Abstract
Alkali-activated materials are alternative building binders, where secondary raw materials are processed. The possibility of using landfilled waste materials in the building industry increases their potential application in construction practice, and they are therefore subject to extensive research, especially in recent years. This paper briefly summarizes the interesting results of an experiment aimed at verifying the possibility of applying cement by-pass dust (CBPD) in the preparation of alkali-activated materials. The research was focused on the possibilities of using these wastes for the preparation of small elements of garden architecture. This work also briefly summarized the interesting results of an experiment aimed at verifying the possibility of applying cement by-pass dust (CBPD) in the preparation of alkali-activated materials. In the experiment, a mixture of blast furnace granulated slag, fly ash and cement by-pass dust was alkali activated with sodium metasilicate.
1. Introduction
In the current construction industry, Portland cement and materials based on it still play a major role in the field of binders [1,2]. However, due to the limited reserves of limestone as well as suitable aggregates, there are already concerns about the long-term development and possibilities of using the raw materials needed for its production. Therefore, many producers of concrete are already looking for alternative binder systems that could replace Portland cement-based materials or considering the possibility that Portland cement could be used at a minimum. Thus, various hybrid cements, ternary binders, alkali-activated systems, and others are slowly beginning to be used [3,4,5].
In the alternative binders, cement is partially or completely replaced by other binders. Hybrid cements are a type of binder that combines the use of small amounts of Portland cement, mineral admixtures, and alkaline activation [3]. Ternary binders are materials based on gypsum binder with pozzolan and exciter (calcium hydroxide, Portland cement) [6,7]. Alkaline activated systems are based on alkaline activation of latently hydraulic or pozzolanic materials. In the preparation of these materials, secondary raw materials are used, most often fly ashes (FA) and slags [4,5,8].
In the preparation of alkali-activated materials, the best required properties are probably reached in the systems based on alkali-activated blast furnace finely ground granular slag (BFS). However, as this is widely used in blast furnace cements, this former waste raw material is now becoming scarce [9,10]. The effort of many research works is therefore directed to the partial replacement of slag by other raw materials of a waste nature. A partial replacement of the fly ash seems possible [11,12,13]. At present, the application of fly ash is focused on fly ash after denitrification. The requirements for nitrogen reduction has led to the introduction of denitrification methods [14]. The denitrification process causes the nitrogen compounds to remain bound in the fly ash in various forms. This phenomenon has a positive effect on the air, but in the field of the application of fly ash to building materials, it brings new research topics, as this modification also changes some properties of the original material [15,16].
Another potential additive usable in alkali activated systems is a cement by-pass dust (CBPD). It is a fine material arising in various stages of cement production. These fine particles are trapped so that they do not escape into the air and do not burden the environment. When installing a by-pass, kiln gases are sucked out and, with rapid cooling, undesired gases condense on the surface of the dust particles. It is therefore a question of how to use these trapped particles, given their diverse composition and properties. One possible application that has been widely tested is in alkali activated systems [17,18,19,20,21,22].
2. Materials and Methods
2.1. Blast-Furnace Granulated Slag (BFS)
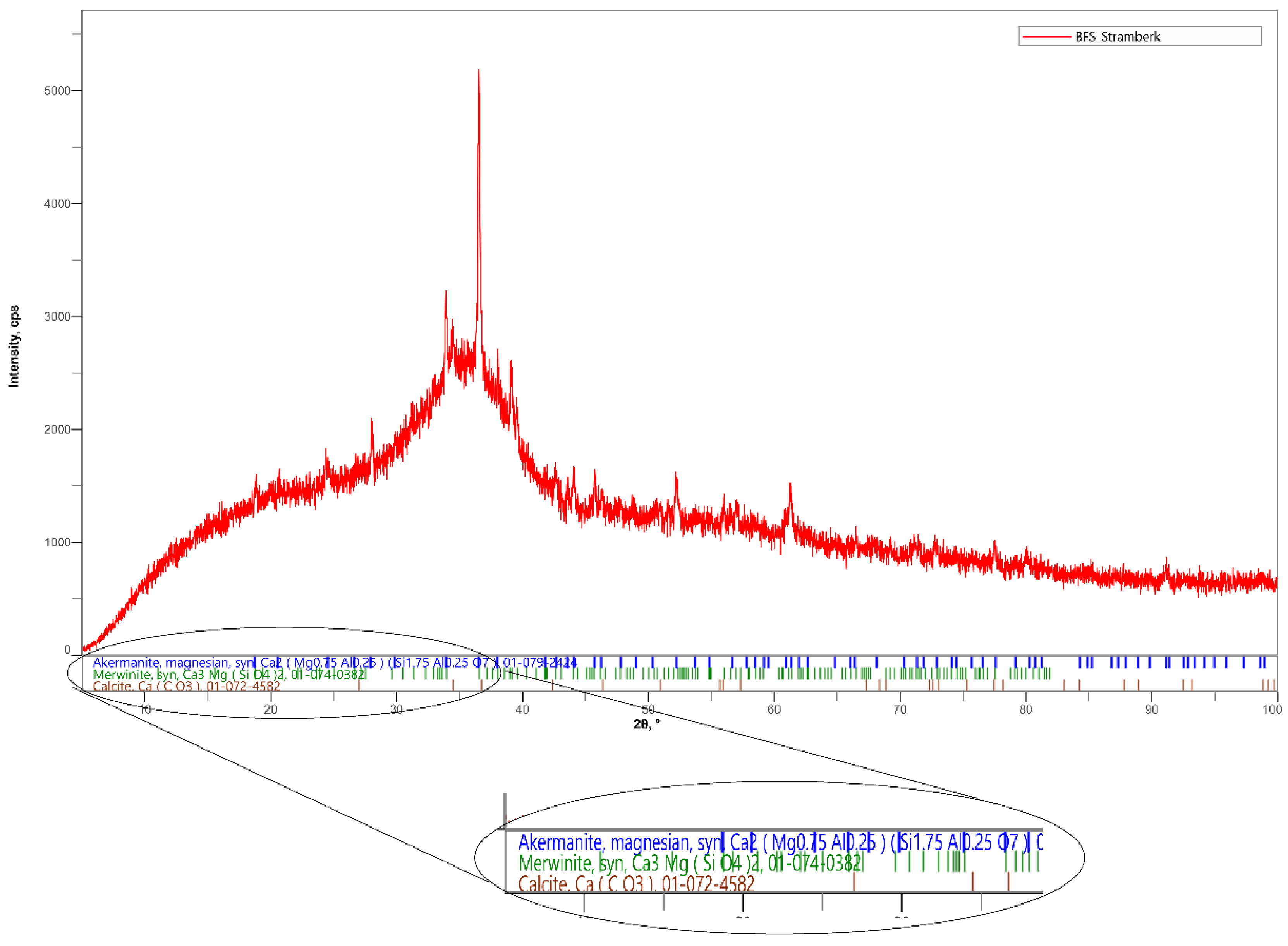
For the experiment, finely ground granulated blast furnace slag was used. This slag has latent hydraulic properties and a surface area of 400 m2/kg [23]. The percentages of individual oxides obtained by a fluorescence spectrometer measurement are shown in Table 1. Mineralogical composition of BFS includes akermanite, merwinite and calcite, as shown in Figure 1 [24].

Table 1.
Content of selected oxides in input raw materials. (FA—fly ashes, LOI—Loss on Ignition).
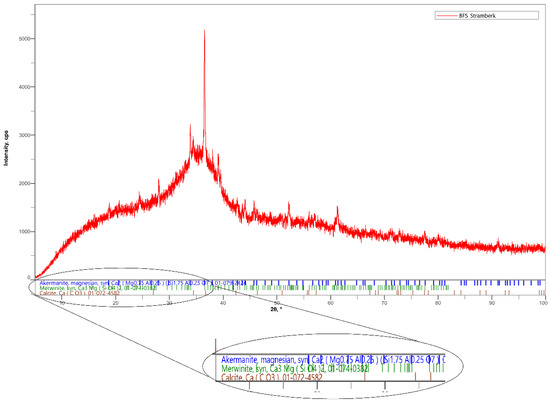
Figure 1.
X-ray diffraction (XRD) pattern of BFS.
2.2. Silica Fly Ash (FA)
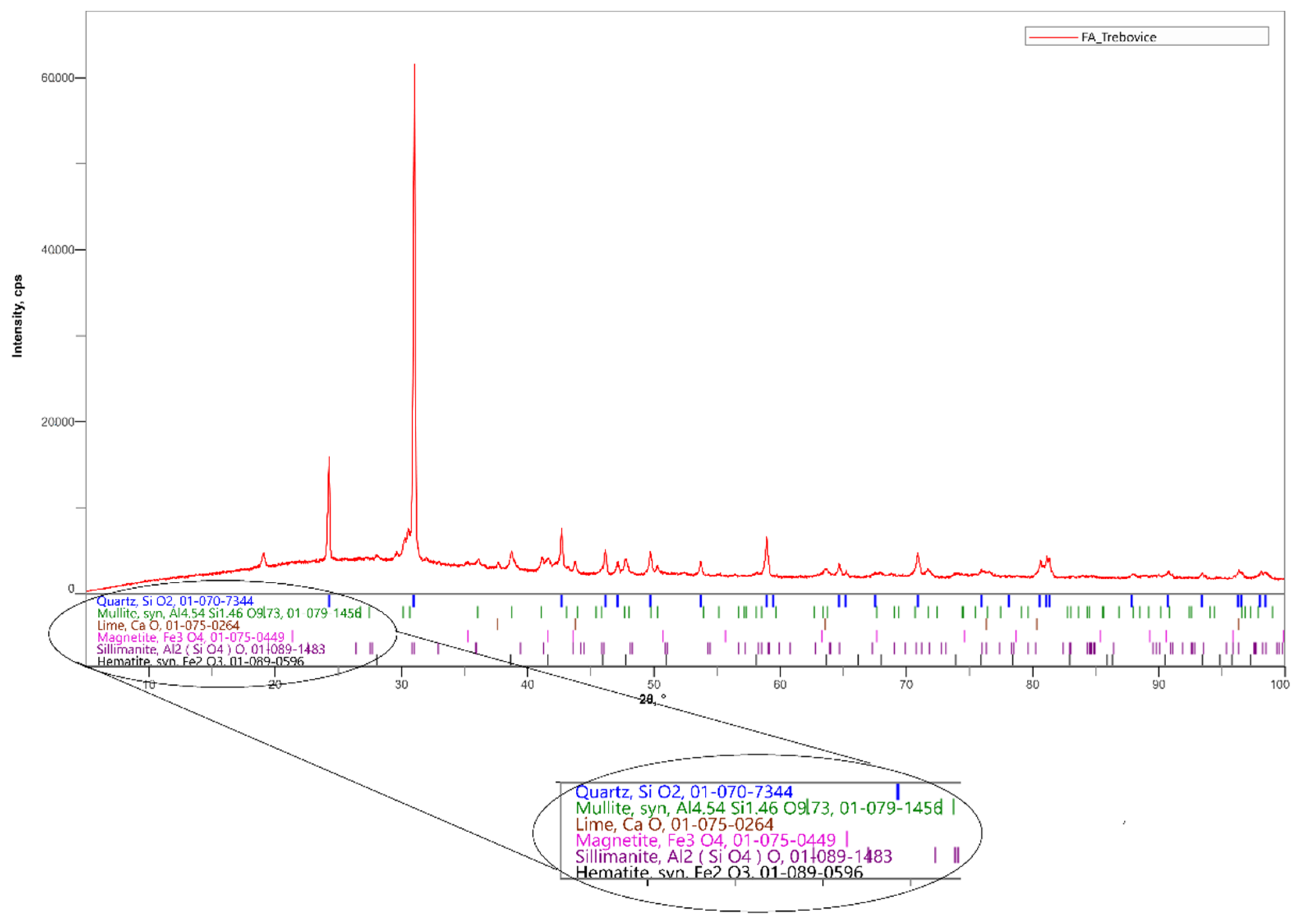
In the experiment, a siliceous fly ash after denitrification by the SNCR (Selective non-catalytic reduction) method and ground fly ash with a surface area of 500 m2/kg were used. This fly ash is produced by a power plant in Ostrava-Třebovice. The content of ammonia released from the aqueous extract is 22.8 mg/kg. The chemical composition is shown in Table 1. The mineralogical composition of the fly ash was determined by X-ray diffraction. SiO2 is present in the fly ash in the form of α-quartz (Figure 2). Other minerals represented in this fly ash include mulite, magnetite, free lime and hematite. The content of the soluble amorphous phase determined by cooking in a 4 M solution of potassium hydroxide was only 4.17%, so it can be assessed that it is a low reactive fly ash [24,25].
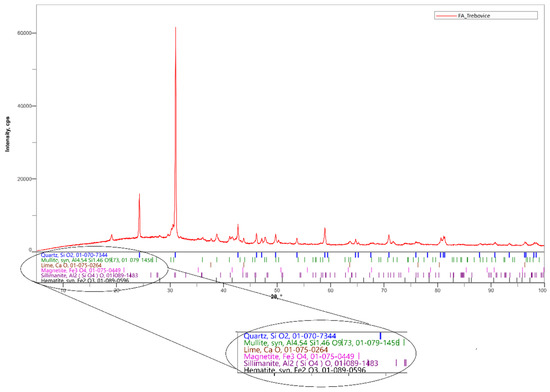
Figure 2.
Mineralogical composition of fly ash determined by X-ray diffraction (XRD).
2.3. Cement By-Pass Dust (CBPD)
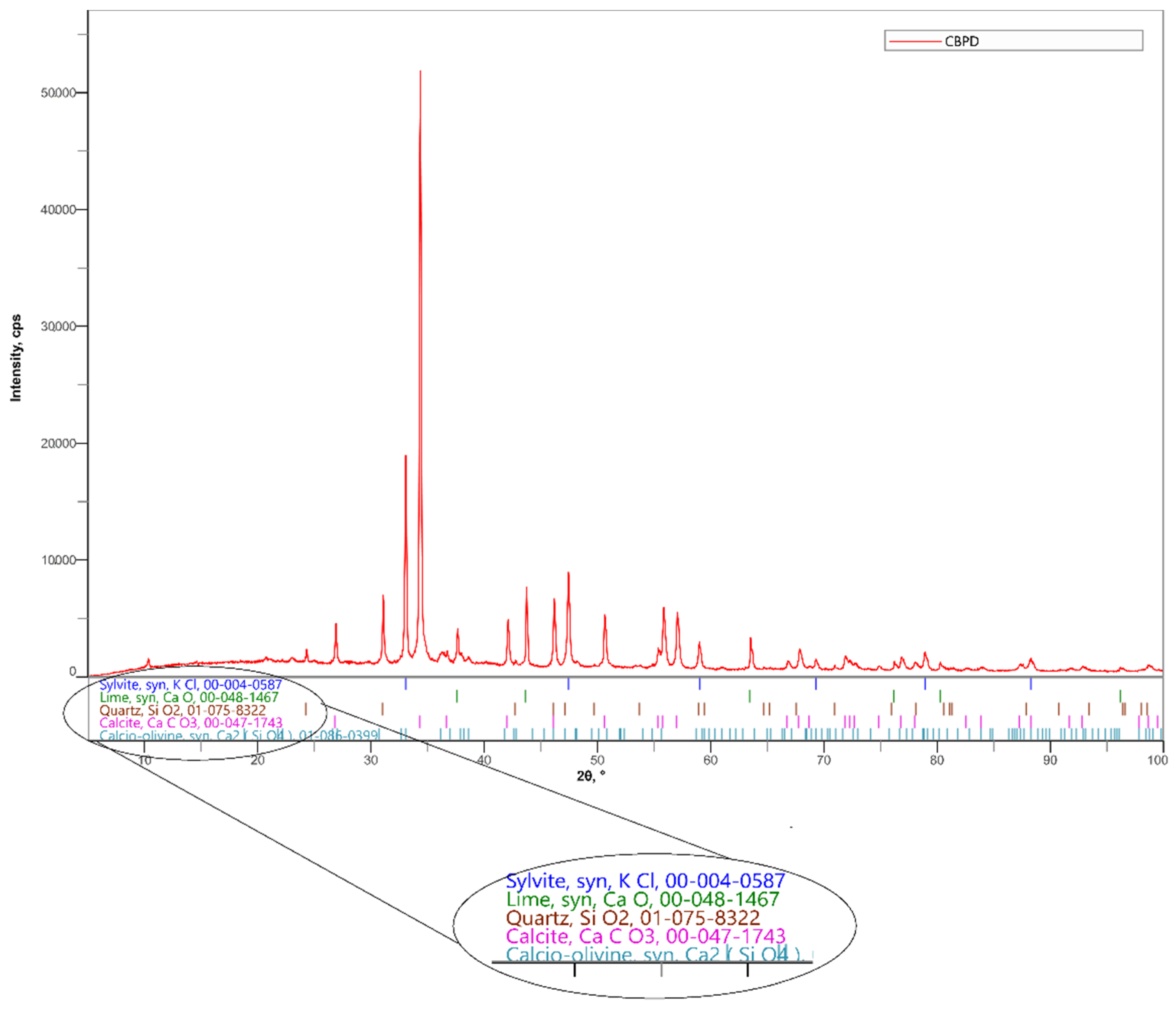
Cement by-pass dust from a cement plant in Horné Srnie was utilized in the experiment. The percentages of individual oxides obtained by a fluorescence spectrometer measurement are shown in Table 1. The chlorine content in CBPD is 10.49%. The phase composition of CBPD is given in Figure 3 and Table 2. The Loss on Ignition (LOI) is 21.9% for CBPD.
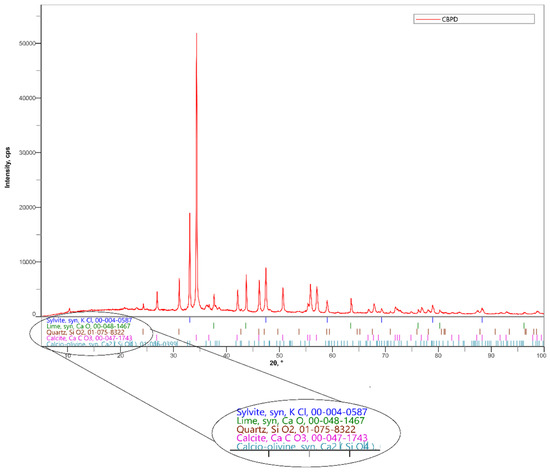
Figure 3.
Mineralogical composition of CBPD determined by X-ray diffraction (XRD).

Table 2.
Cement by-pass dust composition (in wt%) measured by XRD.
The chemical and phase composition of CBPD is taken from the report “Deliverable D1.1 Results from raw materials analyzes” within the GeoDust project. The evaluation of the measurements was carried out using the software HighScore (Malvern, Worcestershire, UK).
2.4. Activator—Anhydrous Disodium Metasilicate (A)
Anhydrous disodium metasilicate (MSS) contains min. 44% SiO2 and it was produced by Penta Chemicals [26]. This activator has a silicate modulus of 1. The basic chemical properties are given in Table 3.

Table 3.
Composition of anhydrous disodium metasilicate.
2.5. Standardized Sand
Standard sand CEN, ČSN EN 196-1 was used as a filler in the experiment. It is a natural quartz sand, which is formed by rounded particles and the silica content is min. 98%, 0/2 mm fraction and less than 0.2% moisture content [27].
2.6. Mixture Preparation
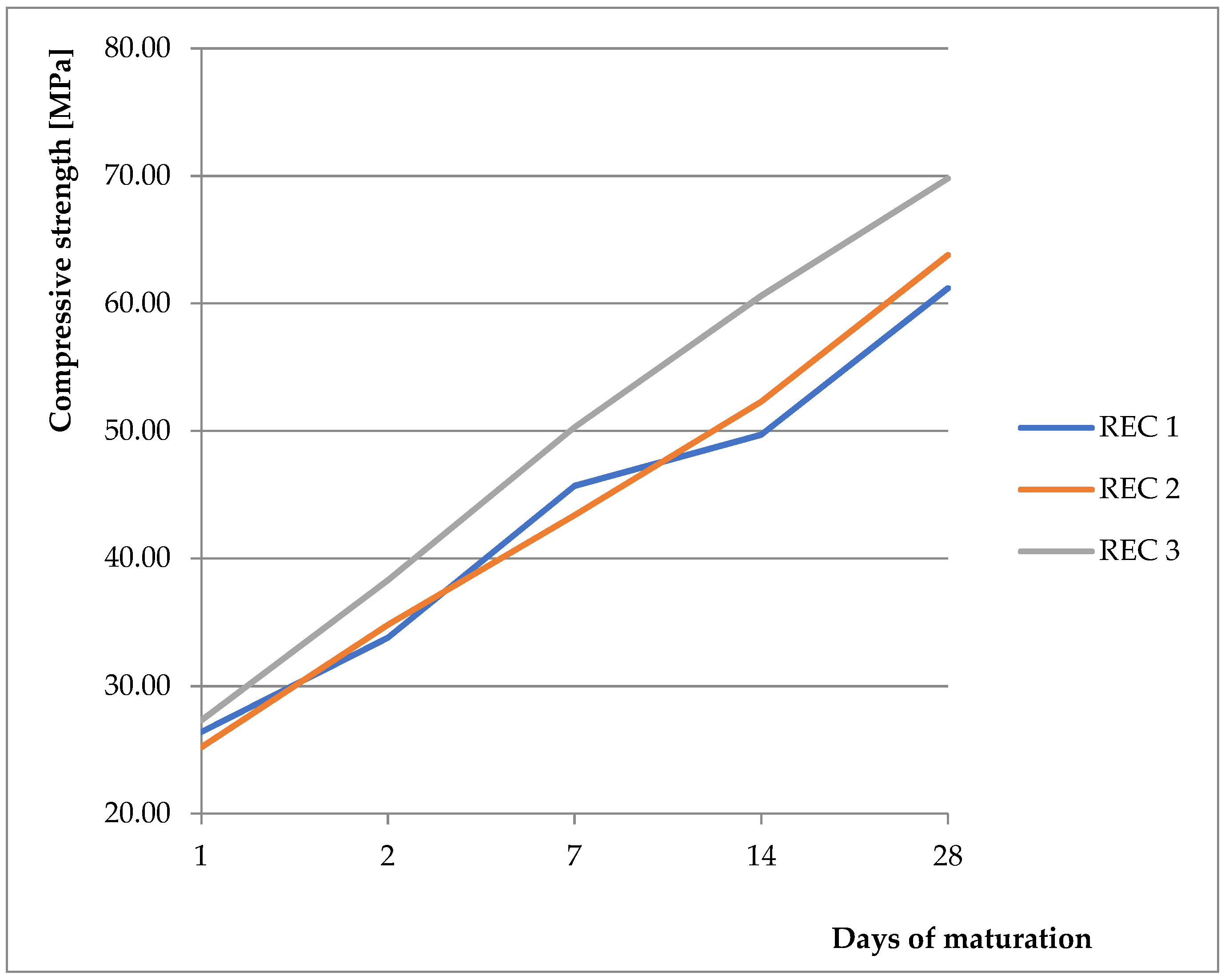
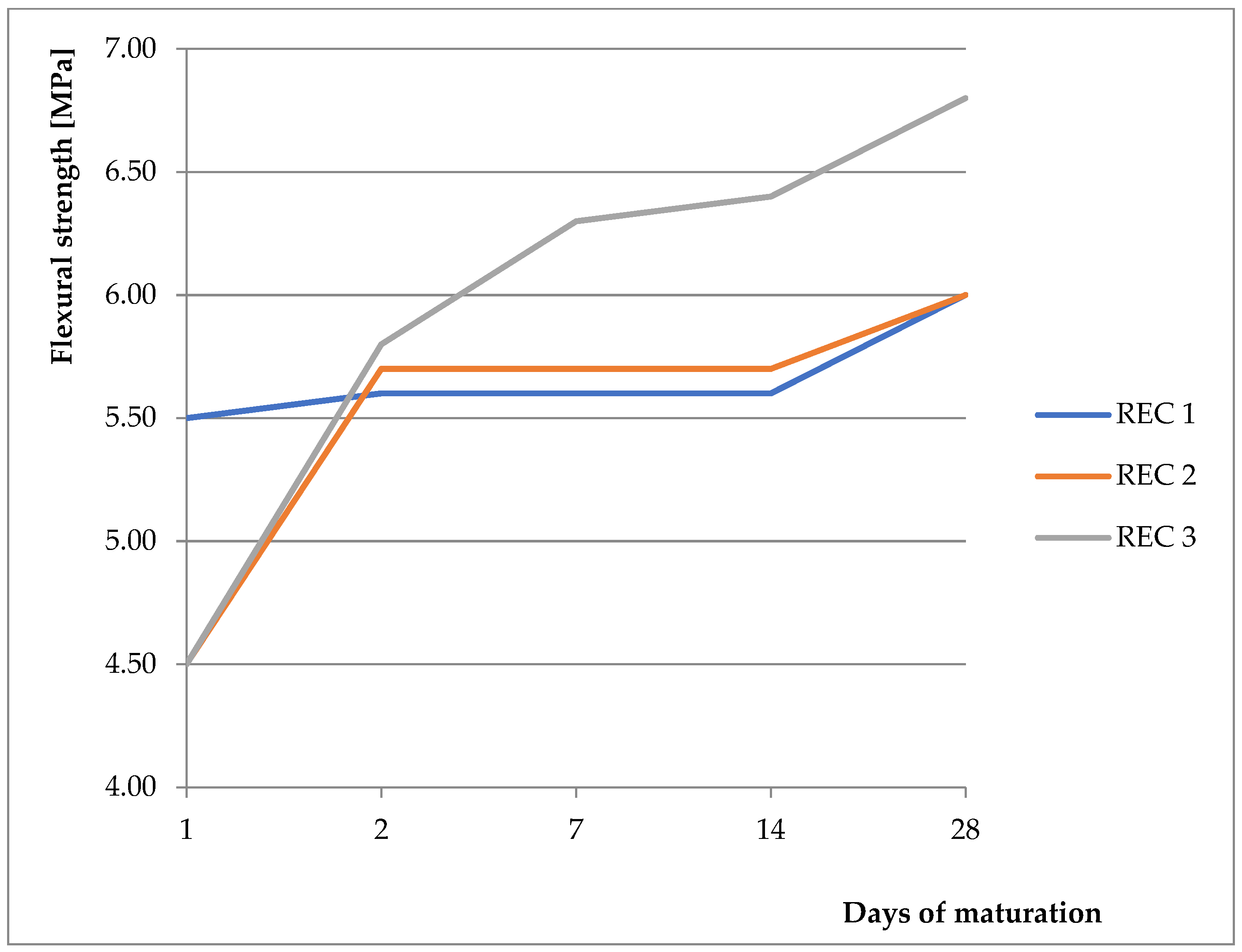
As a basic mixture, the previously verified and tested mixture from the previous work was used [9,28,29,30]. The basic mixture was derived from initial experiments, where the strength properties were monitored with different replacements of BFS with fly ash and CBPD, with the possibility of using different activators. Two activators were compared (anhydrous disodium metasilicate and sodium water glass with silicate modulus of 2). Better results were obtained with the anhydrous disodium metasilicate activator, therefore this activator was chosen for the next phase of the experiment. These mixtures were then tested, and their physical–mechanical properties were verified. Results showing compressive strength and flexural strength can be seen in Figure 4 and Figure 5, respectively. For REC 1, the amount of activator was calculated from the total binder amount. For REC 2, the dose was reduced by 15%, and, for REC 3, the dose was reduced by 30%. The mixture compositions are listed in Table 4. The pH level of the solution prepared by dissolving anhydrous disodium metasilicate in water ranged from 13.95 to 14.00 for all monitored mixtures.
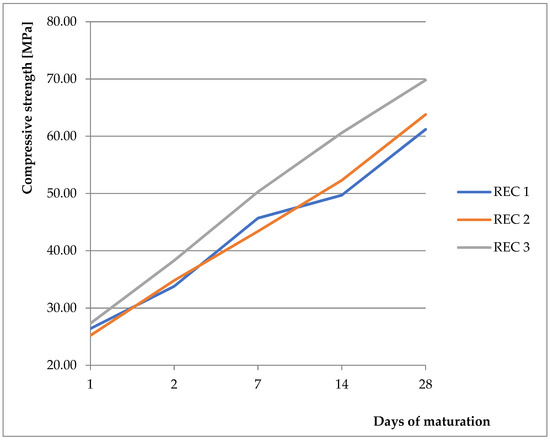
Figure 4.
Compressive strength of prepared mixtures.
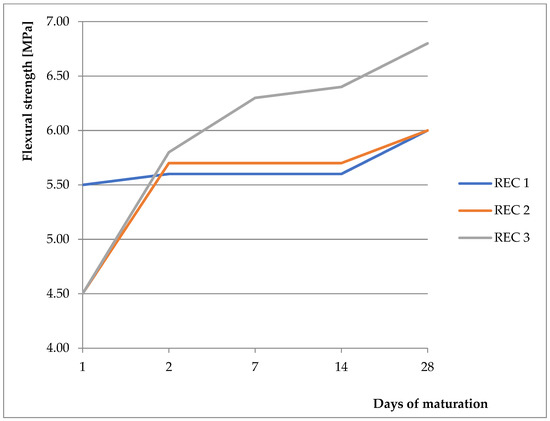
Figure 5.
Flexural strength of prepared mixtures.

Table 4.
Mixture compositions. Recipe of the raw material values is given in [g and wt%].
2.7. X-ray Diffraction
X-ray diffraction was performed on a Rigaku MinyFlex instrument in the range of 5–100° 2theta at a speed of 3°/min. The X-ray diffraction patterns were recorded on pastes. The ratio between the binder components and activator remained the same, according to Table 4. Only the water content was changed to 0.42.
2.8. Strength
To determine the basic strengths, the specimens were stored in a humidity cabinet until testing. The strengths were determined with a hydraulic press. The determination of the flexural strength was carried out by uniform loading at speeds of (50 ± 10) N/s. The compressive strength was determined by uniform loading at a rate of (2400 ± 200) N/s [28].
3. Results
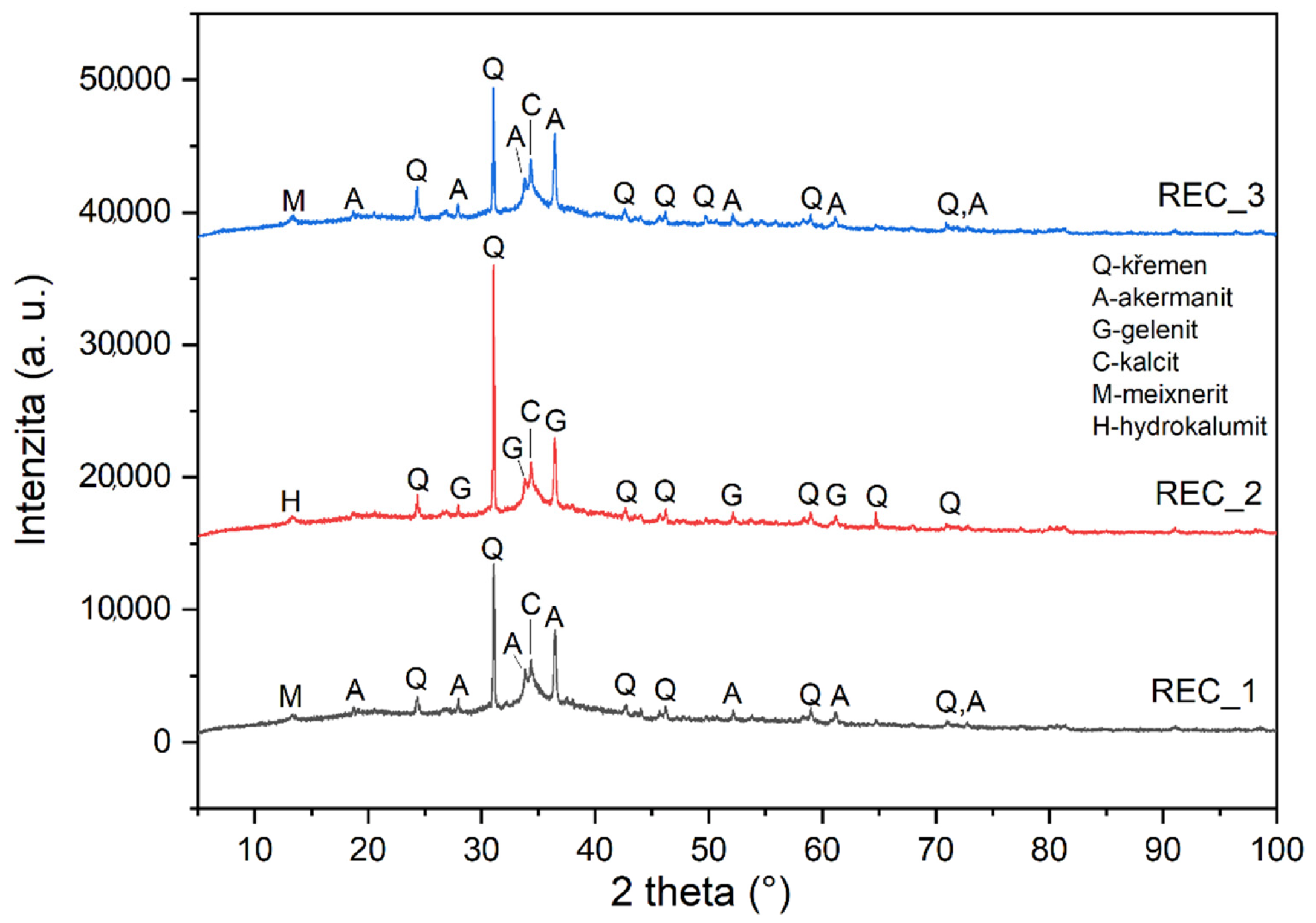
The X-ray diffraction results obtained on alkali-activated pastes are shown in Figure 6. The image is the output of the same software as Figure 1, Figure 2 and Figure 3. However, while it is less detailed, it is the only schematic that better demonstrates the differences in the individual mixture.
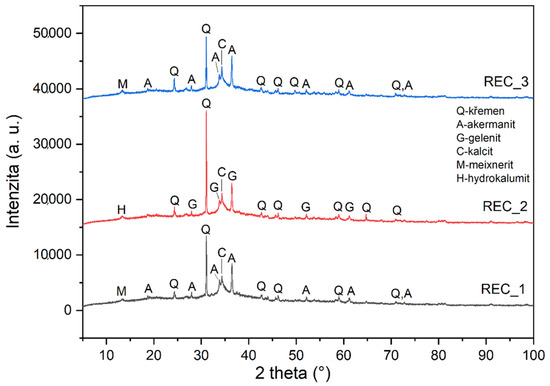
Figure 6.
Mineralogical composition determined by X-ray diffraction (XRD) of prepared alkali activated pastes.
4. Discussion
According to [31], choosing a suitable type of activator according to the slag properties before starting the experiment is important. Most often, the slag is activated by sodium and potassium water glass. (Water glass is the generic term for silicate solutions, such as sodium silicate, potassium silicate, or lithium silicate). These are often treated with hydroxide solutions so that the silicate modulus of the water glass is reduced, and the workability of the prepared composite is extended. However, one of the most common complaints preventing the wider use of alkali-activated systems in construction is the concern that the handling of these liquid strong alkalis is dangerous, due to the low professionalism of construction personnel, and it is an attempt to avoid handling these substances on the construction site. Therefore, the research work of the team included efforts to use a solid activator in the form of sodium metasilicate. This material has a silicate modulus value of 1, and, so, in terms of workability [31,32], it is an ideal activator because, as previous research has shown, the optimal value of silicate modulus is in the range of 1–2; the increasing value significantly decreases workability time [29,30].
One of the essential steps in alkali activation is to determine the optimal amount of activator required for alkali activation of slag, i.e., to introduce an adequate amount of Na2O into the system. The amount of Na2O in the case of slag is generally chosen in the range of 2–8% by weight, depending on the chemical composition of the slag. The properties of the composite together with the amount of Na2O are also affected by the silicate modulus. According to [32], in the case of low values of the introduced Na2O, the lower strength was resulted, and, in the case of the performed experiments, a basic value of 4.2 wt% Na2O, per 100 g of slag was chosen. This value was determined on the basis of the composition of the blast furnace granulated slag as an optimum. This value was subsequently reduced by 15, resp. 30%.
The most interesting results achieved are the following. When the value of the amount of activator decreases by 30%, strengths increase, although the opposite trend was expected. The X-ray diffraction results indicate that the alkali-activation products of the composite with the original and reduced amounts of activator are remarkably similar, although it was expected that the resulting hydration products would be different due to the reduced amount of activator. It is also interesting that gehlenite (Ca2Al [(Si2O7]) was detected by the diffraction in the mixture with the amount of activator reduced by 15%, while akermanite (Ca2Mg[(SiAl)O7]) was detected in two other mixtures (Rec 1 and Rec3). These minerals belong to the group of melilite, they are similar and are commonly found in alkali-activated materials, as they are the basic minerals of blast furnace slags. It is interesting that in the case of akermanite, the mixtures REC1 and REC3 contained in a certain amount of magnesium compound, whereas in the mixture REC2 with gehlenite, magnesium was not present in the system.
The occurrence of quartz is also interesting, and it can be assumed that it was contained in the fly ash. REC1 and REC3 also contained Hydrocalumite Ca2Al(OH)6.5Cl0.5·3(H2O), which has chlorine in its structure, can be assumed to have occurred in cement by-pass dust. Other differences between samples include the Rec 1 samples, which showed Meixnerite and Sylvite minerals, which were included in CPBD.
5. Conclusion
In the next phase of the experiment, X-ray diffraction of individual parts of the alkali-activated system will be performed separately and the individual components of the composite will be monitored. This is necessary in order to determine how significantly the fly ash and cement by-pass dust are involved in the hydration processes, or whether they are less reactive and only replace the fine fractions of the aggregate in the composite. The properties of the precipitated efflorescence which form on the surface of the prepared bodies will also be determined, and it will be verified whether they are sodium salts, which would indicate possible excess of alkalis in the system. However, these may also be precipitated efflorescence of potassium salts, which are abundant in cement by-pass dust. If the type of alkaline element affects the kinetics of the alkaline activation process differently and different hydration products are formed. It will be necessary to deal in detail with the composition of the individual components of the binder. This can be problematic due to the slightly changing composition of cement by-pass dusts.
Author Contributions
Conceptualization, B.V., J.B. and L.P.; methodology, J.B., L.P. and B.V.; software L.P. and member of the Department of Thermal Engineering (see Acknowledgments); validation, B.V., J.B. and L.P.; formal analysis, L.P. and member of the Department of Thermal Engineering (see Acknowledgments); investigation, B.V., J.B. and L.P.; resources, B.V., J.B. and L.P.; data curation, J.B. and L.P.; writing—original draft preparation, J.B. and L.P.; writing—review and editing, B.V.; visualization, B.V. and L.P. supervision, J.B. and B.V.; project administration, B.V.; funding acquisition, B.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by H2020 Marie Skłodowska-Curie Actions, grant number 7348336.
Acknowledgments
The authors would like to thank the Department of Thermal Engineering from VŠB—Technical University of Ostrava for performing XRD and XRF analyzes.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Altwair, N.; Kabir, S. Reducing Environmental Impacts through Green Concrete Technology. In Proceedings of the The 3rd Technology and Innovation for Sustainable Development International Conference (TISD2010) 2010, Faculty of Engineering, Khon Kaen University, Khon Kaen, Thailand, 4–6 March 2010. [Google Scholar]
- Bílek, V.; Khestl, F.; Mec, P. Hybrid Cements with Non Silicate Activators. Solid State Phenom. 2017, 259, 30–34. [Google Scholar] [CrossRef]
- Van Deventer, J.S.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Bílek, V.; Pytlík, D.; Bambuchova, M. High Performance Concrete with Ternary Binders. Key Eng. Mater. 2018, 761, 120–123. [Google Scholar] [CrossRef]
- Dyer, T.; Halliday, J.E.; Dhir, R.K. An investigation of the hydration chemistry of ternary blends containing cement kiln dust. J. Mater. Sci. 1999, 34, 4975–4983. [Google Scholar] [CrossRef]
- Provis, J.L.; Deventer, J.S.J. Geololymers, Structure, Processing, Properties and Industrial Applications; Woodhead Publishing Limited: Sawston, UK, 2009; ISBN 978-1-84569-449-4. [Google Scholar]
- Boháčová, J. Study of Influence of Different Types of Fillers on Properties of Geopolymer Systems Based on Alkali Activated Slags. Bachelor’s Thesis, VŠB—Technical University of Ostrava, Ostrava, Czech Republic, 2008; pp. 30–32. [Google Scholar]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Li, Z.X.; Tang, J.W. The Appropriate Chemical Admixture for Alkali-Activated Cementitious Material. Adv. Mater. Res. 2012, 534, 34–41. [Google Scholar] [CrossRef]
- Chen, K.; Yang, C.H.; Wu, F.; Ye, J.X.; Pan, Q.; Yu, Z.D. Development of Alkali Activated Slag Cement Based Ecomaterial and its Environmental Coordination Evaluation. Mater. Sci. Forum 2009, 610–613, 179–184. [Google Scholar] [CrossRef]
- Chi, M.C.; Chen, H.; Weng, T.L.; Huang, R.; Wang, Y.C. Durability of Alkali-Activated Fly Ash/Slag Concrete. Mater. Sci. Forum 2017, 904, 157–161. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.M.; Yang, D.H.; Qin, W.J.; Yang, G.S.; Zhang, D.L. Research of the SNCR Process and its Application. Adv. Mater. Res. 2014, 953–954, 1307–1314. [Google Scholar] [CrossRef]
- Procházka, L.; Boháčová, J. Verification of Durability Properties of Alkali-Activated Materials Based on Blast Furnace Slag with Fly Ash. Solid State Phenom. 2020, 309, 93–97. [Google Scholar] [CrossRef]
- Procházka, L.; Mec, P. Possibility of using fly ash after denitrification by SNCR as admixture in alkali-activated materials. Mater. Today Proc. 2021, 37, 42–47. [Google Scholar] [CrossRef]
- Štěpánková, E.; Kalina, L.; Belik, V., Jr.; Bartoníčková, E. Utilization of By-Pass Cement Kiln Dust in Alkali-Activated Materials. Key Eng. Mater. 2018, 761, 23–26. [Google Scholar] [CrossRef]
- Tkaczewska, E. The influence of cement bypass dust on the properties of cement curing under normal and autoclave conditions. Struct. Environ. 2019, 11, 5–22. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Utilization of cement kiln dust (CKD) to enhance mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 40, 1002–1011. [Google Scholar] [CrossRef]
- Kubátová, D.; Rybová, A.; Zezulová, A. The Hydrothermal Stability of Alkali-Activated Fly Ash/Slag Pastes by the Incorporation of Cement Kiln Dust. Solid State Phenom. 2019, 296, 15–20. [Google Scholar] [CrossRef]
- Sikorová, V. Methods of Using Cement Kiln By-Pass Dust in Building Materials Technology. Master’s Thesis, Faculty of Chemistry, Brno University of Technology, Brno-Medlánky, Czech Republic, 2019; pp. 33–38. [Google Scholar]
- Adaska, P.W.S.; Taubert, D.H. Beneficial Uses of Cement Kiln Dust. In Proceedings of the 2008 IEEE Cement Industry Technical Conference Record, Miami, FL, USA, 18–22 May 2008; pp. 210–228. [Google Scholar]
- Kotouč Štramberk. 2020. Available online: https://www.cemix.cz/kotouc/cz (accessed on 15 September 2020).
- Ismail, I.; A Bernal, S.; Provis, J.L.; Hamdan, S.; Van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Procházka, L.; Boháčová, J. Effect of Admixtures on Durability Characteristics of Fly Ash Alkali-activated Material. Emerg. Sci. J. 2020, 4, 493–502. [Google Scholar] [CrossRef]
- Penta. Chemical Unlimited—Chemikalie 2020. Available online: https://www.pentachemicals.eu/chemikalie (accessed on 15 September 2020).
- ČSN EN 196-1 Methods of Testing Cement—Part 1: Determination of Strength Prague; Classifier 72 2100; Office for Technical Standardization, Metrology and State Testing: Prague, Czech Republic, 2005; pp. 5–40.
- Koňařík, J. Influence of Activator on Basic Properties of Alkali Activated Systems. Bachelor’s Thesis, VŠB—Technical University of Ostrava, Ostrava, Czech Republic, 2014; pp. 27–35. [Google Scholar]
- Mec, P.; Boháčová, J.; Závrský, P. Testing of Possible Use of Fine-Grained Alkali Activated Composites in the Construction Industry. Mater. Sci. Forum 2016, 865, 47–52. [Google Scholar] [CrossRef]
- Bohacova, J.; Janalík, L. Preparation and Verification of Properties of Alkali-Activated Composite. Solid State Phenom. 2019, 296, 209–214. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.; Rashad, A.; Khalaf, M.A. A review on alkali-activated slag concrete. Ain Shams Eng. J. 2021, 12, 1475–1499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).