Synthesis of Carbon-Supported MnO2 Nanocomposites for Supercapacitors Application
Abstract
:1. Introduction
2. Materials and Methods
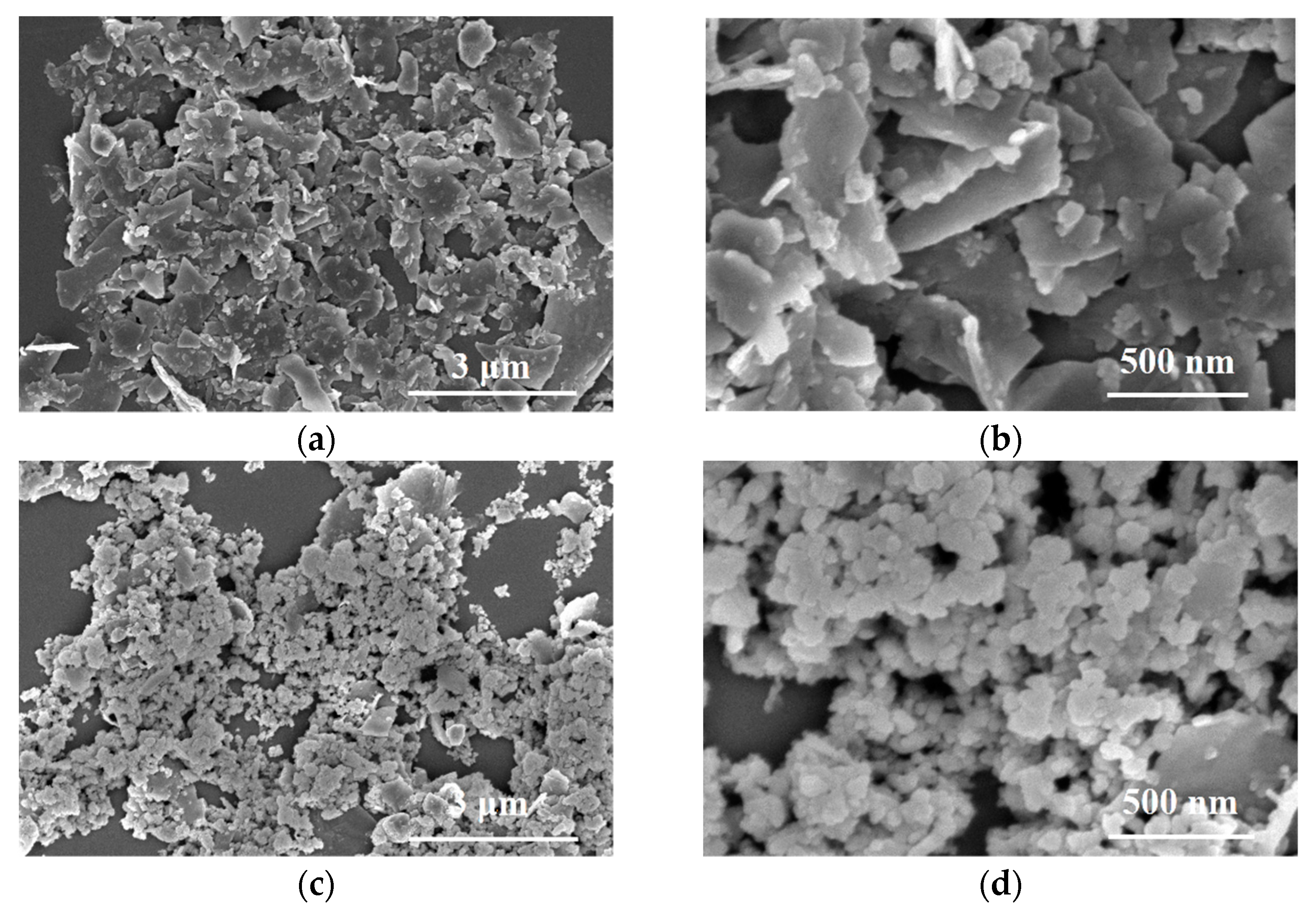
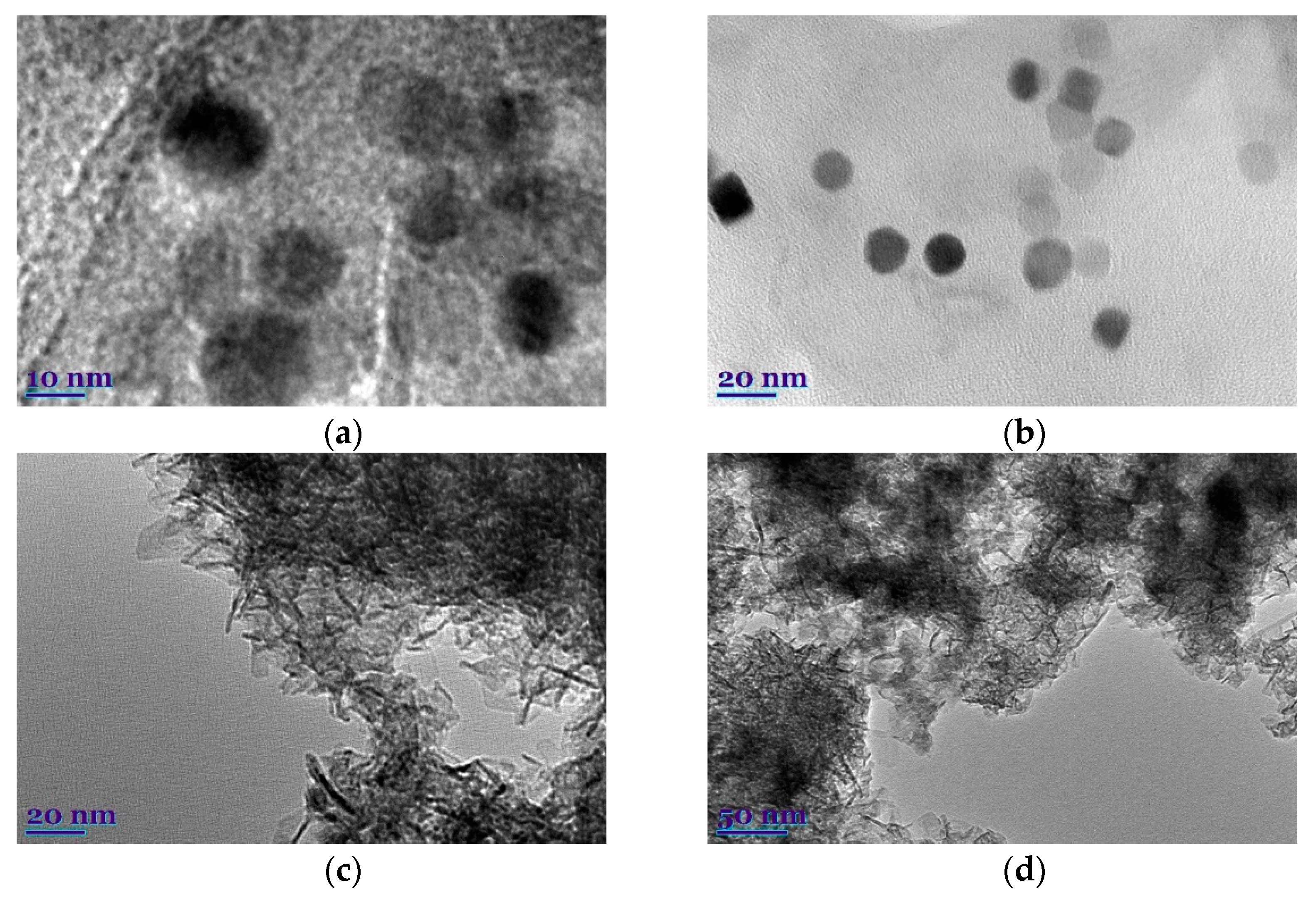
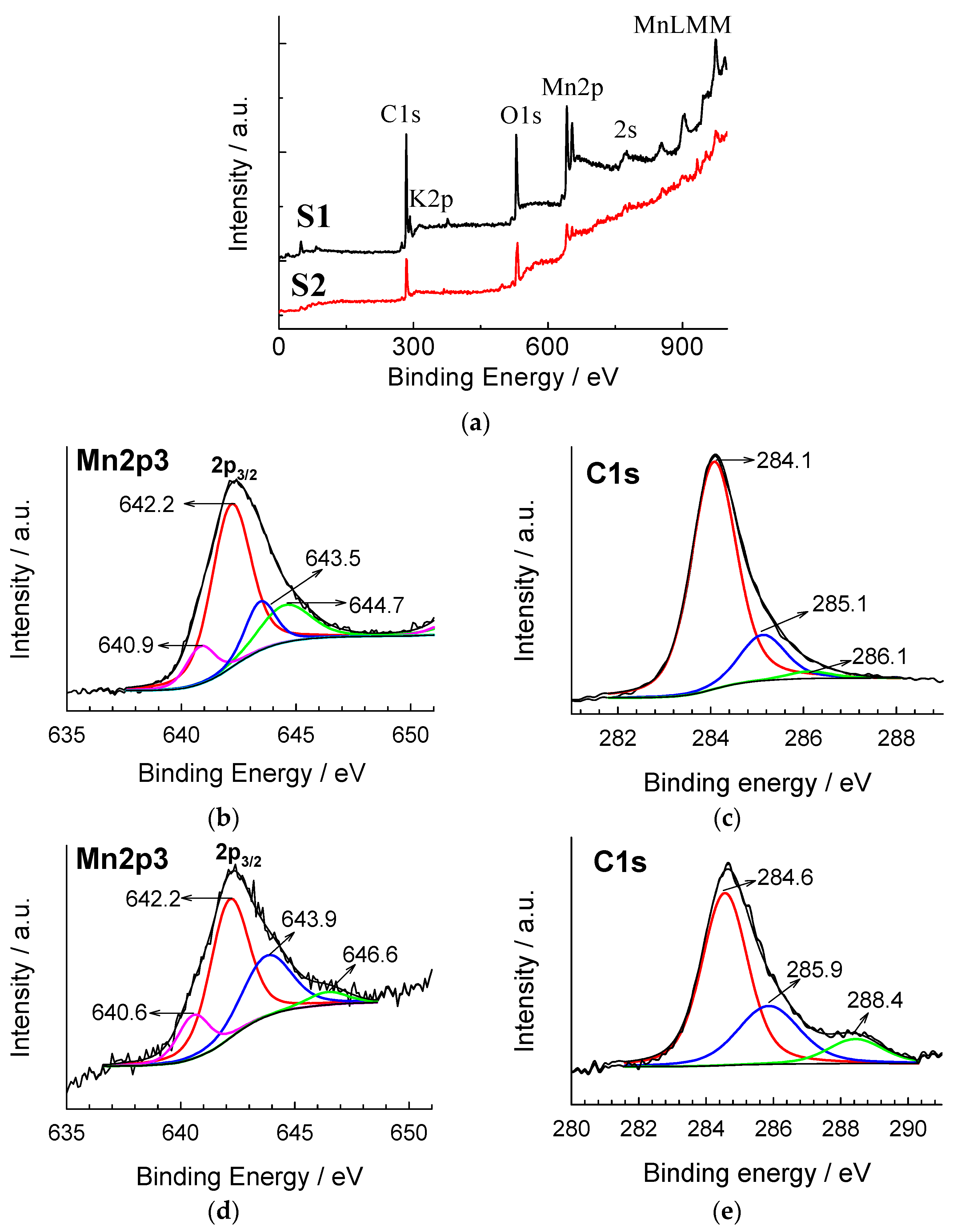
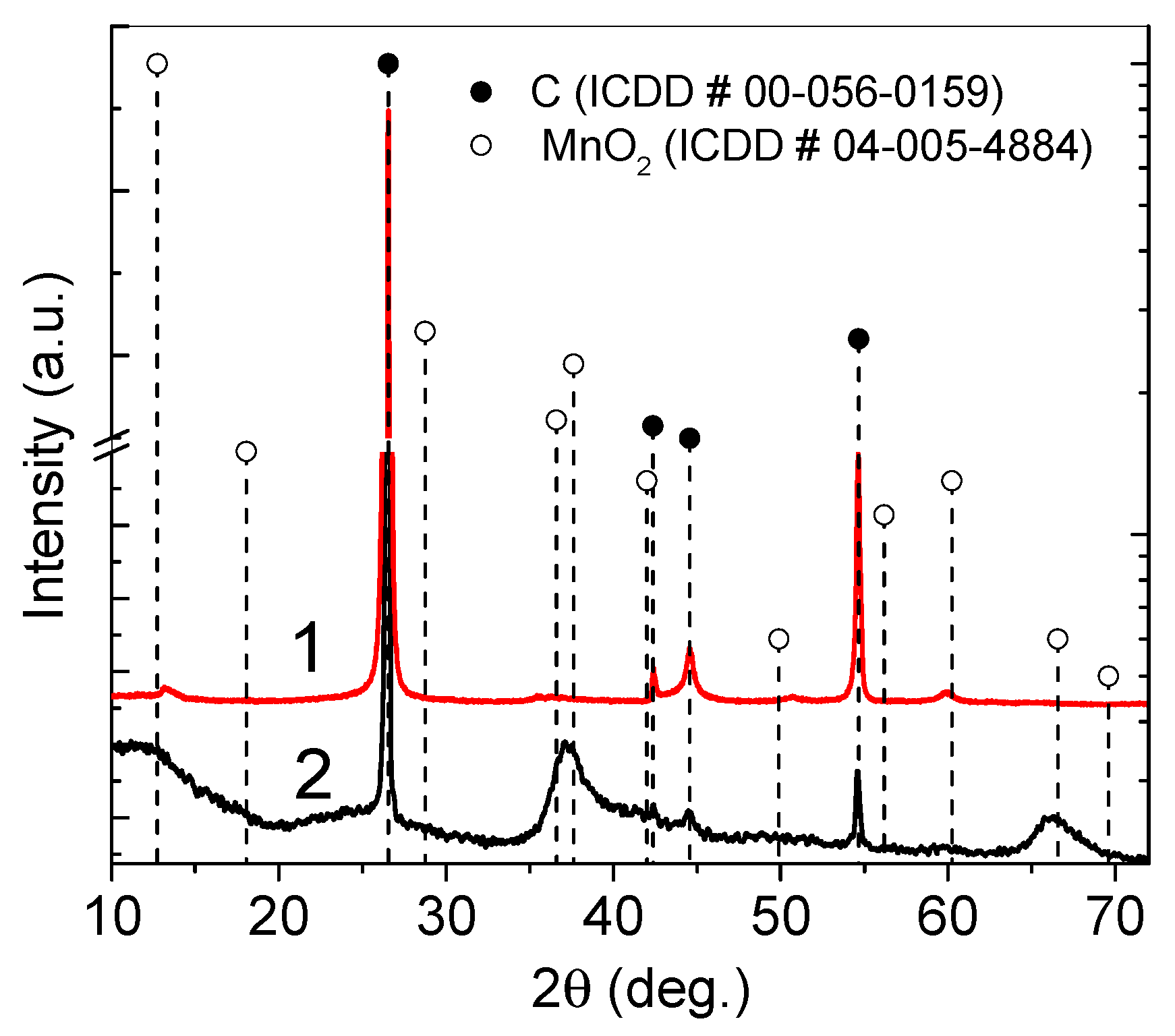
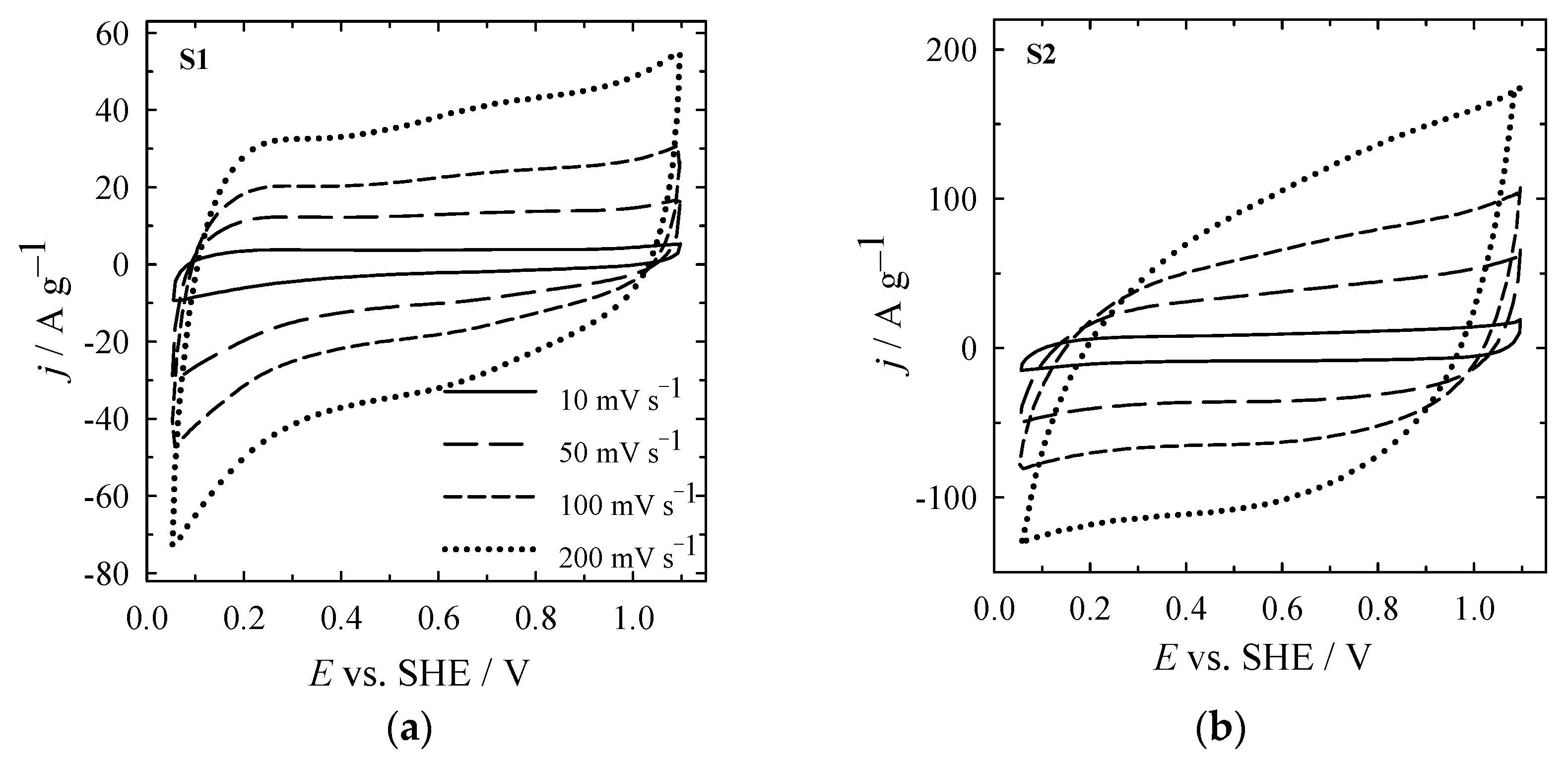
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gopi, C.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Hu, F.; Gao, G.; Wu, G.; Yang, X. Toward Superior Capacitive Energy Storage: Recent Advances in Pore Engineering for Dense Electrodes. Adv. Mater. 2018, 30, e1705713. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Yu, C.; Li, S.; Wang, Z.; Yu, J.; Huang, H.; Qiu, J. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islam, A.; Azad, A. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, Z.; Yang, Q.; Huo, W.; Javed, M.S.; Li, Y.; Huang, L.; Gu, X.; Hu, C. Approaching the lithium-manganese oxides’ energy storage limit with Li2MnO3 nanorods for high-performance supercapacitor. Nano Energy 2018, 43, 168–176. [Google Scholar] [CrossRef]
- Xia, H.; Hong, C.; Li, B.; Zhao, B.; Lin, Z.; Zheng, M.; Savilov, S.V.; Aldoshin, S.M. Facile Synthesis of Hematite Quantum-Dot/Functionalized Graphene-Sheet Composites as Advanced Anode Materials for Asymmetric Supercapacitors. Adv. Funct. Mater. 2015, 25, 627–635. [Google Scholar] [CrossRef]
- Qiu, T.; Luo, B.; Giersig, M.; Akinoglu, E.M.; Hao, L.; Wang, X.; Shi, L.; Jin, M.; Zhi, L. Au@MnO2 Core-Shell Nanomesh Electrodes for Transparent Flexible Supercapacitors. Small 2014, 10, 4136–4141. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Wen, Z.; Liu, Q. Facile synthesis of hierarchical Co3O4@MnO2 core–shell arrays on Ni foam for asymmetric supercapacitors. J. Power Sources 2014, 252, 98–106. [Google Scholar] [CrossRef]
- Radhamani, A.V.; Shareef, K.M.; Rao, M.S.R. ZnO@MnO2 Core–Shell Nanofiber Cathodes for High Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30531–30542. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Li, J.; Cui, X.; Al-Enizi, A.M.; Zhang, L.; Zheng, G. One-dimensional nanostructures for flexible supercapacitors. J. Mater. Chem. A 2015, 3, 16382–16392. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Unconventional supercapacitors from nanocarbon-based electrode materials to device configurations. Chem. Soc. Rev. 2016, 45, 4340–4363. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Ruoff, R.S.; Wen, Z.; Liu, Q. Self-Assembly of Mesoporous Nanotubes Assembled from Interwoven Ultrathin Birnessite-type MnO2 Nanosheets for Asymmetric Supercapacitors. Sci. Rep. 2015, 4, 3878. [Google Scholar] [CrossRef] [Green Version]
- Yin, B.; Zhang, S.; Jiao, Y.; Liu, Y.; Qu, F.; Wu, X. Facile synthesis of ultralong MnO2 nanowires as high performance supercapacitor electrodes and photocatalysts with enhanced photocatalytic activities. CrystEngComm 2014, 16, 9999–10005. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, L.; Seo, J.K.; Zhang, X.; Wang, S.; Shin, J.; Chao, D.; Zhang, H.; Meng, Y.S.; Fan, H.J. Self-branched α-MnO2/δ-MnO2 heterojunction nanowires with enhanced pseudocapacitance. Mater. Horiz. 2017, 4, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Sun, H.; Li, X.; Xiao, J.; Xiao, F.; Liu, L.; Luo, J.; Wang, S. Ultrahigh capacitive performance of three-dimensional electrode nanomaterials based on α-MnO2 nanocrystallines induced by doping Au through Å-scale channels. Nano Energy 2016, 21, 39–50. [Google Scholar] [CrossRef]
- Kang, J.; Hirata, A.; Kang, L.; Zhang, X.; Hou, Y.; Chen, L.; Li, C.; Fujita, T.; Akagi, K.; Chen, M. Enhanced Supercapacitor Performance of MnO2 by Atomic Doping. Angew. Chem. Int. Ed. 2013, 52, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xiao, X.; Chen, C.; Li, T.; Huang, L.; Zhang, C.; Su, J.; Miao, L.; Jiang, J.; Zhang, Y.; et al. Al-doped α-MnO2 for high mass-loading pseudocapacitor with excellent cycling stability. Nano Energy 2015, 11, 226–234. [Google Scholar] [CrossRef]
- Su, X.; Yu, L.; Cheng, G.; Zhang, H.; Sun, M.; Zhang, L.; Zhang, J. Controllable hydrothermal synthesis of Cu-doped δ-MnO2 films with different morphologies for energy storage and conversion using supercapacitors. Appl. Energy 2014, 134, 439–445. [Google Scholar] [CrossRef]
- Peng, R.; Wu, N.; Zheng, Y.; Huang, Y.; Luo, Y.; Yu, P.; Zhuang, L. Large-Scale Synthesis of Metal-Ion-Doped Manganese Dioxide for Enhanced Electrochemical Performance. ACS Appl. Mater. Interfaces 2016, 8, 8474–8480. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Li, Y.; Hu, J.; Lu, Y.; Xu, M. Interlinked multiphase Fe-doped MnO2 nanostructures: A novel design for enhanced pseudocapacitive performance. Nanoscale 2016, 8, 7309–7317. [Google Scholar] [CrossRef]
- Tang, C.-L.; Wei, X.; Jiang, Y.-M.; Wu, X.-Y.; Wang, K.-X.; Chen, J.-S.; Han, L.-N. Cobalt-Doped MnO2 Hierarchical Yolk–Shell Spheres with Improved Supercapacitive Performance. J. Phys. Chem. C 2015, 119, 8465–8471. [Google Scholar] [CrossRef]
- Hashem, A.M.A.; Abuzeid, H.M.; Narayanan, N.; Ehrenberg, H.; Julien, C. Synthesis, structure, magnetic, electrical and electrochemical properties of Al, Cu and Mg doped MnO2. Mater. Chem. Phys. 2011, 130, 33–38. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, X.; Gong, S.; Li, P.; Jin, L.; Cao, Q. Synthesis and characterization of 3D MnO2/carbon microtube bundle for supercapacitor electrodes. Mater. Lett. 2016, 179, 73–77. [Google Scholar] [CrossRef]
- Pandolfo, A.; Hollenkamp, A. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.P.; Reddy, B.P.; Byon, C.; Shim, J. Carbon/CuO nanosphere-anchored g-C3N4 nanosheets as ternary electrode material for supercapacitors. J. Solid State Chem. 2018, 262, 106–111. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Police, A.K.R.; Shim, J.; Byon, C. Sacrificial-template-free synthesis of core-shell C@Bi2S3 heterostructures for efficient supercapacitor and H2 production applications. Sci. Rep. 2018, 8, 4194. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Devarayapalli, K.C.; Dang, N.N.; Shim, J. 1D/1D Na2Ti3O7/SWCNTs electrode for split-cell-type asymmetric supercapacitor device. Ceram. Int. 2021, 47, 11602–11610. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, Z.; Li, J.; Han, J.; Yang, Y.; Xiong, B.; Fang, P.; He, C. Boosting the performance of supercapacitors based hierarchically porous carbon from natural Juncus effuses by incorporation of MnO2. J. Alloys Compd. 2019, 805, 822–830. [Google Scholar] [CrossRef]
- Feng, D.-Y.; Sun, Z.; Huang, Z.-H.; Cai, X.; Song, Y.; Liu, X.-X. Highly loaded manganese oxide with high rate capability for capacitive applications. J. Power Sources 2018, 396, 238–245. [Google Scholar] [CrossRef]
- Sun, L.; Li, N.; Zhang, S.; Yu, X.; Liu, C.; Zhou, Y.; Han, S.; Wang, W.; Wang, Z. Nitrogen-containing porous carbon/α-MnO2 nanowires composite electrode towards supercapacitor applications. J. Alloys Compd. 2019, 789, 910–918. [Google Scholar] [CrossRef]
- Su, X.; Yu, L.; Cheng, G.; Zhang, H.; Sun, M.; Zhang, X. High-performance α-MnO2 nanowire electrode for supercapacitors. Appl. Energy 2015, 153, 94–100. [Google Scholar] [CrossRef]
- Lei, R.; Zhang, H.; Lei, W.; Li, D.; Fang, Q.; Ni, H.; Gu, H. MnO2 nanowires electrodeposited on freestanding graphenated carbon nanotubes as binder-free electrodes with enhanced supercapacitor performance. Mater. Lett. 2019, 249, 140–142. [Google Scholar] [CrossRef]
- Meng, X.; Lu, L.; Sun, C. Green Synthesis of Three-Dimensional MnO2/Graphene Hydrogel Composites as a High-Performance Electrode Material for Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 16474–16481. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, P.; Guo, B.; Cheng, Z.; Fan, H.; Yang, M.; Yang, X.; Li, J. Electrodeposition of manganese dioxide film on activated carbon paper and its application in supercapacitors with high rate capability. RSC Adv. 2014, 4, 64187–64192. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, S.; Yi, F.; Gao, A.; Shu, D.; Ao, Z.; Huang, S.; Zhou, X.; He, C.; Li, S.; et al. Supramolecule-assisted synthesis of in-situ carbon-coated MnO2 nanosphere for supercapacitors. J. Alloys Compd. 2019, 779, 550–556. [Google Scholar] [CrossRef]
- Yang, M.; Kim, D.S.; Hong, S.B.; Sim, J.-W.; Kim, J.; Kim, S.-S.; Choi, B.G. MnO2 Nanowire/Biomass-Derived Carbon from Hemp Stem for High-Performance Supercapacitors. Langmuir 2017, 33, 5140–5147. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Chen, S.; Ye, Y.; Xu, F.; Tan, H.; Li, Z.; Hou, H.; Song, Y. Three-Dimensional Kenaf Stem-Derived Porous Carbon/MnO2 for High-Performance Supercapacitors. Electrochim. Acta 2014, 135, 380–387. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Q.; Pan, C. Green mass synthesis of graphene oxide and its MnO2 composite for high performance supercapacitor. Electrochim. Acta 2019, 312, 11–21. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Chi, M.; Gao, M.; Wang, C. General synthesis of hierarchical C/MOx@MnO2 (M = Mn, Cu, Co) composite nanofibers for high-performance supercapacitor electrodes. J. Colloid Interface Sci. 2018, 509, 235–244. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Li, D.; Sun, S.; Peng, Z.; Komarneni, S.; Yang, D. Direct Interfacial Growth of MnO2 Nanostructure on Hierarchically Porous Carbon for High-Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 633–641. [Google Scholar] [CrossRef]
- Kang, H.G.; Jeong, J.; Hong, S.B.; Lee, G.Y.; Kim, D.H.; Kim, J.W.; Choi, B.G. Scalable exfoliation and activation of graphite into porous graphene using microwaves for high–performance supercapacitors. J. Alloys Compd. 2019, 770, 458–465. [Google Scholar] [CrossRef]
- Bi, Y.; Nautiyal, A.; Zhang, H.; Yan, H.; Luo, J.; Zhang, X. Facile and ultrafast solid-state microwave approach to MnO2-NW@Graphite nanocomposites for supercapacitors. Ceram. Int. 2018, 44, 5402–5410. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, Q.; Li, G.; Wang, Q. Microwave preparation of 3D flower-like MnO2/Ni(OH)2/nickel foam composite for high-performance supercapacitors. J. Alloys Compd. 2017, 700, 185–190. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, W.; Li, C.; Sun, X.; Wang, K.; Ma, Y. Microwave-assisted rapid synthesis of birnessite-type MnO2 nanoparticles for high performance supercapacitor applications. Mater. Res. Bull. 2015, 71, 111–115. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Zhang, H.; Zhang, D.; Ma, Y. Microwave-assisted reflux rapid synthesis of MnO2 nanostructures and their application in supercapacitors. Electrochim. Acta 2013, 87, 637–644. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Enhanced activity of microwave synthesized hierarchical MnO2 for high performance supercapacitor applications. J. Power Sources 2012, 215, 317–328. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, Y.; Banis, M.N.; Liu, J.; Geng, D.; Li, R.; Sun, X. Facile controlled synthesis and growth mechanisms of flower-like and tubular MnO2 nanostructures by microwave-assisted hydrothermal method. J. Colloid Interface Sci. 2012, 369, 123–128. [Google Scholar] [CrossRef]
- Bagotsky, V.S.; Skundin, A.M.; Volfkovich, Y.M. Electrochemical Power Sources—Batteries, Fuel Cells, and Supercapacitors; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Audi, A.A.; Sherwood, P. Valence-band X-ray photoelectron spectroscopic studies of manganese and its oxides interpreted by cluster and band structure calculations. Surf. Interface Anal. 2002, 33, 274–282. [Google Scholar] [CrossRef]
- Zhou, D.; Lin, H.; Zhang, F.; Niu, H.; Cui, L.; Wang, Q.; Qu, F. Freestanding MnO2 nanoflakes/porous carbon nanofibers for high-performance flexible supercapacitor electrodes. Electrochim. Acta 2015, 161, 427–435. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction of Water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 11 May 2021).
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.R.; Artyushkova, K.; Cohen, H.; Easton, C.D.; Engelhard, M.; Gengenbach, T.R.; Greczynski, G.; Mack, P.; Morgan, D.J.; Roberts, A. XPS guide: Charge neutralization and binding energy referencing for insulating samples. J. Vac. Sci. Technol. A 2020, 38, 031204. [Google Scholar] [CrossRef] [Green Version]
- Beyazay, T.; Oztuna, F.E.S.; Unal, U. Self-Standing Reduced Graphene Oxide Papers Electrodeposited with Manganese Oxide Nanostructures as Electrodes for Electrochemical Capacitors. Electrochim. Acta 2019, 296, 916–924. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Grissa, R.; Martinez, H.; Cotte, S.; Galipaud, J.; Pecquenard, B.; Le Cras, F. Thorough XPS analyses on overlithiated manganese spinel cycled around the 3V plateau. Appl. Surf. Sci. 2017, 411, 449–456. [Google Scholar] [CrossRef]
- Dong, X.; Shen, W.; Gu, J.; Xiong, L.; Zhu, Y.; Li, A.H.; Shi, J. MnO2-Embedded-in-Mesoporous-Carbon-Wall Structure for Use as Electrochemical Capacitors. J. Phys. Chem. B 2006, 110, 6015–6019. [Google Scholar] [CrossRef]
- Singu, B.S.; Hong, S.E.; Yoon, K.R. Ultra-thin and ultra-long α-MnO2 nanowires for pseudocapacitor material. J. Solid State Electrochem. 2017, 21, 3215–3220. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, H.; Qin, F.; Guo, Z.; Wang, J.; Ni, G.; Zuo, P.; Qu, S.; Shen, W. MnO2 doped carbon nanosheets prepared from coal tar pitch for advanced asymmetric supercapacitor. Electrochim. Acta 2020, 354, 136667. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Xiong, D.; Lee, Y.R. Direct electro-synthesis of MnO2 nanoparticles over nickel foam from spent alkaline battery cathode and its supercapacitor performance. J. Taiwan Inst. Chem. Eng. 2019, 97, 414–423. [Google Scholar] [CrossRef]
- Xue, C.; Hao, Y.; Luan, Q.; Wang, E.; Ma, X.; Hao, X. Porous manganese dioxide film built from arborization-like nanoclusters and its superior electrochemical supercapacitance with attractive cyclic stability. Electrochim. Acta 2019, 296, 94–101. [Google Scholar] [CrossRef]



| Materials | Scan Rate, mV s−1 | Specific Capacitance, F g−1 | Ref. |
|---|---|---|---|
| MnO2/C (S2) | 10 | 980.7 | This work |
| MnO2/C (S1) | 10 | 535.8 | This work |
| Self-branched α-MnO2/δ-MnO2 heterojunction nanowires | 10 | 152.0 | [22] |
| MnO2 | 5 | 380.0 | [24] |
| MnO2 | 10 | 154.0 | [29] |
| MnO2/3D-PC | 1 | 416.0 | [47] |
| MnO2 | 5 | 547.0 | [68] |
| Ultra-long MnO2 nanowires | 2 | 495.0 | [69] |
| MnO2 NPs/Ni foam | 5 | 549.0 | [71] |
| MnO2/MWCNT | 2 | 553.0 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonskiene, J.; Simkunaite, D.; Vaiciuniene, J.; Stalnionis, G.; Drabavicius, A.; Jasulaitiene, V.; Pakstas, V.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Synthesis of Carbon-Supported MnO2 Nanocomposites for Supercapacitors Application. Crystals 2021, 11, 784. https://doi.org/10.3390/cryst11070784
Jablonskiene J, Simkunaite D, Vaiciuniene J, Stalnionis G, Drabavicius A, Jasulaitiene V, Pakstas V, Tamasauskaite-Tamasiunaite L, Norkus E. Synthesis of Carbon-Supported MnO2 Nanocomposites for Supercapacitors Application. Crystals. 2021; 11(7):784. https://doi.org/10.3390/cryst11070784
Chicago/Turabian StyleJablonskiene, Jolita, Dijana Simkunaite, Jurate Vaiciuniene, Giedrius Stalnionis, Audrius Drabavicius, Vitalija Jasulaitiene, Vidas Pakstas, Loreta Tamasauskaite-Tamasiunaite, and Eugenijus Norkus. 2021. "Synthesis of Carbon-Supported MnO2 Nanocomposites for Supercapacitors Application" Crystals 11, no. 7: 784. https://doi.org/10.3390/cryst11070784
APA StyleJablonskiene, J., Simkunaite, D., Vaiciuniene, J., Stalnionis, G., Drabavicius, A., Jasulaitiene, V., Pakstas, V., Tamasauskaite-Tamasiunaite, L., & Norkus, E. (2021). Synthesis of Carbon-Supported MnO2 Nanocomposites for Supercapacitors Application. Crystals, 11(7), 784. https://doi.org/10.3390/cryst11070784







