In-Situ Reduction of Mo-Based Composite Particles during Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Experimental
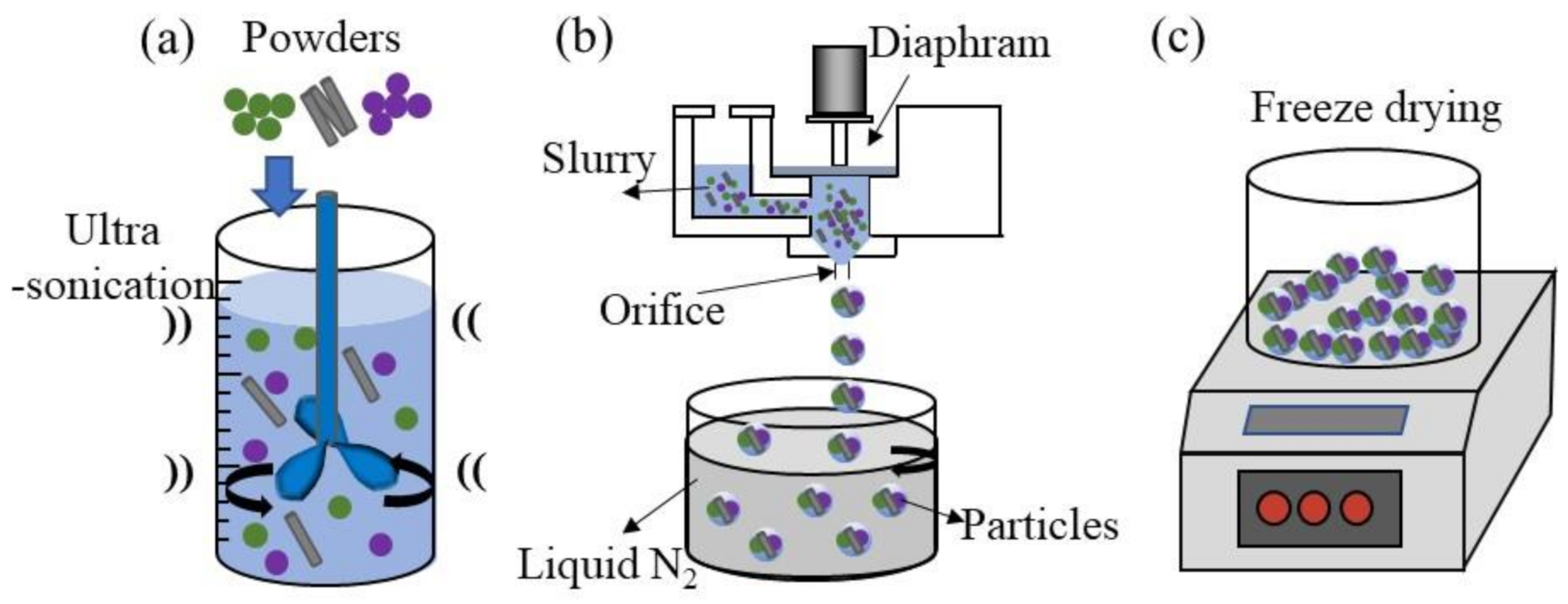
2.1. Fabrication of CNT/Mo Particles Using the FD-POEM
2.2. Reduction of Particles Processed by FD-POEM
2.3. L-PBF Processing of Particles Processed by FD-POEM
2.4. Characterizations
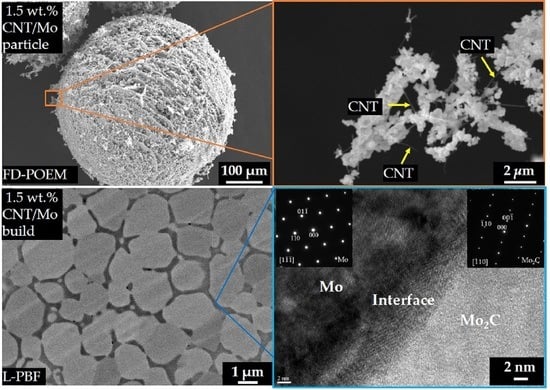
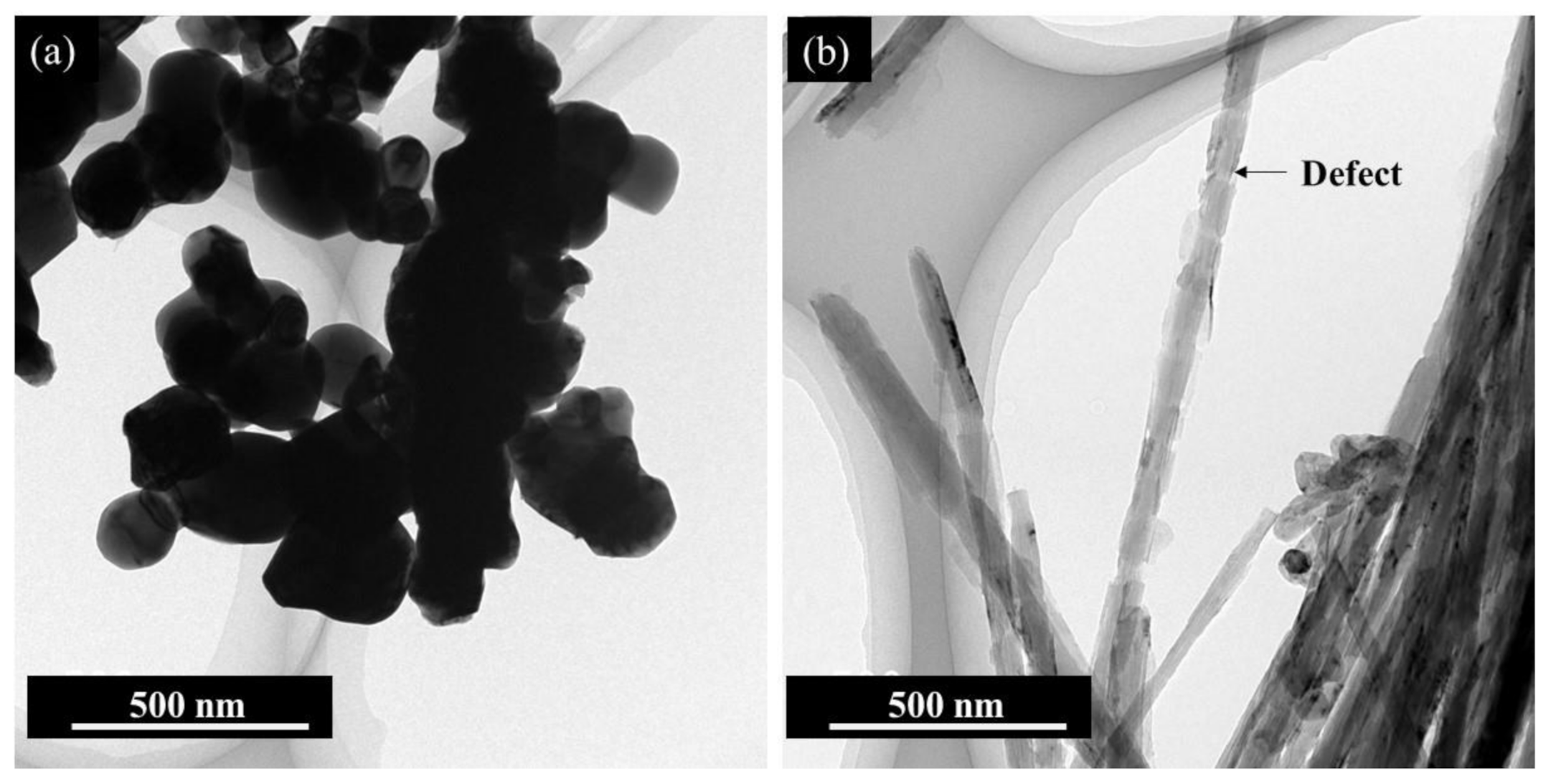
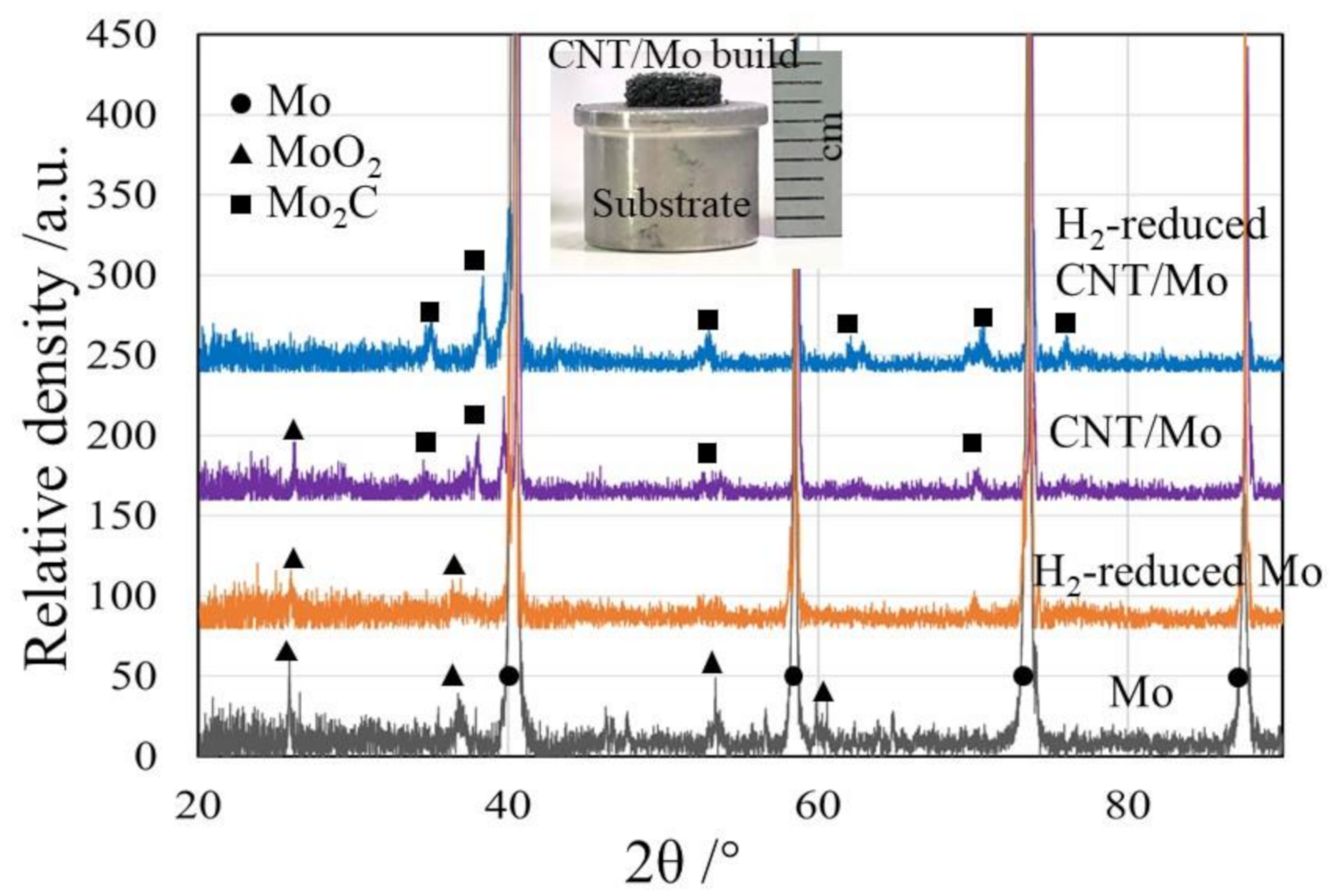
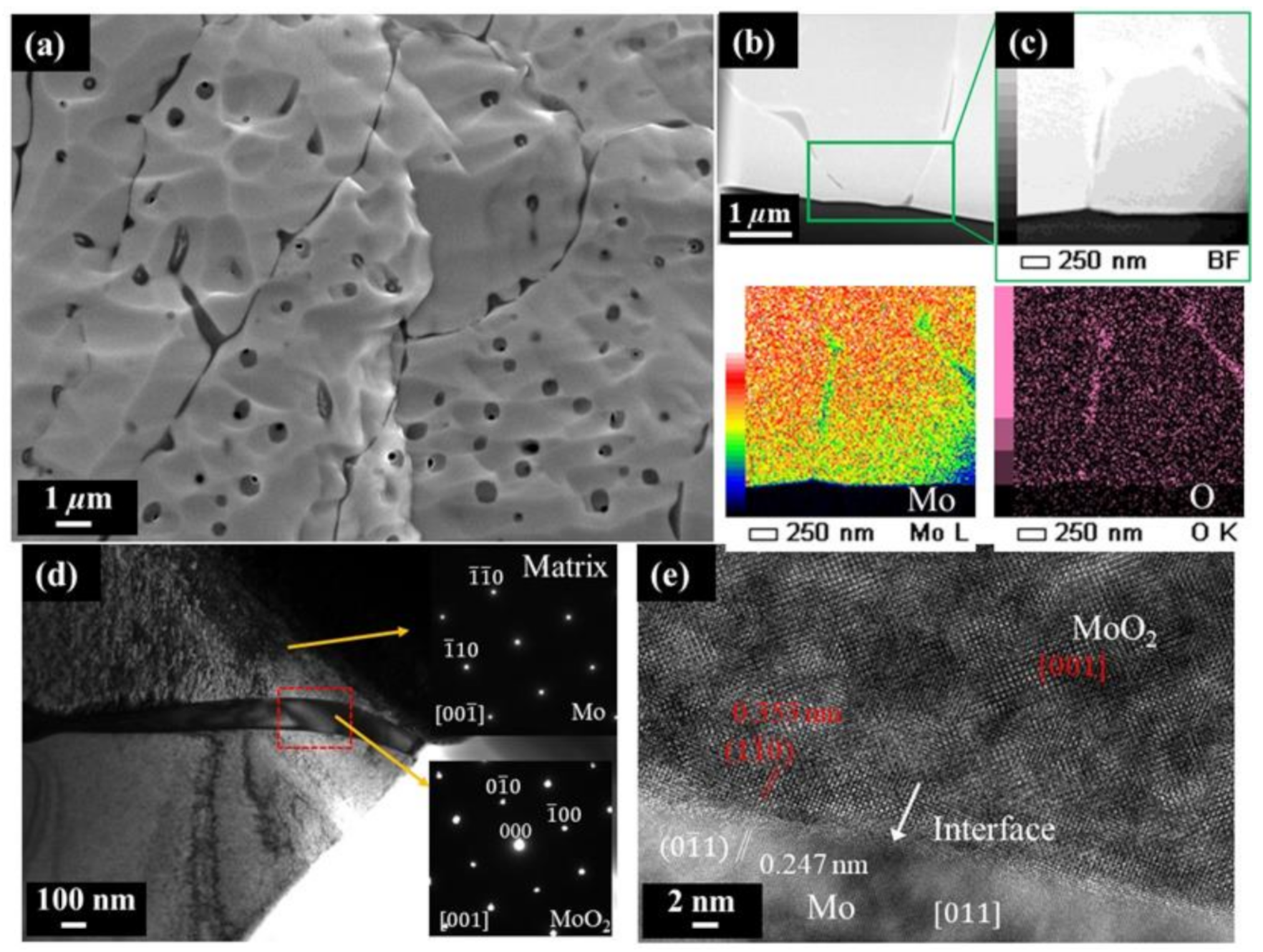
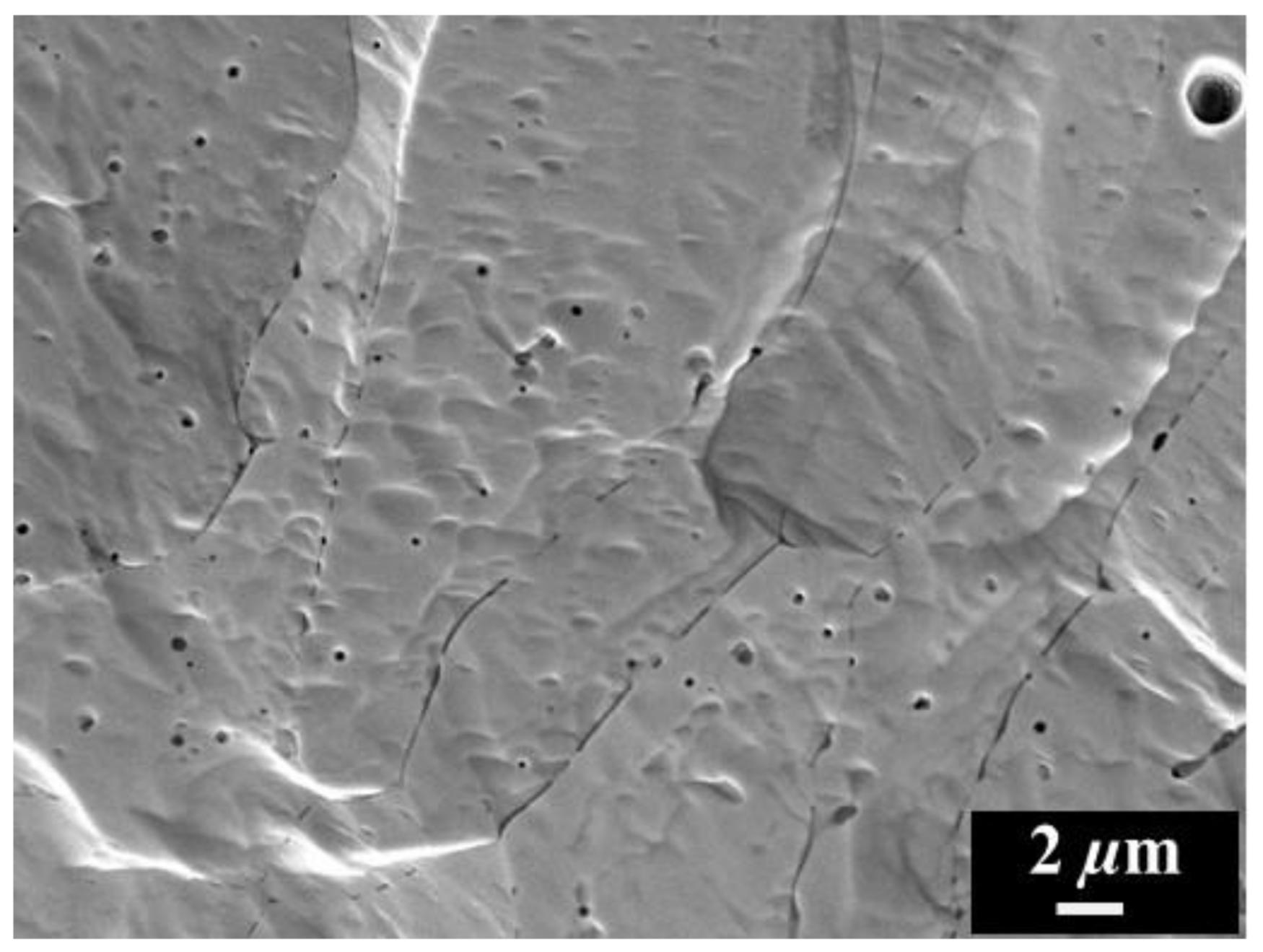
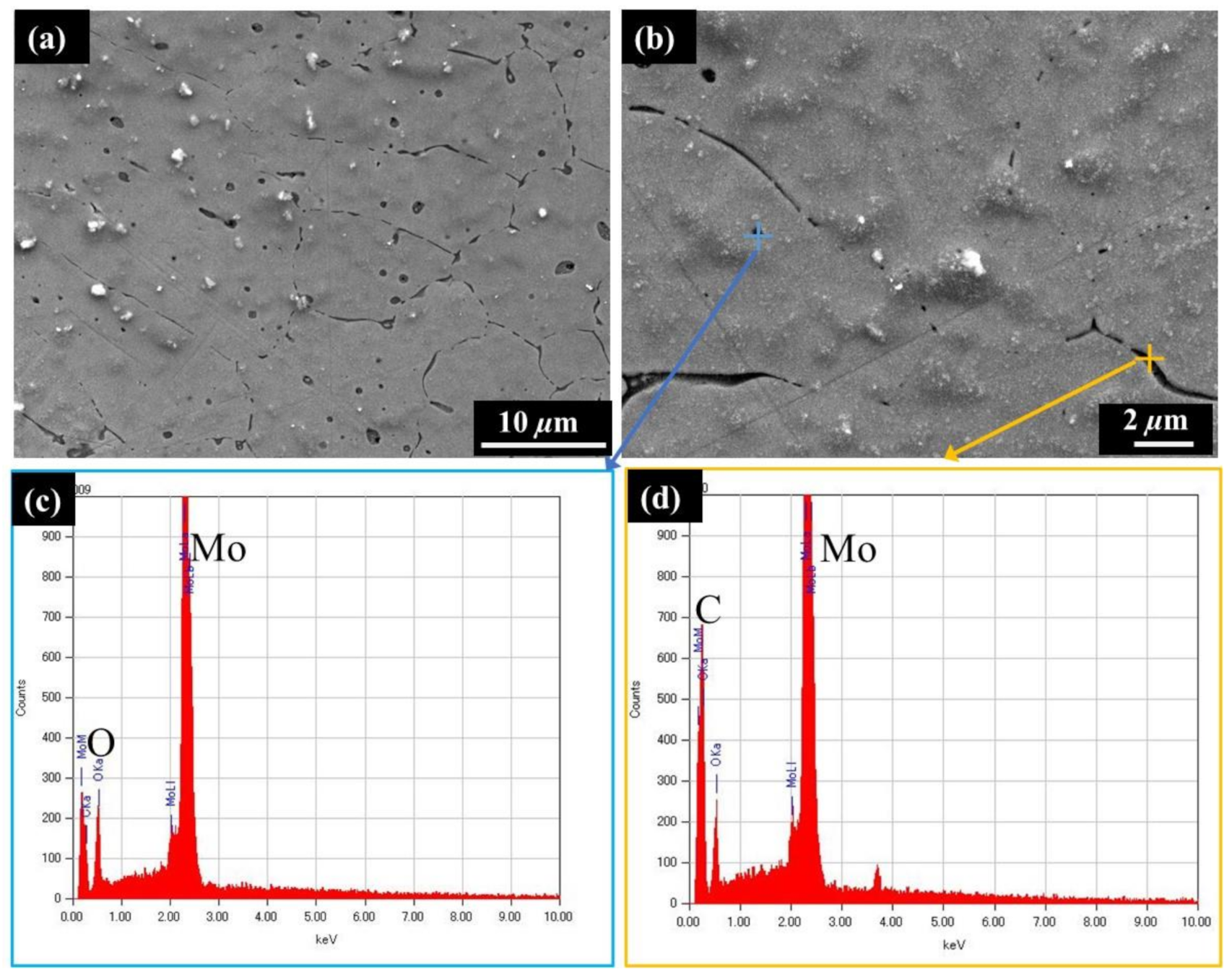
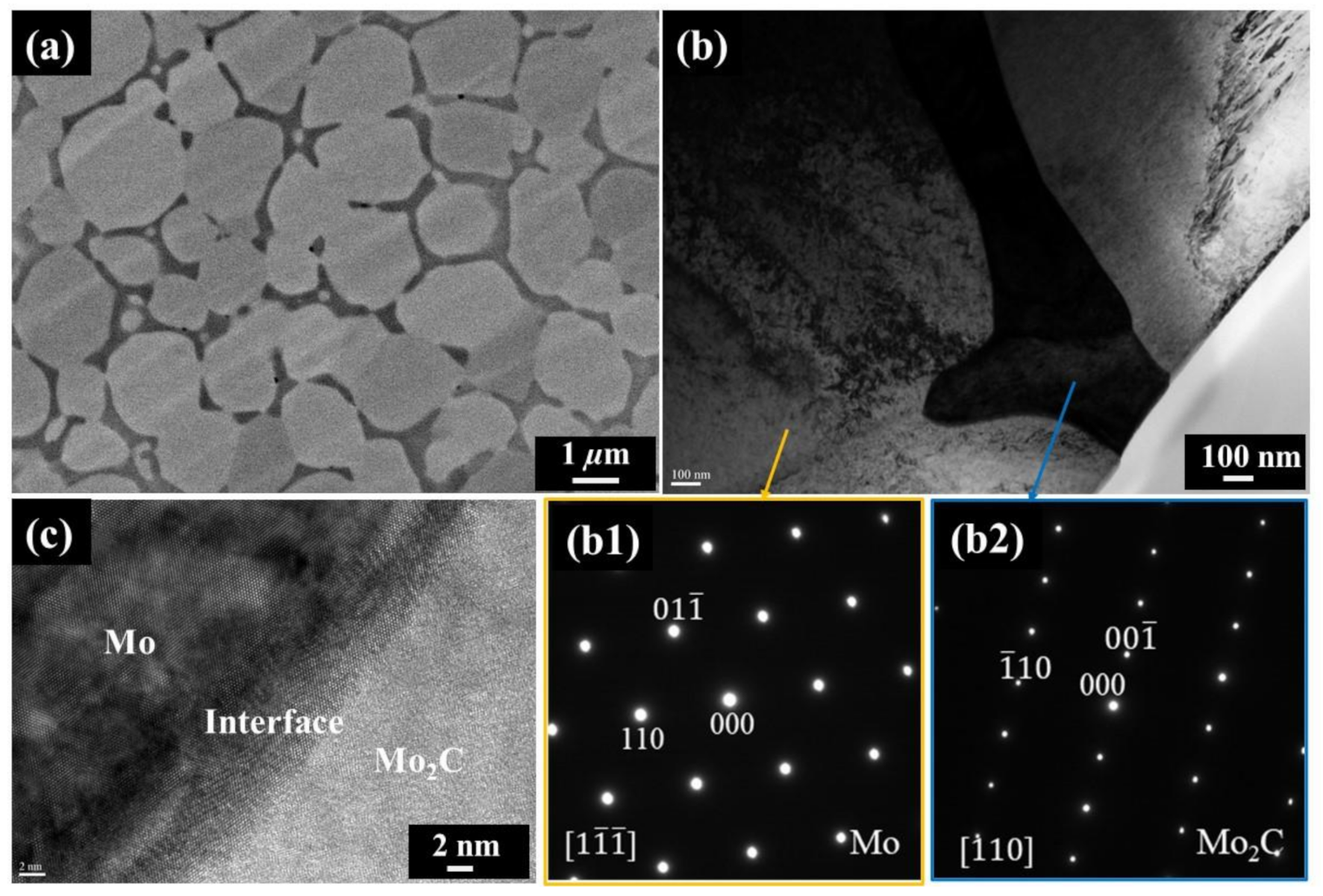
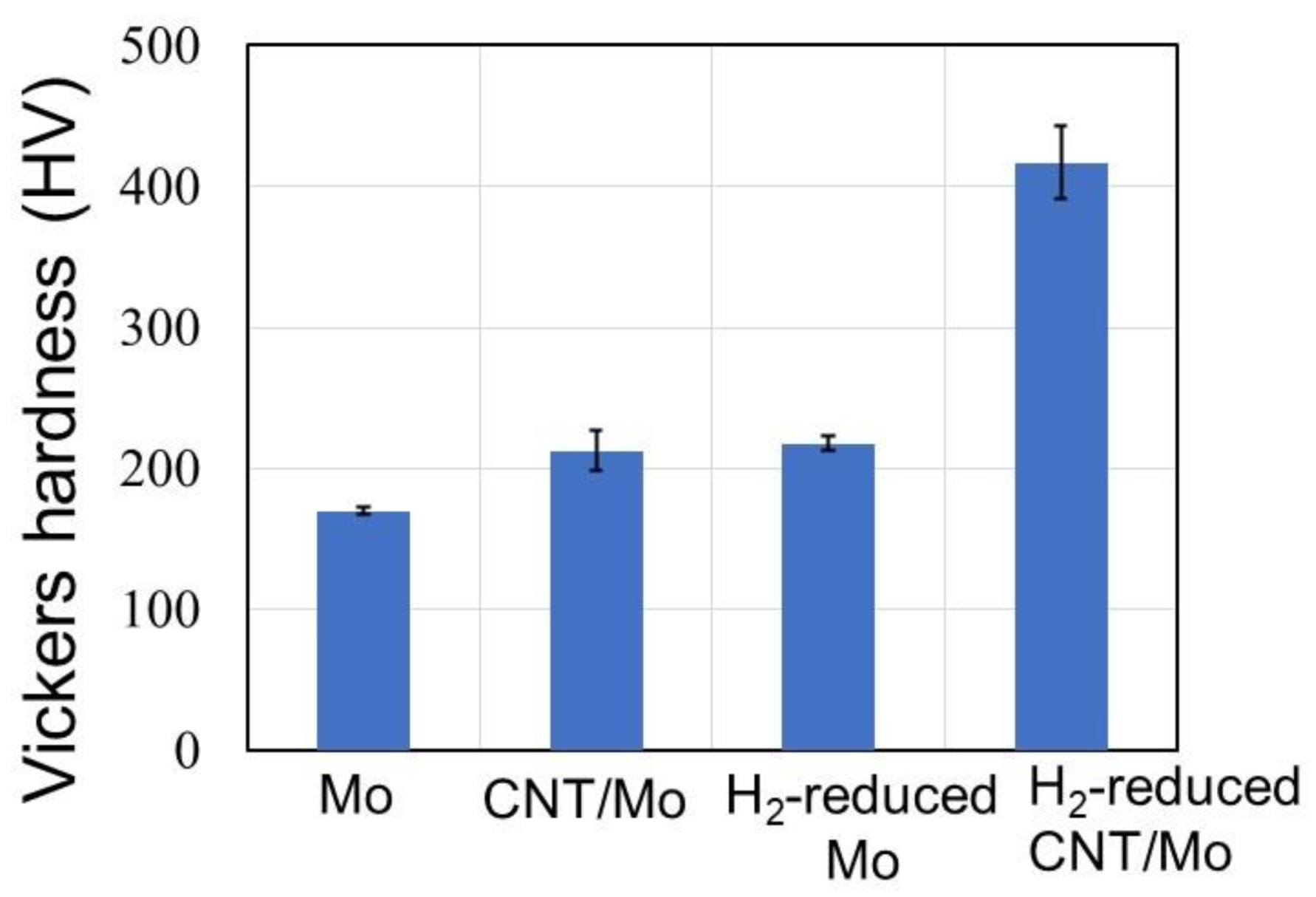
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perepezko, J.H. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Yoshimi, K.; Ha, S.-H.; Kaneko, T.; Nakamura, J.; Sato, T.; Maruyama, K.; Tu, R.; Goto, T. Phase equilibria, microstructure, and high-temperature strength of TiC-added Mo-Si-B alloys. Metall. Mater. Trans. 2014, 45, 1112–1123. [Google Scholar] [CrossRef]
- Yoshimi, K.; Nakamura, J.; Kanekon, D.; Yamamoto, S.; Maruyama, K.; Katsui, H.; Goto, T. High-temperature compressive properties of TiC-added Mo-Si-B alloys. JOM 2014, 66, 1930–1938. [Google Scholar] [CrossRef]
- Shields, J.A. Applications of Molybdenum Metal and Its Alloys, 2nd ed.; IMOA: London, UK, 2013; pp. 4–8. [Google Scholar]
- Stiefel, E.I. Molybdenum and molybdenum alloys. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: Hoboken, NJ, USA, 2000; pp. 1–16. [Google Scholar]
- Jéhanno, P.; Böning, M.; Kestler, H.; Heilmaier, M.; Saage, H.; Krüger, M. Molybdenum alloys for high temperature applications in air. Powder Metall. 2008, 51, 99–102. [Google Scholar] [CrossRef]
- Zhao, M.; Nakayama, S.; Hatakeyama, T.; Nakamura, J.; Yoshimi, K. Microstructure, high-temperature deformability and oxidation resistance of a Ti5Si3-containing multiphase MoSiBTiC alloy. Intermetallics 2017, 90, 169–179. [Google Scholar] [CrossRef]
- Cagirici, M.; Wang, P.; Ng, F.L.; Nai, M.L.S.; Ding, J.; Wei, J. Additive manufacturing of high-entropy alloys by thermophysical calculations and in situ alloying. J. Mater. Sci. Technol. 2021, 94, 53–66. [Google Scholar] [CrossRef]
- Wang, P.; Huang, P.; Ng, F.L.; Sin, W.J.; Lu, S.; Nai, M.L.S.; Dong, Z.; Wei, J. Additively manufactured CoCrFeNiMn high-entropy alloy via pre-alloyed powder. Mater. Des. 2019, 168, 107576. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, W.; Kamata, K.; Nomura, N. Microstructure and mechanical property of graphene oxide/AlSi10Mg composites fabricated by laser additive manufacturing. Mater. Charact. 2020, 170, 110678. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Rong, T.; Gu, D.; Shi, Q.; Cao, S.; Xia, M. Effects of tailored gradient interface on wear properties of WC/Inconel 718 composites using selective laser melting. Surf. Coat. Technol. 2016, 307, 418–427. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Dai, D.; Gu, D. Thermal behavior and densification mechanism during selective laser melting of copper matrix composites: Simulation and experiments. Mater. Des. 2014, 55, 482–491. [Google Scholar] [CrossRef]
- Han, Q.; Geng, Y.; Setchi, R.; Lacan, F.; Gu, D.; Evans, S.L. Macro and nanoscale wear behaviour of Al-Al2O3 nanocomposites fabricated by selective laser melting. Compos. Part B Eng. 2017, 127, 26–35. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Tsunoda, K.; Kikuchi, K.; Nomura, N.; Yoshimi, K.; Kawasaki, A. Powder fabrication and laser additive manufacturing of MoSiBTiC alloy. Intermetallics 2019, 104, 33–42. [Google Scholar] [CrossRef]
- Faidel, D.; Jonas, D.; Natour, G.; Behr, W. Investigation of the selective laser melting process with molybdenum powder. Addit. Manuf. 2015, 8, 88–94. [Google Scholar] [CrossRef]
- Kaserer, L.; Braun, J.; Stajkovic, J.; Leitz, K.H.; Singer, P.; Letofsky-Papst, I.; Leichtfried, G. Microstructure and mechanical properties of molybdenum-titanium-zirconium-carbon alloy TZM processed via laser powder-bed fusion. Int. J. Refract. Met. Hard Mater. 2020, 93, 105369. [Google Scholar] [CrossRef]
- Bajaj, P. SLM Manufacturing of Molybdenum. Master’s Thesis, The University of Sheffield, Sheffield, UK, 2016. [Google Scholar]
- Wang, D.; Yu, C.; Ma, J.; Liu, W.; Shen, Z. Densification and crack suppression in selective laser melting of pure molybdenum. Mater. Des. 2017, 129, 44–52. [Google Scholar] [CrossRef]
- Braun, J.; Kaserer, L.; Stajkovic, J.; Leitz, K.-H.; Tabernig, B.; Singer, P.; Leibenguth, P.; Gspan, C.; Kestler, H.; Leichtfried, G. Molybdenum and tungsten manufactured by selective laser melting: Analysis of defect structure and solidification mechanisms. Int. J. Refract. Met. Hard Mater. 2019, 84, 104999. [Google Scholar] [CrossRef]
- Spierings, A.B.; Herres, N.; Levy, G. Influence of the particle size distribution on surface quality and mechanical properties in AM steel parts. Rapid Prototyp. J. 2011, 17, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Yablokova, G.; Speirs, M.; Van Humbeeck, J.; Kruth, J.-P.; Schrooten, J.; Cloots, R.; Boschini, F.; Lumay, G.; Luyten, J. Rheological behavior of β-Ti and NiTi powders produced by atomization for SLM production of open porous orthopedic implants. Powder Technol. 2015, 283, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Naboychenko, S.; Neikov, O.D. Handbook of Non-Ferrous Metal Powders: Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kassym, K.; Perveen, A. Atomization processes of metal powders for 3D printing. Mater. Today Proc. 2020, 26, 1727–1733. [Google Scholar] [CrossRef]
- Higashi, M.; Ozaki, T. Selective laser melting of pure molybdenum: Evolution of defect and crystallographic texture with process parameters. Mater. Des. 2020, 191, 108588. [Google Scholar] [CrossRef]
- Leung, C.L.A.; Marussi, S.; Towrie, M.; Atwood, R.C.; Withers, P.J.; Lee, P.D. The effect of powder oxidation on defect formation in laser additive manufacturing. Acta Mater. 2019, 166, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.-Y.; Song, J.-L.; Lee, J.-S. Reaction kinetics and phase transformation during hydrogen reduction of spherical Fe2O3 nanopowder agglomerates. Powder Technol. 2016, 302, 215–221. [Google Scholar] [CrossRef]
- Kaserer, L.; Braun, J.; Stajkovic, J.; Leitz, K.-H.; Tabernig, B.; Singer, P.; Letofsky-Papst, I.; Kestler, H.; Leichtfried, G. Fully dense and crack free molybdenum manufactured by Selective Laser Melting through alloying with carbon. Int. J. Refract. Met. Hard Mater. 2019, 84, 105000. [Google Scholar] [CrossRef]
- Zhou, W.; Sasaki, S.; Kawasaki, A. Effective control of nanodefects in multiwalled carbon nanotubes by acid treatment. Carbon 2014, 78, 121–129. [Google Scholar] [CrossRef]
- Zhou, W.; Kikuchi, K.; Nomura, N.; Yoshimi, K.; Kawasaki, A. Novel laser additive-manufactured Mo-based composite with enhanced mechanical and oxidation properties. J. Alloy. Compd. 2020, 819, 152981. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| Laser power, P (W) | 20.6 |
| Scanning speed, ν (mm·s−1) | 20 |
| Hatch distance, h (μm) | 100 |
| Layer thickness, t (μm) | 25 |
| Oxygen in atmosphere | <0.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Zhou, W.; Zhou, Z.; Fan, Y.; Luo, W.; Nomura, N. In-Situ Reduction of Mo-Based Composite Particles during Laser Powder Bed Fusion. Crystals 2021, 11, 702. https://doi.org/10.3390/cryst11060702
Guo S, Zhou W, Zhou Z, Fan Y, Luo W, Nomura N. In-Situ Reduction of Mo-Based Composite Particles during Laser Powder Bed Fusion. Crystals. 2021; 11(6):702. https://doi.org/10.3390/cryst11060702
Chicago/Turabian StyleGuo, Suxia, Weiwei Zhou, Zhenxing Zhou, Yuchi Fan, Wei Luo, and Naoyuki Nomura. 2021. "In-Situ Reduction of Mo-Based Composite Particles during Laser Powder Bed Fusion" Crystals 11, no. 6: 702. https://doi.org/10.3390/cryst11060702
APA StyleGuo, S., Zhou, W., Zhou, Z., Fan, Y., Luo, W., & Nomura, N. (2021). In-Situ Reduction of Mo-Based Composite Particles during Laser Powder Bed Fusion. Crystals, 11(6), 702. https://doi.org/10.3390/cryst11060702