Abstract
A new organic-inorganic hybrid structure based on copper (I) iodide staircase chain 1D-Cu2I2(5-chloropyrimidine)2 (1) has been synthesized by a slow-diffusion method. It emits red emission peaking at 620 nm. The internal quantum yield (IQY) measured for this compound is 6.5% under 360 nm excitation. This compound exhibits potential as a non-rare-earth light-emitting phosphor alternative.
1. Introduction
Replacement of traditional energy-costly incandescent lamps by light-emitting diode (LED) lamps could be an important step on reducing the amount of electricity usage worldwide [1,2,3]. Since modern LEDs are typically monochromatic emitters, phosphors are usually used in combination with a monochromatic LED to generate target light with high quality for general lighting usage [4]. Examples, such as white light LED (WLED), whose emission spectrum covers the whole visible light region (400 nm–700 nm), are majorly produced by a green-yellow phosphor, Ce3+ intermingled yttrium aluminum garnet (YAG), coating onto a blue emitting InGaN/GaN diode [5]. The combination of emission from yellow phosphor and light from blue LED through the phosphor would generate white light. This type of LEDs is called phosphor-converted (pc-) LEDs. Except yellow ones, phosphors with other colors are also in significant demand for specific usages. Examples include blue phosphors, such as BaCa2MgSi2O8: Eu2+ [6] and red phosphors, such as (Ba,Sr)2Si5N8:Eu2+ [7] and so on [8]. Rare-earth (RE) metal doped with inorganic ligand, like sulfides, nitrides, oxides, oxysulfides, and oxynitrides, is the major formation of commercial phosphors today. They possess the advantages of high efficiency and stability. However, the RE metal is essential either for building the inorganic structures or to control the light quality, and RE metals, such as europium and yttrium, are at supply risk and have environmental issues [8]. What is more, their synthesis usually involves extremely high temperatures (>1000 °C) and synthetic processes are always complicated. Because of these drawbacks, developing non-rare-earth new types of phosphors that could overcome these issues is of significant importance [4,9].
There have been several types of non-rare-earth phosphors studied recently, such as inorganic nanocrystals and quantum dots [10,11]. Complicated synthetic procedure and particle size dependence are the limitation of their practical application. Organic-inorganic hybrid materials are promising phosphor candidates for their novel optical properties that arise from their hybrid nature and several structure systems have been published as rare-earth free phosphors for lighting usages [12,13,14,15,16,17,18,19,20]. Among them, structure diversity, optical tunability and facile synthesis are the particular interesting characteristics of the copper(I) iodide (I-VII semiconductors)-based hybrid materials [21,22,23,24]. Copper iodide-based organic-inorganic hybrid structures have a variety of inorganic modules [25,26]. Among them, structures that are based on copper iodide rhomboid dimer are particular interesting due to the tunable band gaps and facile synthesis [27,28,29,30]. Research reveals that the valence band is majorly made of I(5p) and Cu(3d) orbitals while the lowest unoccupied molecular orbital (LUMO) of the organic ligands contribute to the conduction band. Their band gaps could be synthetically modulated through incorporating ligands with various LUMO energies into the structures, which could lead to band gap emission at various wavelengths. This is also in agreement with the two luminescence mechanisms, i.e., halide-to-ligand charge transfer (XLCT), and metal-to-ligand charge transfer (MLCT), observed for staircase chain structures [25,31,32,33]. Among all the reported copper iodide phosphors, highly efficient low-energy emitters are particularly rare. In this paper, we report a new organic-inorganic hybrid structure on the basis of copper halide staircase chain 1D-Cu2I2(5-chloropyrimidine)2 (1) as a promising candidate for light emitting phosphors. This compound emits red emission peaking at 620 nm. The internal quantum yield (IQY) measured for 1 is 6.5% under 360 nm excitation.
2. Experimental
2.1. Materials
Acetonitrile (>99%), CuI (98%), 5-chloropyrimidine (98%), KI (99%) are obtained from Aladdin. These materials were used as received, which means no further purification was applied.
2.2. Synthesis Procedure of Compound 1
1D-Cu2I2(5-chloropyrimidine)2 single crystals were cultured by slow diffusion that has been reported previously [23]. The reactions were operated in glass bottles with lid at room temperature. There were three layers in this reaction. The bottom layer was a CuI/KI saturated aqueous solution. The middle was acetonitrile. The top layer was ligand in ethanol. The single crystals were typically generated at the middle layer in three to five days under room temperature condition. To obtain the samples of pure phase powder, the CuI (0.019 g, 0.1 mmol) in saturated KI solution was directly mixed with ligand (0.1 mmol) in ethanol. After stirring, the powder samples would normally be generated straight away. Mild heating (60 °C for 12 h) may be helpful for obtaining samples of higher crystallinity.
2.3. Single Crystal X-ray Diffraction (SC-XRD)
To collect the SXRD data, a Bruker APEX-II CCD diffractometer was used at low temperature (193 K) with graphite-monochromatic MoKα radiation (λ = 0.71073 Å). Direct methods were applied to solve the structures, and the Bruker SHELXTL package was employed to refine the data by full-matrix least-squares on F2. These data can be acquired from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_ request/cif, accessed on 24 May 2021 without any charge. The structure was loaded into the Cambridge Structural Database (CSD) with the number 1999510 (see in Supplementary Materials). Crystal data of compound 1 are shown in Table 1.

Table 1.
X-ray diffraction data of compound 1.
2.4. Powder X-ray Diffraction (PXRD)
The PXRD spectrum of compound 1 was detected by a Bruker D8 Advance automated diffraction system, in which Cu Kα radiation was used with a λ value of 1.5406 Å. A 3–50° 2θ range and a 1 °/min scan speed was adopted to gather the data. The system was operated with a power of 40 kV/40 mA at room temperature.
2.5. UV–vis Diffuse Reflectance Spectra
A Shimadzu UV-3600 UV/VIS/NIR spectrometer was used to obtain the UV–vis diffuse reflectance spectra at room temperature. The reflectance data were then converted to the Kubelka–Munk function of α/S = (1 − R)2/2R (where α is the absorption coefficient, S represents the scattering coefficient and R represents the reflectance), from which the band gap was estimated. To prepare the samples for reflectance measurements, the ground powder sample was evenly distributed between two quartz slides.
2.6. Thermogravimetric (TG) Analysis
A computer-controlled TG 550 (TA Instrument) was used to conduct the TG analysis. Pure powder samples were placed into platinum pans. Then, they were heated to 400 °C from room temperature with a 10 °C/min heating rate.
2.7. Photoluminescence Measurements
A FLS1000 spectrofluorometer was used to obtain steady-state photoluminescence spectra under room temperature condition.
2.8. Internal Quantum Yield (IQY) Measurements
To measure the IQY of crystals, a Hamamatsu Photonics C9920-03 absolute quantum yield measurement system was used, for which a 3.3 inch integrating sphere and 150 W xenon monochromatic light source were applied.
3. Results and Discussion
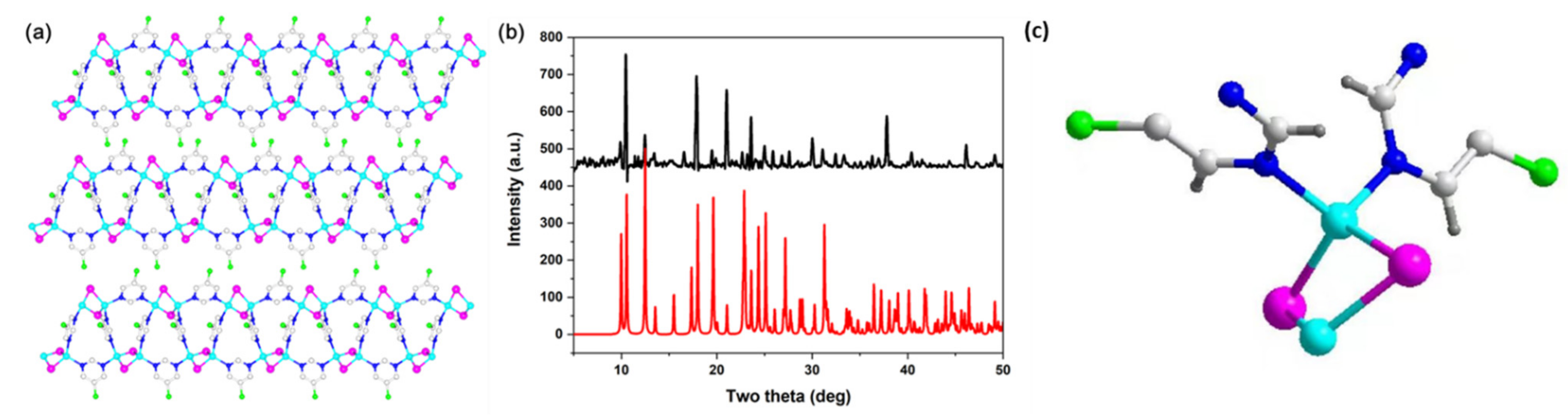
Various approaches have been developed to grow single crystals of copper iodide-based hybrid structures. Among them, the slow-diffusion method is an efficient method for obtaining high-purity crystals of Cu2I2 rhomboid dimer type structures. Because of the fast nucleation rate, these compounds generally precipitate out immediately after direct mixing of the copper iodide with the ligands at room temperature. To slow down the reaction and to promote the crystallization process, a third solvent, namely acetonitrile, was used in our synthesis as a buffer layer and was placed between two solutions containing the copper iodide (CuI/KI aqueous solution) and the ligand (in ethanol solution). With this approach, compound 1 was obtained with fine crystal samples. The analysis from single crystal X-ray diffraction demonstrated a crystallization in the orthorhombic space group Cmca for compound 1. The inorganic part of compound 1 is discrete Cu2I2 dimer. As shown in Figure 1a, the copper ions link to two iodide ions and two ligand molecules, generating a tetrahedral geometry. The Cu2I2 inorganic modules have been connected by the bidentate organic ligand molecules. The phase purity was revealed by PXRD analyses. As shown in Figure 1b, the peak positions of PXRD patterns are consistent with those simulated from single crystal X-ray data, which indicates that a pure phase is gained. It is interesting to note that the two N atoms in the pyrimidine-based ligand coordinate to Cu atoms, generating one-dimensional (1D) extended hybrid structures. Excepted pyrimidine derivatives, other bidentate ligands, such as pyrazine derivatives, are commonly used for the synthesis of multidimensional copper iodide-based hybrid structures [34]. The asymmetric unit of compound 1 has been plotted in Figure 1c.
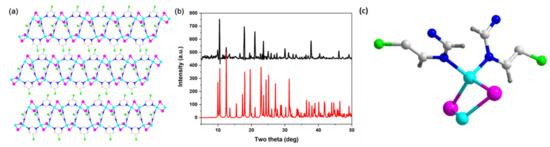
Figure 1.
(a) Structural plot of compound 1. (b) PXRD patterns of simulated 1 (red) and as made 1 (black). (c) Asymmetric unit of compound 1. (Cyan: Cu atoms; pink: I atoms; grey: C atoms; blue: N atoms; green: Cl atoms; dark grey: H atoms).
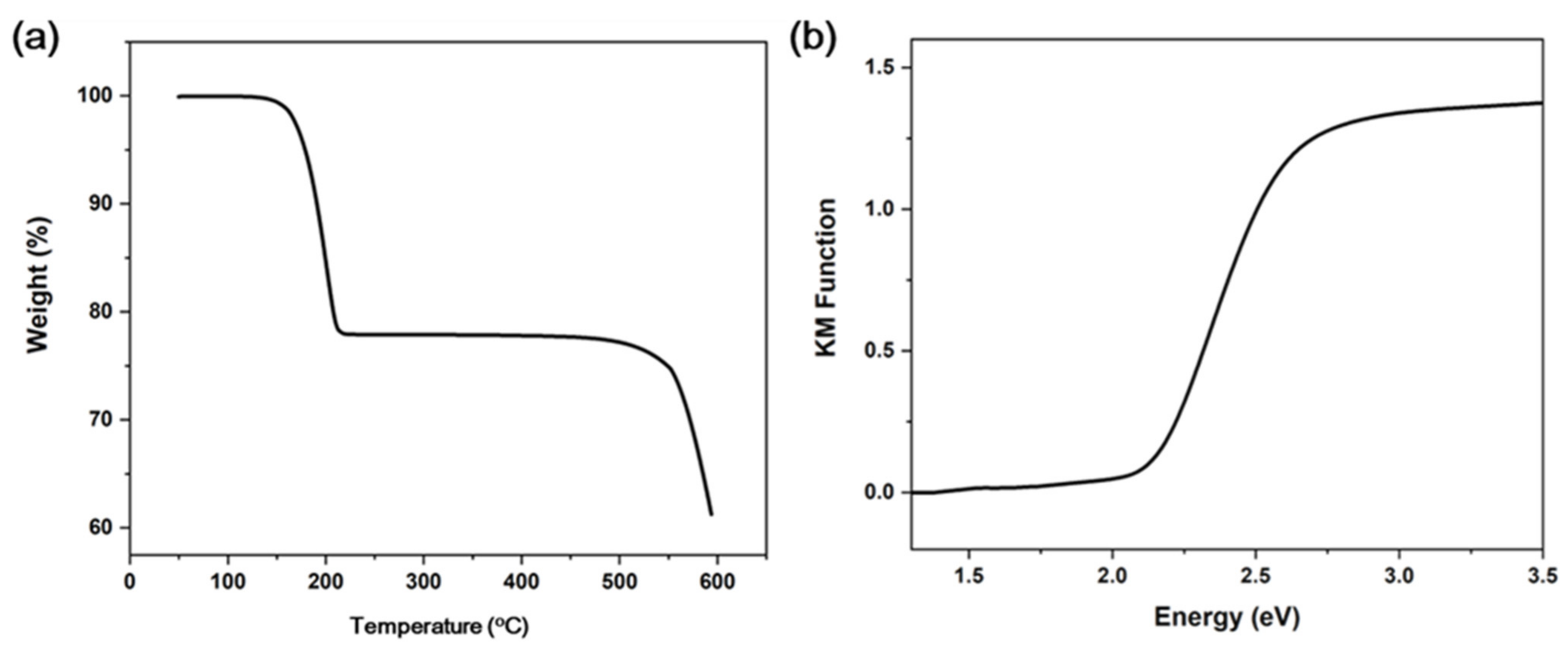
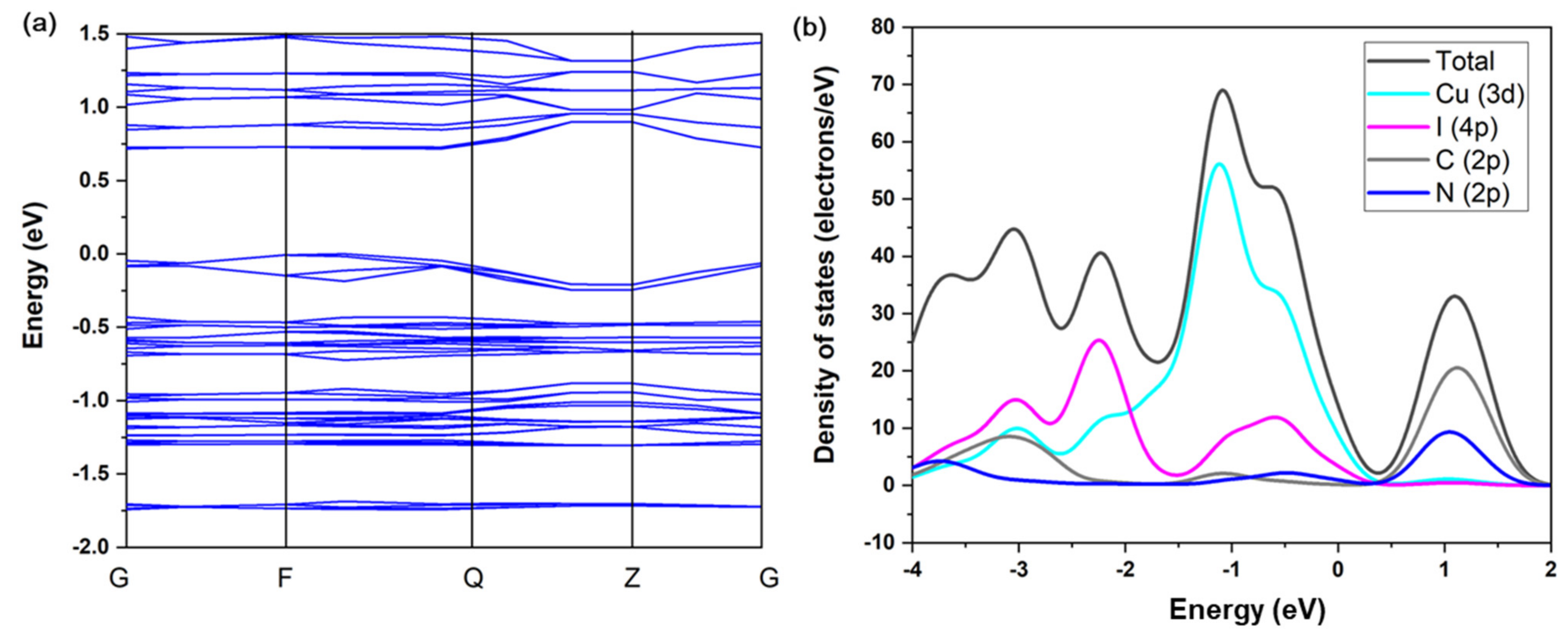
The TG plot shows that 120 °C is the decomposition temperature of compound 1 (Figure 2a). This is in accordance with other 1D copper iodide staircase chain-based structures with pyrimidine/pyrazine derivatives [35]. The use of other organic ligands forming stronger Cu-N bonds may improve the stability of these compounds. The UV absorption spectrum of compound 1 is shown in Figure 2b. The estimated energy gap of compound 1 is ∼2.1 eV. The commercial CASTEP code implemented in the Material Studio 5.0 package was used to conduct the first-principles calculations of compound 1, through which the band structure (BS) and density of states (DOS) were calculated. Generalized gradient approximations (GGA) with the Perdew–Burke–Ernzerhof (PBE) exchange-correlation functional (xc) were adopted for the simulations. The calculation result indicates that the band gap of compound 1 is 1.193 eV (Figure 3a). Such a discrepancy of the experimental and calculated band gap values results from the fact that the GGA functional typically underestimates the band gaps [36]. Energy states for the region in the valence band maximum are mainly contributed by the inorganic ingredients (i.e., Cu 3d and I 5p atomic orbitals), in contrast, the region of the conduction band minimum is majorly originated from the organic ingredients (i.e., C and N 2p atomic orbitals) (Figure 3b). Based on the calculation results, it is suggested that the luminescence mechanism of compound 1 is a combination of XLCT and MLCT. This property is similar to CuI staircase chain-based structures, the band gap of 1 may be affected by altering the organic ligands with various LUMO energies [23].
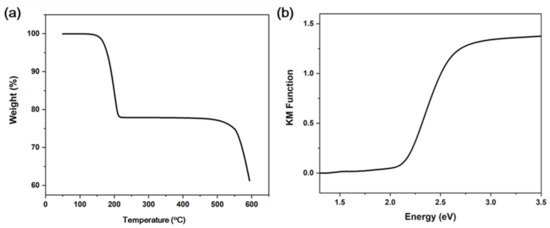
Figure 2.
(a) TGA plot of 1. (b) UV-vis absorption spectrum of 1.
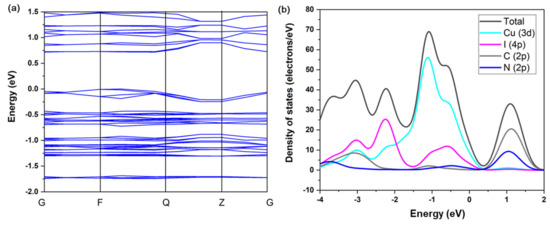
Figure 3.
(a) Simulated band structure of 1. (b) DOS plot of 1.
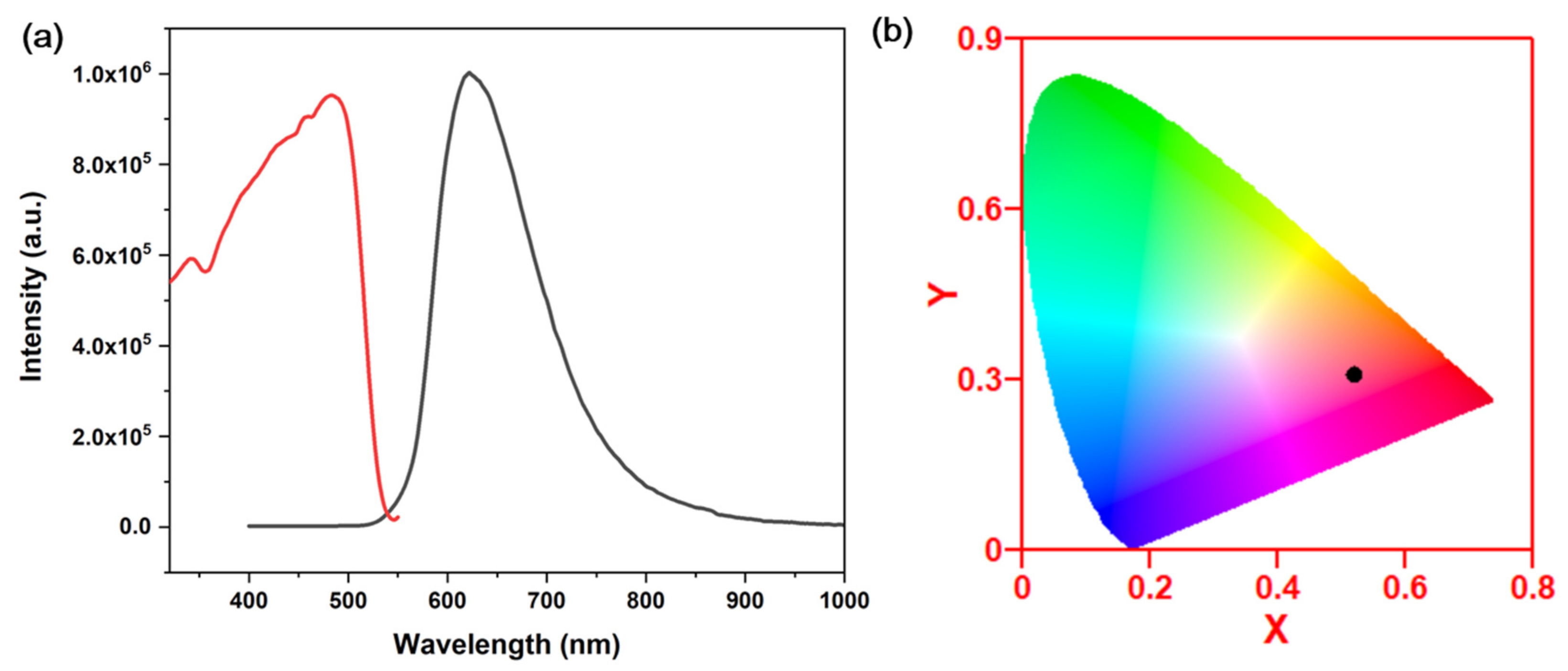
When the organic ligand is placed under UV excitation, it emits no detectable emission. Single crystals of 1 are red under natural light and emit strong red light under 360 nm wavelength UV irradiation. Figure 4a shows the emission spectra under room temperature and UV excitation. The red emission of compound 1 reaches the peak at 620 nm under room temperature condition, with a full width at half-maximum (FWHM) of around 100 nm. The Commission Internationale de l’Eclairage (CIE) chromaticity coordinates were calculated to be (0.52, 0.32) for compound 1 (Figure 4b). The IQYs were tested with a C9920-03 absolute quantum yield measurement system. In addition, the IQYs of compound 1 is 6.5% under an excitation wavelength of 360 nm. Compared to other staircase-chain-based structures, compound 1 shows a relatively higher IQY [25]. This might be because of the substituted group on the pyrimidine ring. It has been found that a structure with organic ligands with electron donating groups would enhance the IQYs of the hybrid, such as the chloro-group in this case [23].
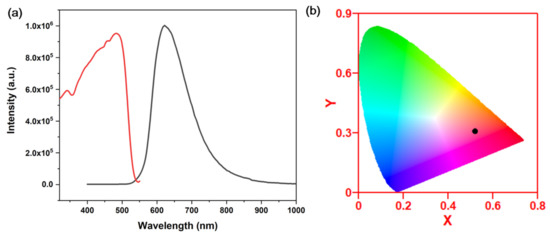
Figure 4.
(a) Excitation (red curve) and emission spectrum (black curve) of 1. λex = 360 nm, λem = 620 nm. (b) CIE coordinates of compound 1.
4. Conclusions
In summary, a new copper(I) iodide staircase-chain-based inorganic-organic hybrid structure has been cultured and characterized. This compound is demonstrated as a red-light emitter, and an IQY of 6.5% under 360 nm excitation was revealed. This implies that the compound 1 has the potential to be a rare-earth-free lighting phosphor alternative.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11060594/s1, CIF file of compound 1.
Author Contributions
Conceptualization, X.W. and W.L.; data curation, B.L.; formal analysis, B.L.; investigation, B.L. and W.L.; methodology, X.W. and W.L.; project administration, W.L.; visualization, X.W.; writing—original draft, B.L.; writing—review and editing, X.W. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 21901167 and U20A20233 and Shenzhen Science and Technology Innovation Commission, grant number JCYJ20180307102051326.
Acknowledgments
Financial support from the National Natural Science Foundation of China (grant no. 21901167 and U20A20233), Shenzhen Science and Technology Innovation Commission (grant no. JCYJ20180307102051326) is gratefully acknowledged.
Conflicts of Interest
There is no conflict of interest.
References
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Liang, J.; Ying, L.; Huang, F.; Cao, Y. Recent advances in high performance solution processed WOLEDs for solid-state lighting. J. Mater. Chem. C 2016, 4, 10993–11006. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Huang, X. Red phosphor converts white LEDs. Nat. Photonics 2014, 8, 748. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R Rep. 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Chen, L.-T.; Sun, I.L.; Hwang, C.-S.; Chang, S.-J. Luminescence properties of BAM phosphor synthesized by TEA coprecipitation method. J. Lumin. 2006, 118, 293–300. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.; Jeong, H.G.; Sohn, K.-S. Solid-State Combinatorial Screening of (Sr,Ca,Ba,Mg)2Si5N8:Eu2+ Phosphors. ACS Comb. Sci. 2011, 13, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lin, C.C.; Luo, W.; Shu, S.; Liu, Z.; Liu, Y.; Kong, J.; Ma, E.; Cao, Y.; Liu, R.-S.; et al. Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 2014, 5, 4312. [Google Scholar] [CrossRef]
- Hei, X.; Teat, S.J.; Liu, W.; Li, J. Eco-friendly, solution-processable and efficient low-energy lighting phosphors: Copper halide based hybrid semiconductors Cu4X6(L)2 (X = Br, I) composed of covalent, ionic and coordinate bonds. J. Mater. Chem. C 2020, 8, 16790–16797. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef]
- Yang, K.; Li, F.; Liu, Y.; Xu, Z.; Li, Q.; Sun, K.; Qiu, L.; Zeng, Q.; Chen, Z.; Chen, W.; et al. All-Solution-Processed Perovskite Quantum Dots Light-Emitting Diodes Based on the Solvent Engineering Strategy. ACS Appl. Mater. Interfaces 2018, 10, 27374–27380. [Google Scholar] [CrossRef]
- Wang, M.-S.; Guo, G.-C. Inorganic–organic hybrid white light phosphors. Chem. Commun. 2016, 52, 13194–13204. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Roushan, M.; Zhang, R.; Peng, J.; Zeng, H.; Li, J. Tuning and Enhancing White Light Emission of II–VI Based Inorganic–Organic Hybrid Semiconductors as Single-Phased Phosphors. Chem. Mater. 2012, 24, 1710–1717. [Google Scholar] [CrossRef]
- Ki, W.; Li, J. A Semiconductor Bulk Material That Emits Direct White Light. J. Am. Chem. Soc. 2008, 130, 8114–8115. [Google Scholar] [CrossRef]
- Mao, L.; Guo, P.; Kepenekian, M.; Hadar, I.; Katan, C.; Even, J.; Schaller, R.D.; Stoumpos, C.C.; Kanatzidis, M.G. Structural Diversity in White-Light-Emitting Hybrid Lead Bromide Perovskites. J. Am. Chem. Soc. 2018, 140, 13078–13088. [Google Scholar] [CrossRef]
- Mao, L.; Wu, Y.; Stoumpos, C.C.; Traore, B.; Katan, C.; Even, J.; Wasielewski, M.R.; Kanatzidis, M.G. Tunable White-Light Emission in Single-Cation-Templated Three-Layered 2D Perovskites (CH3CH2NH3)4Pb3Br10–xClx. J. Am. Chem. Soc. 2017, 139, 11956–11963. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, C.; Tian, Y.; Shu, Y.; Messier, J.; Wang, J.C.; van de Burgt, L.J.; Kountouriotis, K.; Xin, Y.; Holt, E.; et al. One-dimensional organic lead halide perovskites with efficient bluish white-light emission. Nat. Commun. 2017, 8, 14051. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Tian, Y.; Yuan, Z.; Clark, R.; Chen, B.; van de Burgt, L.J.; Wang, J.C.; Zhou, Y.; Hanson, K.; et al. Luminescent zero-dimensional organic metal halide hybrids with near-unity quantum efficiency. Chem. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Worku, M.; Neu, J.; Zhou, Y.; Tian, Y.; Lee, S.; Djurovich, P.; Siegrist, T.; Ma, B. Blue Emitting Single Crystalline Assembly of Metal Halide Clusters. J. Am. Chem. Soc. 2018, 140, 13181–13184. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Peng, R.; Li, M.; Li, D. Copper(I) halides: A versatile family in coordination chemistry and crystal engineering. Coord. Chem. Rev. 2010, 254, 1–18. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Wei, G.Z.; Banerjee, D.; Hu, Z.; Li, J. Systematic Approach in Designing Rare-Earth-Free Hybrid Semiconductor Phosphors for General Lighting Applications. J. Am. Chem. Soc. 2014, 136, 14230–14236. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.M.; Pike, R.D.; Sabat, M.; Bailey, R.D.; Pennington, W.T. Coordination Polymers of Copper(I) Halides. Inorg. Chem. 2000, 39, 5121–5132. [Google Scholar] [CrossRef]
- Benito, Q.; Le Goff, X.F.; Maron, S.; Fargues, A.; Garcia, A.; Martineau, C.; Taulelle, F.; Kahlal, S.; Gacoin, T.; Boilot, J.-P.; et al. Polymorphic Copper Iodide Clusters: Insights into the Mechanochromic Luminescence Properties. J. Am. Chem. Soc. 2014, 136, 11311–11320. [Google Scholar] [CrossRef]
- Liu, W.; Fang, Y.; Li, J. Copper Iodide Based Hybrid Phosphors for Energy-Efficient General Lighting Technologies. Adv. Funct. Mater. 2018, 28, 1705593. [Google Scholar] [CrossRef]
- Liu, W.; Banerjee, D.; Lin, F.; Li, J. Strongly luminescent inorganic–organic hybrid semiconductors with tunable white light emissions by doping. J. Mater. Chem. C 2019, 7, 1484–1490. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, K.; Teat, S.J.; Deibert, B.J.; Yuan, W.B.; Li, J. A mechanochemical route toward the rational, systematic, and cost-effective green synthesis of strongly luminescent copper iodide based hybrid phosphors. J. Mater. Chem. C, 5 2017, 5, 5962–5969. [Google Scholar] [CrossRef]
- Araki, H.; Tsuge, K.; Sasaki, Y.; Ishizaka, S.; Kitamura, N. Luminescence Ranging from Red to Blue: A Series of Copper(I)−Halide Complexes Having Rhombic {Cu2(μ-X)2} (X = Br and I) Units with N-Heteroaromatic Ligands. Inorg. Chem. 2005, 44, 9667–9675. [Google Scholar] [CrossRef]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent copper(I) complexes with halogenido-bridged dimeric core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3648. [Google Scholar] [CrossRef]
- Kyle, K.R.; Ryu, C.K.; Ford, P.C.; DiBenedetto, J.A. Photophysical studies in solution of the tetranuclear copper(I) clusters Cu4I4L4 (L = pyridine or substituted pyridine). J. Am. Chem. Soc. 1991, 113, 2954–2965. [Google Scholar] [CrossRef]
- Perruchas, S.; Le Goff, X.F.; Maron, S.; Maurin, I.; Guillen, F.; Garcia, A.; Gacoin, T.; Boilot, J.-P. Mechanochromic and Thermochromic Luminescence of a Copper Iodide Cluster. J. Am. Chem. Soc. 2010, 132, 10967–10969. [Google Scholar] [CrossRef]
- Näther, C.; Wriedt, M.; Jeß, I. Synthesis, Crystal Structures, and Properties of the Copper(I) Halide Coordination Polymers 2[CuX(μ-2-chloropyrazine-N, N’)] (fX = Cl, Br), 1[CuI(2-chloropyrazine-N)], and [Cu2I2(2-chloropyrazine)]. Z. Anorg. Allg. Chem. 2002, 628, 394–400. [Google Scholar] [CrossRef]
- Xu, C.; Lv, L.; Luo, D.; Liu, W. Synthesis, structure and photoluminescence properties of three copper(i) iodide based inorganic–organic hybrid structures with pyrazine derivatives. New J. Chem. 2020, 44, 14103–14107. [Google Scholar] [CrossRef]
- Muscat, J.; Wander, A.; Harrison, N.M. On the prediction of band gaps from hybrid functional theory. Chem. Phys. Lett. 2001, 342, 397–401. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).