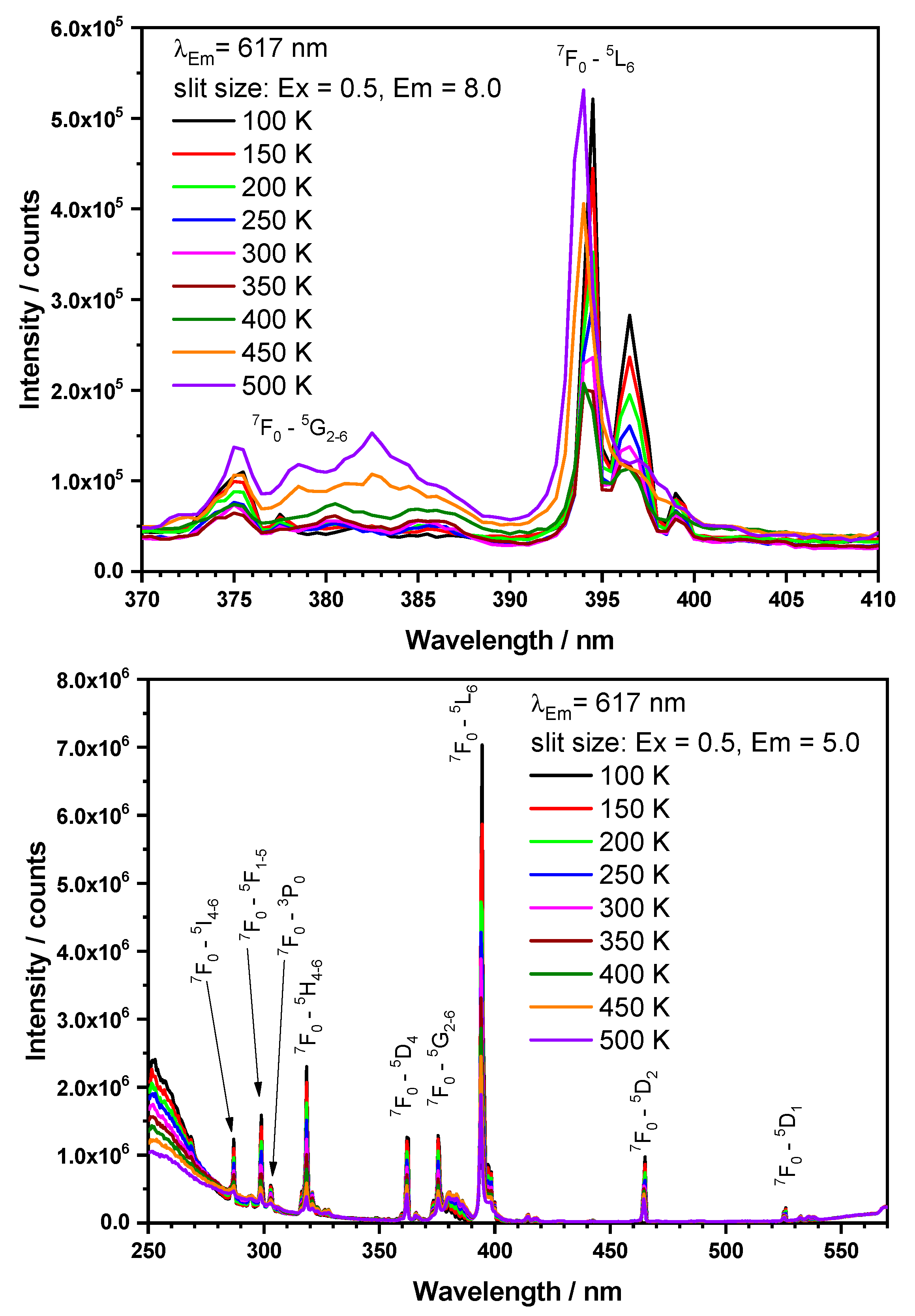
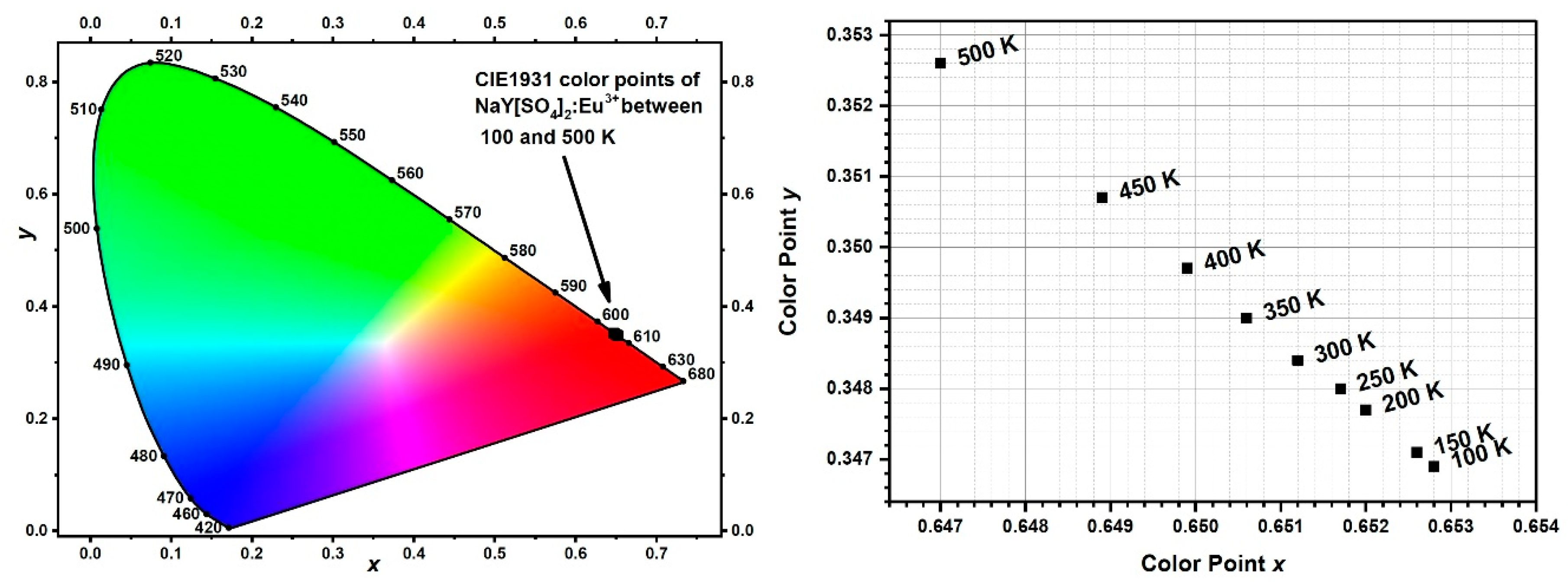
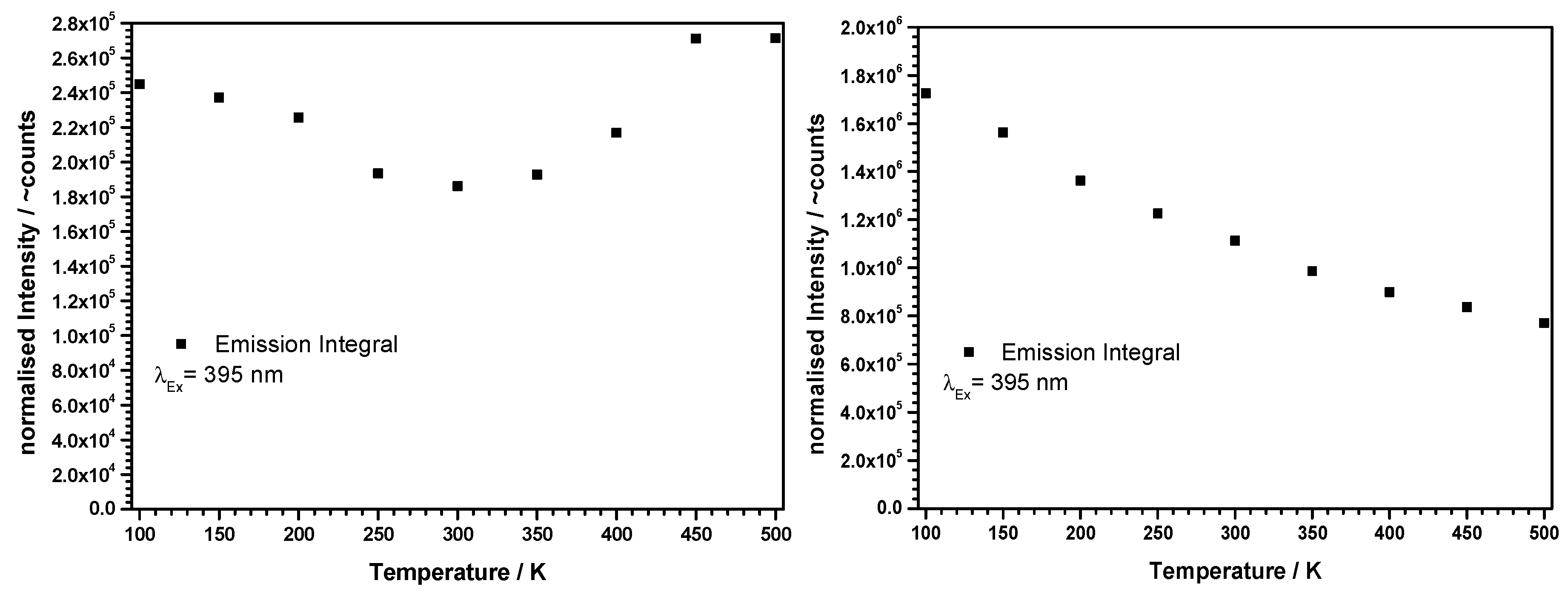
3.3. Luminescence-Spectroscopic Properties
3.4. IR and Raman Studies of NaY[SO4]2 ∙ H2O and NaY[SO4]2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jüstel, T.; Nikol, H.; Ronda, C. New Developments in the Field of Luminescent Materials for Lighting and Displays. Angew. Chem. Int. Ed. 1998, 37, 3084–3103. [Google Scholar] [CrossRef]
- Feldmann, C.; Jüstel, T.; Ronda, C.R.; Schmidt, P.J. Inorganic Luminescent Materials: 100 Years of Research and Application. Adv. Funct. Mater. 2003, 13, 511–516. [Google Scholar] [CrossRef]
- Skaudzius, R.; Katelnikovas, A.; Enseling, D.; Kareiva, A.; Jüstel, T. Dependence of the 5D0→7F4 transitions of Eu3+ on the local environment in phosphates and garnets. J. Lumin. 2014, 147, 290–294. [Google Scholar] [CrossRef]
- Laufer, S.; Strobel, S.; Schleid, T.; Cybinska, J.; Mudring, A.-V.; Hartenbach, I. Yttrium(III) Oxomolybdates(VI) as Potential Host Materials for Luminescence Applications: An Investigation of Eu3+-Doped Y2[MoO4]3 and Y2[MoO4]2[Mo2O7]. New J. Chem. 2013, 37, 1919–1926. [Google Scholar] [CrossRef]
- Goerigk, F.C.; Paterlini, V.; Dorn, K.V.; Mudring, A.-V.; Schleid, T. Synthesis and Crystal Structure of the Short LnSb2O4Br Series (Ln = Eu–Tb) and Luminescence Properties of Eu3+-Doped Samples. Crystals 2020, 10, 1089. [Google Scholar] [CrossRef]
- Popovic, E.J.; Imre-Lucaci, F.; Muresan, L.; Stefan, M.; Bica, E.; Grecu, R.; Indrea, E. Spectral investigations on niobium and rare earth activated yttrium tantalate powders. J. Optoelectron. Adv. Mater. 2008, 10, 2334–2337. [Google Scholar]
- Ledderboge, F.; Nowak, J.; Massonne, H.-J.; Förg, K.; Höppe, H.A.; Schleid, T. High-Pressure Investigations of Yttrium(III) Oxoarsenate(V): Crystal Structure and Luminescence Properties of Eu3+-Doped Scheelite-Type Y[AsO4] from Xenotime-Type Precursors. J. Solid State Chem. 2018, 263, 65–71. [Google Scholar] [CrossRef]
- Cybińska, J. Temperature dependent morphology variation of red emitting microcrystalline YPO4:Eu3+ fabricated by hydrothermal method. Opt. Mater. 2017, 65, 88–94. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Chen, F.-S.; Lu, C.-H. Preparation and luminescence characterization of new carbonate (Y2(CO3)3 · n H2O:Eu3+) phosphors via the hydrothermal route. J. Alloys Compd. 2011, 509, 10014–10019. [Google Scholar] [CrossRef]
- Lindgren, O. The Crystal Structure of Sodium Cerium(III) Sulfate Hydrate, NaCe(SO4)2 ∙ H2O. Acta Chem. Scand. 1977, 31, 591–594. [Google Scholar] [CrossRef]
- Wu, C.-D.; Liu, Z.-Y. Hydrothermal synthesis of a luminescent europium(III) sulfate with three-dimensional chiral framework structure. J. Solid State Chem. 2006, 179, 3500–3504. [Google Scholar] [CrossRef]
- Zhai, B.; Li, Z.; Zhang, C.; Zhang, F.; Zhang, X.; Zhang, F.; Cao, G.; Li, S.; Yang, X. Three rare Ln–Na heterometallic 3D polymers based on sulfate anion: Syntheses, structures, and luminescence properties. Inorg. Chem. Commun. 2016, 63, 16–19. [Google Scholar] [CrossRef]
- Perles, J.; Fortes-Revilla, C.; Gutiérrez-Puebla, E.; Iglesias, M.; Monge, M.Á.; Ruiz-Valero, C.; Snejko, N. Synthesis, Structure, and Catalytic Properties of Rare-Earth Ternary Sulfates. Chem. Mater. 2005, 17, 2701–2706. [Google Scholar] [CrossRef]
- Paul, A.K.; Kanagaraj, R. Synthesis, Characterization, and Crystal Structure Analysis of New Mixed Metal Sulfate NaPr(SO4)2(H2O). J. Struct. Chem. 2019, 60, 477–484. [Google Scholar] [CrossRef]
- Blackburn, A.C.; Gerkin, R.E. Sodium lanthanum(III) sulfate monohydrate, NaLa(SO4)2 ∙ H2O. Acta Crystallogr. 1994, 50, 835–838. [Google Scholar] [CrossRef]
- Blackburn, A.C.; Gerkin, R.E. Redetermination of sodium cerium(III) sulfate monohydrate, NaCe(SO4)2 ∙ H2O. Acta Crystallogr. 1995, 51, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Kolcu, Ö.; Zümreoğlu-Karan, B. Nonisothermal Dehydration Kinetics of Sodium-Light Lanthanoid Double Sulfate Monohydrates. Thermochim. Acta 1997, 296, 135–139. [Google Scholar] [CrossRef]
- Kolcu, Ö.; Zümreoǧlu-Karan, B. Thermal Properties of Sodium-Light-Lanthanoid Double Sulfate Monohydrates. Thermochim. Acta 1994, 240, 185–198. [Google Scholar] [CrossRef]
- Iyer, P.N.; Natarajan, P.R. Double Sulphates of Plutonium(III) and lanthanides with sodium. J. Less Common Met. 1989, 146, 161–166. [Google Scholar] [CrossRef]
- Kazmierczak, K.; Höppe, H.A. Syntheses, crystal structures and vibrational spectra of KLn(SO4)2 · H2O (Ln = La, Nd, Sm, Eu, Gd, Dy). J. Solid State Chem. 2010, 183, 2087–2094. [Google Scholar] [CrossRef]
- Jemmali, M.; Walha, S.; Ben Hassen, R.; Vaclac, P. Potassium cerium(III) bis(sulfate) monohydrate, KCe(SO4)2 ∙ H2O. Acta Crystallogr. 2005, 61, i73–i75. [Google Scholar] [CrossRef]
- Iskhakova, L.D.; Sarukhanyan, N.L.; Shchegoleva, T.M.; Trunov, V.K. The crystal structure of KPr(SO4)2 ∙ H2O. Kristallografiya 1985, 30, 474–479. [Google Scholar]
- Lyutin, V.I.; Safyanov, Y.N.; Kuzmin, E.A.; Ilyukhin, V.V.; Belov, N.V. Rhomb evaluation of the crystal structure of K-Tb double sulfate. Kristallografiya 1974, 19, 376–378. [Google Scholar]
- Robinson, P.D.; Jasty, S. Rubidium Cerium Sulfate Monohydrate, RbCe(SO4 )2 ∙ H2O. Acta Crystallogr. 1998, C54, IUC9800021. [Google Scholar] [CrossRef]
- Prokofev, M.V. Crystal structure of the double sulfate of rubidium and holmium. Kristallografiya 1981, 26, 598–600. [Google Scholar]
- Sarukhanyan, N.L.; Iskhakova, L.D.; Trunov, V.K.; Ilyukhin, V.V. Crystal structure of RbLn(SO4)2(H2O) (Ln = Gd, Ho, Yb). Koord. Khim. 1984, 10, 981–987. [Google Scholar]
- Audebrand, N.; Auffrédic, J.-P.; Louër, D. Crystal structure of silver cerium sulfate hydrate, AgCe(SO4)2 · H2O. Z. Kristallogr. 1998, 213, 481. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, Y.; Mei, D.; Wen, S.; Doert, T.H. Synthesis, Crystal Structures, Spectroscopic Characterization, and Thermal Analyses of the New Bismuth Sulfates NaBi(SO4)2 · H2O and ABi(SO4)2 (A = K, Rb, Cs). Z. Anorg. Allg. Chem. 2020, 646, 1688–1695. [Google Scholar] [CrossRef]
- Song, Y.; Zou, H.; Sheng, Y.; Zheng, K.; You, H. 3D Hierarchical Architectures of Sodium Lanthanide Sulfates: Hydrothermal Synthesis, Formation Mechanisms, and Luminescence Properties. J. Phys. Chem. C 2011, 115, 19463–19469. [Google Scholar] [CrossRef]
- Kampf, A.R.; Nash, B.P.; Marty, J. Chinleite-(Y), NaY(SO4)2 · H2O, a new rare-earth sulfate mineral structurally related to bassanite. Mineral. Mag. 2017, 81, 909–916. [Google Scholar] [CrossRef]
- Sirotinkine, S.P.; Tchijov, S.M.; Pokrovskii, A.N.; Kovba, L.M. Structure cristalline de sulfates doubles de sodium et de terres rares. J. Less Common Met. 1978, 58, 101–105. [Google Scholar] [CrossRef]
- Chizhov, S.M.; Pokrovskii, A.N.; Kovba, L.M. The crystal structure of alpha-NaTm(SO4)2. Kristallografiya 1982, 27, 997–998. [Google Scholar]
- Chizhov, S.M.; Pokrovskii, A.N.; Kovba, L.M. The crystal structure of NaLa(SO4)2. Kristallografiya 1981, 26, 834–836. [Google Scholar]
- Wickleder, M.S.; Büchner, O. The Gold Sulfates MAu(SO4)2 (M = Na, K, Rb). Z. Naturforsch. 2001, 56, 1340–1343. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Volkova, S.S.; Andreev, O.V. Structure, Thermal Stability, and Spectroscopic Properties of Triclinic Double Sulfate AgEu(SO4)2 with Isolated SO4 Groups. Inorg. Chem. 2018, 57, 13279–13288. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Program. Suite for the Solution and Refinement of Crystal Structures; Univeristy of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Bärnighausen, W.; Herrendorf, H. Habitus; Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE; Karlsruhe: Gießen, Germany, 1993. [Google Scholar]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction analysis. In Materials Science Forum. Proceedings of the European Powder Diffraction Conference; CiteSeerX: State College, PA, USA, 2001. [Google Scholar]
- Kawamura, Y.; Sasabe, H.; Adachi, C. Simple Accurate System for Measuring Absolute Photoluminescence Quantum Efficiency in Organic Solid-State Thin Films. Jpn. J. Appl. Phys. 2004, 43, 7729–7730. [Google Scholar] [CrossRef]
- Downloaded at 10 April 2021. Available online: https://www.osram.us/cb/tools-and-resources/applications/led-colorcalculator/index.jsp (accessed on 27 April 2021).
- Smet, P.F.; Parmentier, A.B.; Poelman, D. Selecting Conversion Phosphors for White Light-Emitting Diodes. J. Electrochem. Soc. 2011, 158, 37. [Google Scholar] [CrossRef]
- Fischer, R.X.; Tillmanns, E. The equivalent isotropic displacement factor. Acta Crystallogr. 1988, 44, 775–776. [Google Scholar] [CrossRef]
- Held, P.; Wickleder, M.S. Yttrium(III) sulfate octahydrate. Acta Crystallogr. 2003, 59, i98–i100. [Google Scholar] [CrossRef]
- Wickleder, M.S. Wasserfreie Sulfate der Selten-Erd-Elemente: Synthese und Kristallstruktur von Y2(SO4)3 und Sc2(SO4)3. Z. Anorg. Allg. Chem. 2000, 626, 1468–1472. [Google Scholar] [CrossRef]
- Degtyarev, P.A.; Pokrovskii, A.N.; Kovba, L.M. Crystal structure of the anhydrous double sulfate KPr(SO4)2. Kristallografiya 1978, 23, 840–843. [Google Scholar]
- Degtyarev, P.A.; Korytnaya, F.M.; Pokrovskii, A.N.; Kovba, L.M. Crystal structure of the anhydrous double sulfate of potassium and neodymium KNd(SO4)2. Vestn. Mosk. Univ. Seriya 2 Khimiya 1997, 16, 705–708. [Google Scholar]
- Iskhakova, L.D.; Gasanov, Y.M.; Trunov, V.K. Crystal structure of the monoclinic modification of KNd(SO4)2. J. Struct. Chem. 1988, 29, 242–246. [Google Scholar] [CrossRef]
- Sarukhanyan, N.L.; Iskhakova, L.D.; Trunov, V.K. The crystal structure of KEr(SO4)2. Kristallografiya 1985, 30, 274–278. [Google Scholar]
- Zachariasen, W.H.; Ziegler, G.E. The crystal structure of anhydrous sodium sulfate Na2SO4. Z. Kristallogr. 1932, 81, 92–101. [Google Scholar] [CrossRef]
- Ruben, H.W.; Templeton, D.H.; Rosenstein, R.D.; Olovsson, I. Crystal Structure and Entropy of Sodium Sulfate Decahydrate. J. Am. Chem. Soc. 1961, 83, 820–824. [Google Scholar] [CrossRef][Green Version]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, 47, 192. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Wu, T.; Huang, Z.; Zhang, C. Deep-ultraviolet transparent alkali metal-rare earth metal sulfate NaY(SO4)2 · H2O as a nonlinear optical crystal: Synthesis and characterization. CrystEngComm 2021, 23, 2945–2951. [Google Scholar] [CrossRef]
- Kijima, T.; Shinbori, T.; Sekita, M.; Uota, M.; Sakai, G. Abnormally enhanced Eu3+ emission in Y2O2SO4:Eu3+ inherited from their precursory dodecylsulfate-templated concentric-layered nanostructure. J. Lumin. 2008, 128, 311–316. [Google Scholar] [CrossRef]
- Zhukov, S.; Yatsenko, A.; Chernyshev, V.; Trunov, V.; Tserkovnaya, E.; Antson, O.; Hölsä, J.; Baulés, P.; Schenk, H. Structural study of lanthanum oxysulfate (LaO)2SO4. Mater. Res. Bull. 1997, 32, 43–50. [Google Scholar] [CrossRef]
- Hartenbach, I.; Schleid, T. Serendipitous Formation of Single-Crystalline Eu2O2[SO4]. Z. Anorg. Allg. Chem. 2002, 628, 2171. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V. Synthesis and thermal transformation of a neodymium(III) complex [Nd(HTBA)2(C2H3O2)(H2O)2]· 2 H2O to non-centrosymmetric oxosulfate Nd2O2SO4. J. Coord. Chem. 2015, 68, 1865–1877. [Google Scholar] [CrossRef]
- Niggli, A. Die Raumgruppe von Na2CrO4. Acta Crystallogr. 1954, 7, 776. [Google Scholar] [CrossRef]
- Brauer, G.; Gradinger, H. Über heterotype Mischphasen bei Seltenerdoxyden. Z. Anorg. Allg. Chem. 1954, 276, 209–226. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751. [Google Scholar] [CrossRef]
- Blasse, B.; Grabmaier, C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1994; ISBN 3-540-58019-0. [Google Scholar]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Dorenbos, P. The Eu3+ charge transfer energy and the relation with the band gap of compounds. J. Lumin. 2005, 111, 89–104. [Google Scholar] [CrossRef]
- Baur, F.; Jüstel, T. New Red-Emitting Phosphor La2Zr3(MoO4)9:Eu3+ and the Influence of Host Absorption on its Luminescence Efficiency. Aust. J. Chem. 2015, 68, 1727–1734. [Google Scholar] [CrossRef]
- Baur, F.; Jüstel, T. Eu3+ activated molybdates—Structure property relations. Opt. Mater. X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Blasse, G. Reminiscencies of a quenched luminescence investigatory. J. Lumin. 2002, 100, 65–67. [Google Scholar] [CrossRef]
- Frech, R.; Cole, R.; Dharmasena, G. Raman Spectroscopic Studies of Y2(SO4)3 Substitution in LiNaSO4 and LiKSO4. J. Solid State Chem. 1993, 105, 151–160. [Google Scholar] [CrossRef]
- Supkowski, R.M.; Horrocks, W.D.W. On the determination of the number of water molecules, q, coordinated to europium(III) ions in solution from luminescence decay lifetimes. Inorg. Chim. Acta 2002, 340, 44–48. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Fdez-Ortiz de Vallejuelo, S.; Veneranda, M.; Lama, E.; Castro, K.; Arana, G.; Larrañaga, A.; Madariaga, J.M. The Raman spectra of the Na2SO4–K2SO4 system: Applicability to soluble salts studies in built heritage. J. Raman Spectrosc. 2019, 50, 175–183. [Google Scholar] [CrossRef]
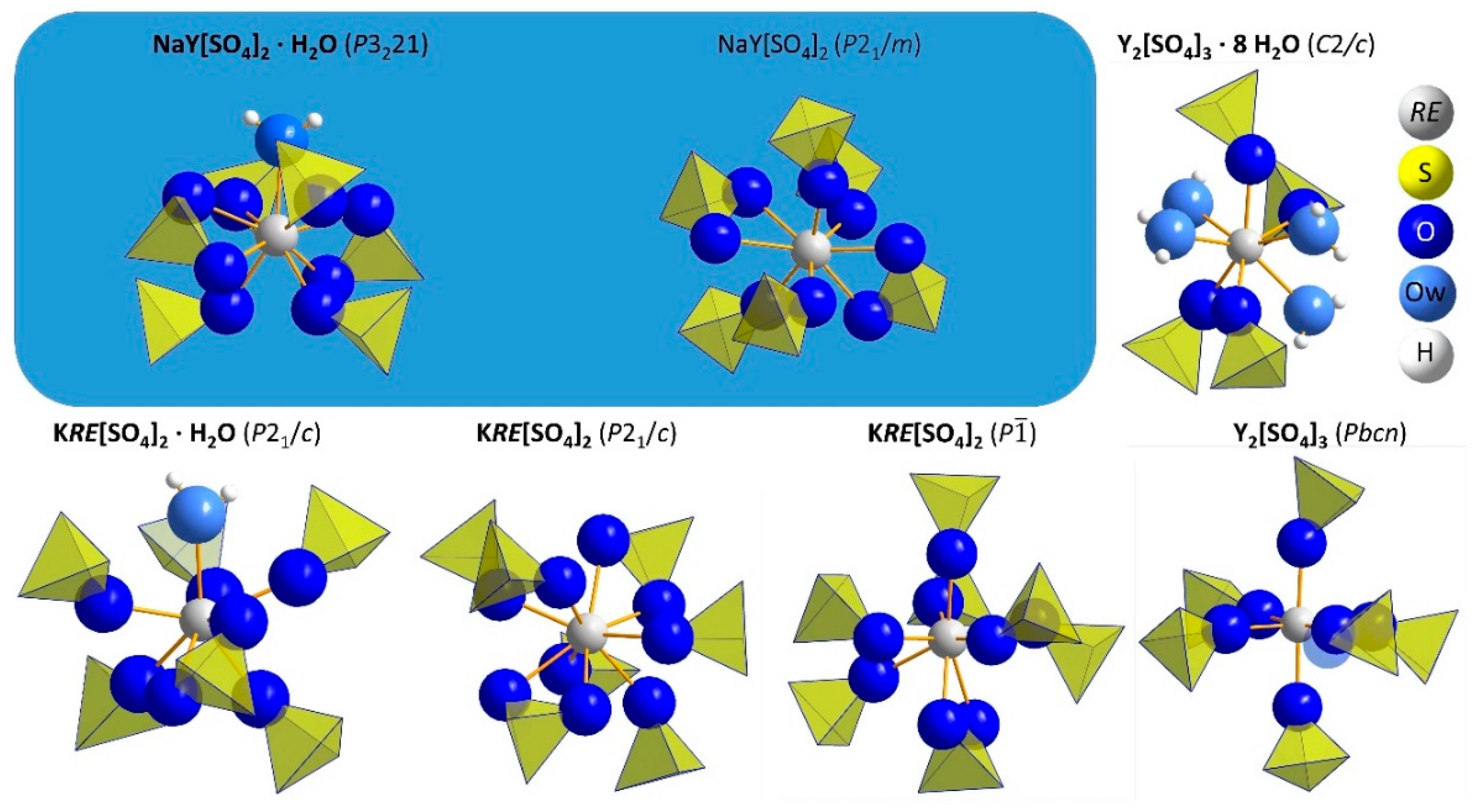
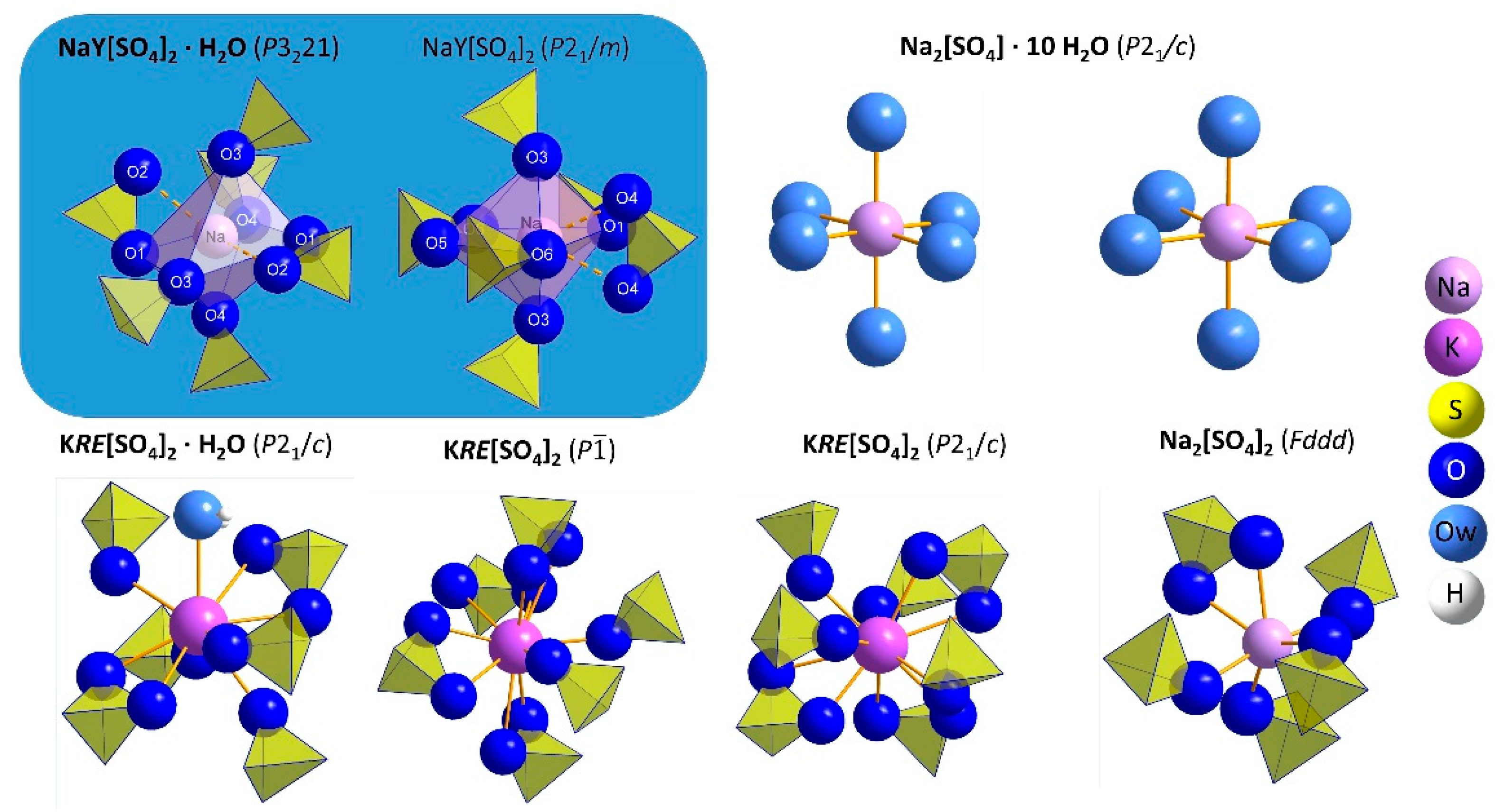

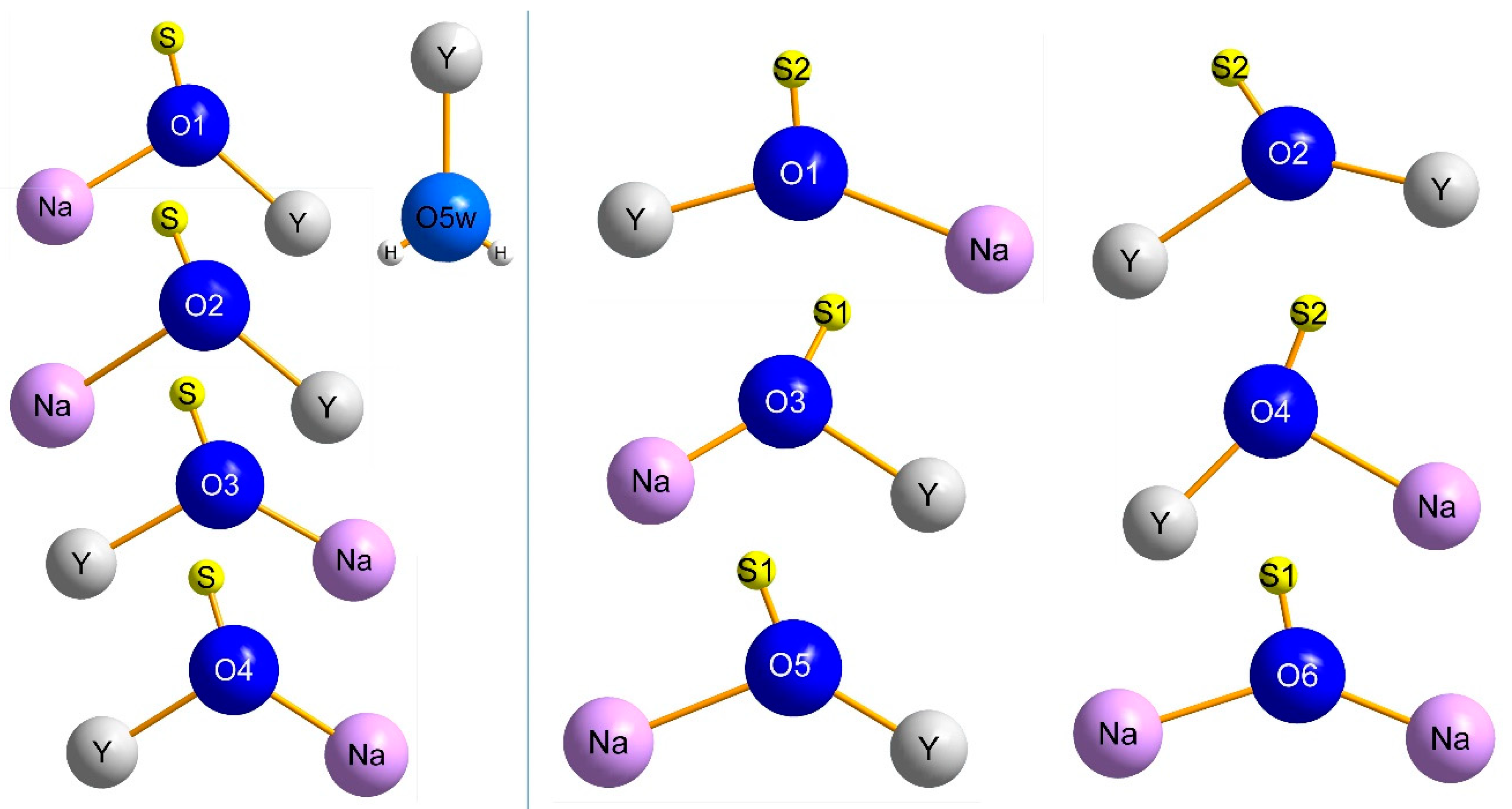






| Compound | NaY[SO4]2 ∙ H2O | NaY[SO4]2 | |
|---|---|---|---|
| Crystal system | trigonal | monoclinic | |
| Space group | P3221 (no. 154) | P21/m (no. 11) | |
| Lattice parameters, | |||
| a [pm] | 682.24(5) | 467.697(5) | |
| b [pm] | = a | 686.380(6) | |
| c [pm] | 1270.65(9) | 956.597(9) | |
| β [°] | 90 | 96.8079(5) | |
| Number of formula units, Z | 3 | 2 | |
| Unit-cell volume, Vuc [nm³] | 0.51219(4) | 0.304919(5) | |
| Molar volume, Vm [cm3 ∙ mol−1] | 102.81 | 91.81 | |
| Calculated density, Dx [g ∙ cm−3] | 3.132 | 3.311 | |
| Diffraction method | single crystal | powder | |
| Instrument | κ-CCD | Stadi-P (transmission) | |
| Radiation | Mo-Kα, λ = 71.07 pm | Cu-Kα, λ = 154.06 pm | |
| Structure resolution and refinement | SHELX-97 | FULLPROF | |
| Range in ±h, ±k, ±l | 8, 8, 16 | 4, 7, 10 | |
| Range of 2ϴ [°] | 3–55 | 8–110 | |
| Absorption coefficient, μ [mm−1] | 19.25 | − | |
| Extinction coefficient, g | 0.0174(15) | − | |
| Reflections collected | 8159 | 438 | |
| and unique | 786 | − | |
| Rint / Rσ | 0.080/0.036 | − | |
| R1 / wR2 for all reflections | 0.031/0.070 | − | |
| Goodness of Fit (GooF) | 1.074 | − | |
| Residual e— density (max. / min.) | 0.60 and −0.48 | − | |
| Flack-x parameter | −0.021(9) | − | |
| Rp | − | 4.67 | |
| Rwp | − | 7.52 | |
| Rexp | − | 4.33 | |
| χ2 | − | 3.02 | |
| CSD number | 2016596 | 2072719 |
| Atom | Wyckoff Site | Symmetry | x/a | y/b | z/c | U/pm2 |
|---|---|---|---|---|---|---|
| Na | 3b | .2. | 0.5299(3) | 0 | 1/6 | 211(5) |
| Y | 3a | .2. | 0 | 0.56341(8) | 1/3 | 145(2) |
| S | 6c | 1 | 0.9864(2) | 0.5437(2) | 0.09243(6) | 134(2) |
| O1 | 6c | 1 | 0.1273(5) | 0.5055(5) | 0.0180(2) | 217(7) |
| O2 | 6c | 1 | 0.8273(5) | 0.5829(5) | 0.0316(2) | 210(7) |
| O3 | 6c | 1 | 0.8677(5) | 0.3517(5) | 0.1655(2) | 192(7) |
| O4 | 6c | 1 | 0.1249(5) | 0.7408(5) | 0.1610(2) | 196(7) |
| O5w | 3a | .2. | 0 | 0.9123(8) | 1/3 | 369(14) |
| H | 6c | 1 | 0.063(11) | 0.957(11) | 0.042(4) | 554(36) |
| Na | 2e | m | 0.6289(11) | 1/4 | 0.3506(4) | 195(12) |
| Y | 2e | m | 0.6536(3) | 1/4 | 0.82110(12) | 167(3) |
| S1 | 2e | m | 0.1619(7) | 1/4 | 0.5875(3) | 163(9) |
| S2 | 2e | m | 0.1407(6) | 1/4 | 0.0715(3) | 183(9) |
| O1 | 2e | m | 0.8254(13) | 1/4 | 0.0738(6) | 114(18) |
| O2 | 2e | m | 0.2317(13) | 1/4 | 0.9259(6) | 105(17) |
| O3 | 4f | 1 | 0.3075(10) | 0.0730(6) | 0.6574(4) | 177(14) |
| O4 | 4f | 1 | 0.2628(10) | 0.0699(6) | 0.1470(4) | 176(13) |
| O5 | 2e | m | 0.8757(14) | 1/4 | 0.6311(6) | 119(18) |
| O6 | 2e | m | 0.1881(13) | 1/4 | 0.4401(6) | 106(18) |
| NaY[SO4]2 ∙ H2O | NaY[SO4]2 | ||||
|---|---|---|---|---|---|
| d(Y–O2) | (1×) | 236.7(3) | d(Y–O5) | (1×) | 219.8(7) |
| d(Y–O2) | (1×) | 239.7(4) | d(Y–O4) | (2×) | 224.5(4) |
| d(Y–O5W) | (1×) | 238.0(3) | d(Y–O2) | (1×) | 231.7(6) |
| d(Y–O1) | (2×) | 239.1(3) | d(Y–O3) | (2×) | 243.9(4) |
| d(Y–O4) | (2×) | 224.0(3) | d(Y–O1) | (1×) | 245.5(6) |
| d(Y–O3) | (2×) | 247.9(3) | d(Y–O2) | (1×) | 277.0(6) |
| (Y–O) | (C.N. = 9) | 241.5 | (Y–O) | (C.N. = 8) | 238.8 |
| d(Na–O3) | (2×) | 235.4(4) | d(Na–O3) | (2×) | 223.9(4) |
| d(Na–O4) | (2×) | 242.5(4) | d(Na–O6) | (1×) | 232.4(8) |
| d(Na–O1) | (2×) | 253.6(3) | d(Na–O6) | (1×) | 265.4(8) |
| d(Na–O2) | (2×) | 287.9(3) | d(Na–O4) | (2×) | 273.2(5) |
| (Na–O) | (C.N. = 8) | 254.9 | d(Na–O5) | (1×) | 279.3(7) |
| d(Na–O1) | (1×) | 290.4(7) | |||
| (Na–O) | (C.N. = 8) | 257.7 | |||
| d(S–O1) | (1×) | 146.2(3) | |||
| d(S–O2) | (1×) | 146.2(4) | d(S1–O6) | (1×) | 141.0(7) |
| d(S–O3) | (1×) | 147.4(3) | d(S1–O5) | (1×) | 144.9(8) |
| d(S–O4) | (1×) | 148.0(3) | d(S1–O3) | (2×) | 151.0(4) |
| (S–O) | (C.N. = 4) | 147.2 | (S1–O) | (C.N. = 4) | 147.4 |
| d(S2–O1) | (1×) | 147.7(7) | |||
| d(S2–O2) | (1×) | 150.4(7) | |||
| d(S2–O4) | (2×) | 150.9(4) | |||
| (S2–O) | (C.N. = 4) | 150.0 |
| NaY[SO4]2 ∙ H2O | ||||||||||
| for Y | O2 | O2’ | O5 | O1 | O1’ | O4 | O4’ | O3 | O3’ | |
| d(Y–O) [pm] | 236.68 | 236.70 | 238.03 | 239.12 | 239.13 | 243.98 | 244.06 | 247.86 | 247.93 | ∑(vij) |
| vij | 0.385 | 0.385 | 0.372 | 0.361 | 0.361 | 0.316 | 0.316 | 0.285 | 0.284 | 3.065 |
| for Na | O3 | O3’ | O4 | O4’ | O1 | O1’ | O2 | O2’ | ||
| d(Na–O) [pm] | 235.35 | 235.35 | 242.50 | 242.50 | 253.58 | 253.66 | 287.92 | 287.99 | ∑(vij) | |
| vij | 0.224 | 0.224 | 0.185 | 0.185 | 0.137 | 0.137 | 0.054 | 0.054 | 1.199 | |
| for S | O1 | O2 | O3 | O4 | for H | O5w | ||||
| d(S–O) [pm] | 101.46 | 101.46 | 101.47 | 101.48 | ∑(vij) | d(H–O) [pm] | 97.86 | |||
| vij | 1.550 | 1.549 | 1.500 | 1.477 | 6.076 | vij | 0.926 | |||
| NaY[SO4]2 | ||||||||||
| for Y | O5 | O4 | O4 | O2 | O3 | O3 | O1 | O2 | ||
| d(Y–O) [pm] | 219.77 | 224.48 | 224.48 | 231.68 | 243.87 | 243.87 | 245.53 | 277.00 | ∑(vij) | |
| vij | 0.609 | 0.536 | 0.536 | 0.441 | 0.317 | 0.317 | 0.303 | 0.130 | 3.189 | |
| for Na | O3 | O3’ | O6 | O6’ | O4 | O4’ | O5 | O1 | ||
| d(Na–O) [pm] | 223.94 | 223.94 | 232.42 | 265.37 | 272.18 | 273.18 | 279.25 | 290.39 | ∑(vij) | |
| vij | 0.305 | 0.305 | 0.242 | 0.100 | 0.083 | 0.081 | 0.068 | 0.051 | 1.234 | |
| for S1 | O6 | O5 | O3 (2×) | for S2 | O1 | O2 | O4 (2×) | |||
| d(S1–O) [pm] | 142.97 | 144.85 | 150.96 | ∑(vij) | d(S2–O) [pm] | 147.74 | 150.39 | 150.93 | ∑(vij) | |
| vij | 1.691 | 1.607 | 1.362 | 6.022 | vij | 1.486 | 1.383 | 1.363 | 5.597 | |
| Rij constant from [53] for | Y | Na | S | H | ||||||
| distance to O | 2.014 | 1.80 | 1.624 | 0.95 | Å | |||||
| NaY[SO4]2 ∙ H2O | O1 | O2 | O3 | O4 | O5w | C.N. | |
| Y | 2/1 | 2/1 | 2/1 | 2/1 | 1/1 | 9 | |
| Na | 2/1 | 2/1 | 2/1 | 2/1 | 0/0 | 8 | |
| S | 1/1 | 1/1 | 1/1 | 1/1 | 0/0 | 4 | |
| H | 0/0 | 0/0 | 0/0 | 0/0 | 1/2 | 1 | |
| C.N. | 3 | 3 | 3 | 3 | 3 | ||
| NaY[SO4]2 | O1 | O2 | O3 | O4 | O5 | O6 | C.N. |
| Y | 1/1 | 2/2 | 2/1 | 2/1 | 1/1 | 0/0 | 8 |
| Na | 1/1 | 0/0 | 2/1 | 2/1 | 1/1 | 2/2 | 8 |
| S1 | 0/0 | 0/0 | 2/1 | 0/0 | 1/1 | 1/1 | 4 |
| S2 | 1/1 | 1/1 | 0/0 | 2/1 | 0/0 | 0/0 | 4 |
| C.N. | 3 | 3 | 3 | 3 | 3 | 3 | |
| NaY[SO4]2 | NaY[SO4]2 ∙ H2O | Y2[SO4]3 ∙ 8 H2O | Y2[SO4]3 * | Na2[SO4] (thenardite) * | |
|---|---|---|---|---|---|
| [SO4]2– | |||||
δs | 413, 479 | 429, 492 | 442, 450, 467 | 452, 484, 504 | 451, 466 |
δas | 608, 629, 669 667, 685 | 628, 669 600, 626, 660 | 618 638, 652, 688, 743 | 609, 654 | 621, 632, 647 609, 634, 668 |
νs | 1015, 1046 1007, 1010, 1068 | 1020 1012, 1032, 1092 | 1015 1002, 1080, 1032 | 1013 | 933 |
νas | 1080, 1085, 1140, 1172 1120, 1140, 1289 | 1145, 1168 1135, 1165 | 1080, 1088, 1112, 1146 1132 | 1122, 1145, 1184 | 1102, 1129, 1152 1090 |
| H2O | |||||
νas,s | 3536, 3592 | 3229, 3348, 3467 | |||
δ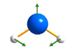 | 1606 | 1640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyer, C.; Enseling, D.; Jüstel, T.; Schleid, T. Hydrothermal Synthesis, Crystal Structure, and Spectroscopic Properties of Pure and Eu3+-Doped NaY[SO4]2 ∙ H2O and Its Anhydrate NaY[SO4]2. Crystals 2021, 11, 575. https://doi.org/10.3390/cryst11060575
Buyer C, Enseling D, Jüstel T, Schleid T. Hydrothermal Synthesis, Crystal Structure, and Spectroscopic Properties of Pure and Eu3+-Doped NaY[SO4]2 ∙ H2O and Its Anhydrate NaY[SO4]2. Crystals. 2021; 11(6):575. https://doi.org/10.3390/cryst11060575
Chicago/Turabian StyleBuyer, Constantin, David Enseling, Thomas Jüstel, and Thomas Schleid. 2021. "Hydrothermal Synthesis, Crystal Structure, and Spectroscopic Properties of Pure and Eu3+-Doped NaY[SO4]2 ∙ H2O and Its Anhydrate NaY[SO4]2" Crystals 11, no. 6: 575. https://doi.org/10.3390/cryst11060575
APA StyleBuyer, C., Enseling, D., Jüstel, T., & Schleid, T. (2021). Hydrothermal Synthesis, Crystal Structure, and Spectroscopic Properties of Pure and Eu3+-Doped NaY[SO4]2 ∙ H2O and Its Anhydrate NaY[SO4]2. Crystals, 11(6), 575. https://doi.org/10.3390/cryst11060575








