Highly Dispersed and Stable Ni/SBA-15 Catalyst for Reverse Water-Gas Shift Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Test
3. Results and Discussion
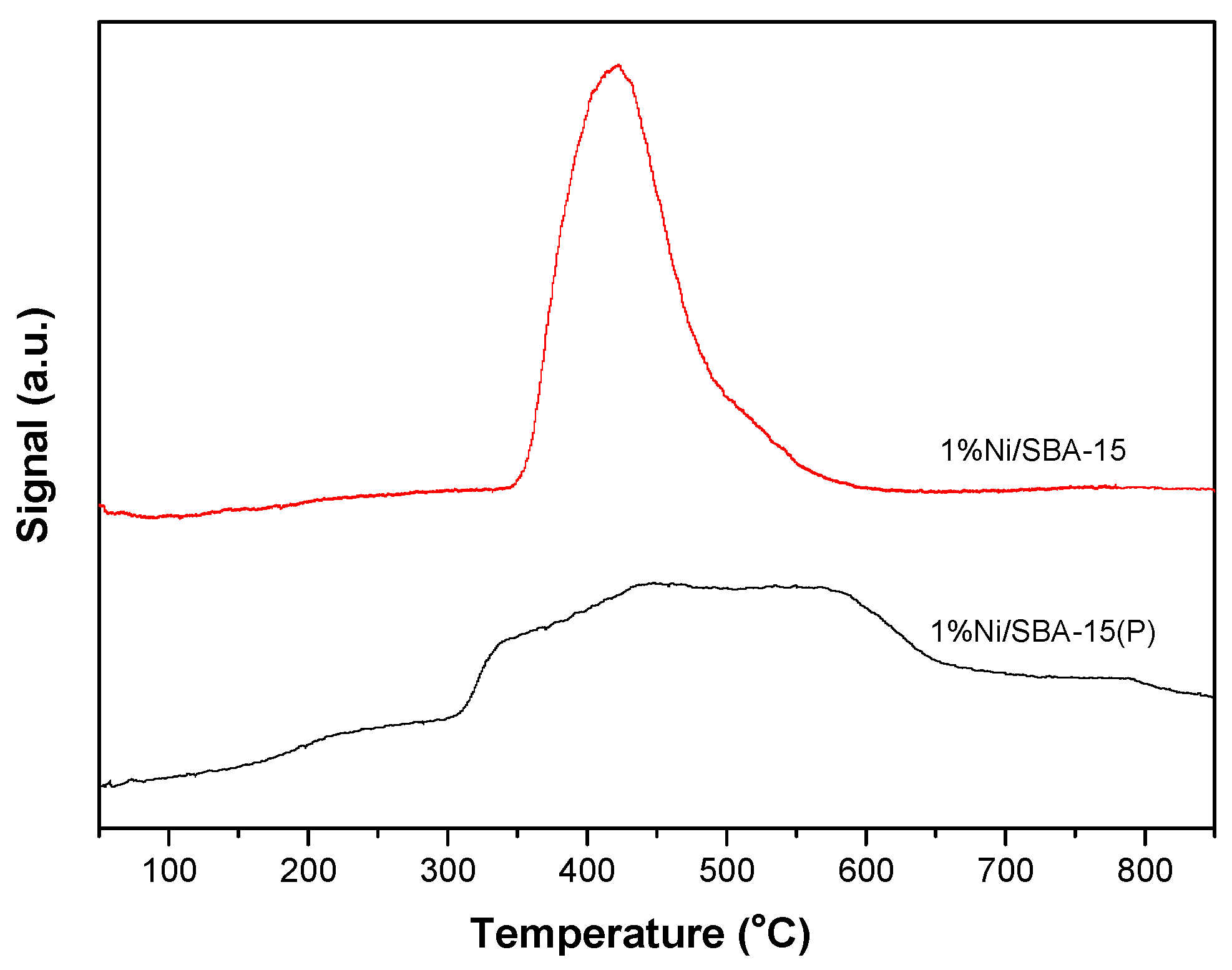
3.1. Catalyst Characterization
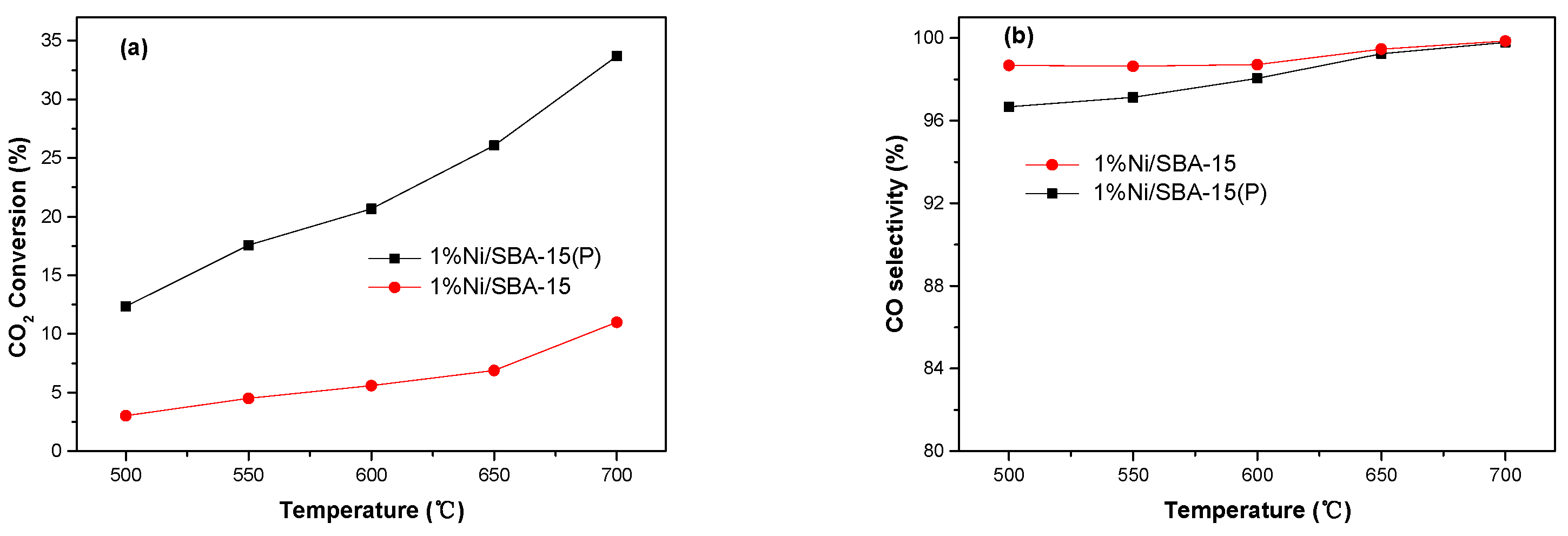
3.2. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nedolivko, V.V.; Zasypalov, G.O.; Vutolkina, A.V.; Gushchin, P.A.; Vinokurov, V.A.; Kulikov, L.A.; Egazar’yants, S.V.; Karakhanov, E.A.; Maksimov, A.L.; Glotov, A.P. Carbon Dioxide Reforming of Methane. Russ. J. Appl. Chem. 2020, 93, 765–787. [Google Scholar] [CrossRef]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Chotirach, M.; Tantayanon, S.; Tungasmita, D.N.; Sun, J.; Tungasmita, S. Synthesis and characterizations of TiN–SBA-15 mesoporous materials for CO2 dry reforming enhancement. Pure Appl. Chem. 2020, 92, 545–556. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent Advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- González-Castaño, M.; Dorneanu, B.; Arellano-García, H. The reverse water gas shift reaction: A process systems engineering perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Gorbunov, D.N.; Semernina, V.A.; Terenina, M.V.; Kardasheva, Y.S.; Maksimov, A.L.; Karakhanov, E.A. Catalytic Decomposition of Methyl Formate in the Presence of Transition Metal Complexes, Phosphine Ligands and Water. Pet. Chem. 2019, 59, 412–419. [Google Scholar] [CrossRef]
- Gorbunov, D.N.; Nenasheva, M.V.; Kardasheva, Y.S.; Karakhanov, E.A. Alternative sources of syngas for hydroformylation of unsaturated compounds. Russ. Chem. Bull. 2020, 69, 625–634. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Liu, Y. Reverse water gas shift reaction over Co-precipitated Ni-CeO2 catalysts. J. Rare Earths 2008, 26, 66–70. [Google Scholar] [CrossRef]
- Zonetti, P.C.; Letichevsky, S.; Gaspar, A.B.; Sousa-Aguiar, E.F.; Appel, L.G. The NixCe0.75Zr0.25−xO2 solid solution and the RWGS. Appl. Catal. A: Gen. 2014, 475, 48–54. [Google Scholar] [CrossRef]
- Sun, F.-m.; Yan, C.-f.; Wang, Z.-d.; Guo, C.-q.; Huang, S.-l. Ni/Ce–Zr–O catalyst for high CO2 conversion during reverse water gas shift reaction (RWGS). Int. J. Hydrogen Energy 2015, 40, 15985–15993. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Vono, L.L.R.; Wojcieszak, R.; Dias, C.S.B.; Wender, H.; Teixeira-Neto, E.; Rossi, L.M. Selective hydrogenation of CO2 into CO on a highly dispersed nickel catalyst obtained by magnetron sputtering deposition: A step towards liquid fuels. Appl. Catal. B Environ. 2017, 209, 240–246. [Google Scholar] [CrossRef]
- Nie, W.; Zou, X.; Chen, C.; Wang, X.; Ding, W.; Lu, X. Methanation of Carbon Dioxide over Ni–Ce–Zr Oxides Prepared by One-Pot Hydrolysis of Metal Nitrates with Ammonium Carbonate. Catalysts 2017, 7, 104. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Vutolkina, A.V.; Glotov, A.P.; Zanina, A.V.; Makhmutov, D.F.; Maximov, A.L.; Egazar’yants, S.V.; Karakhanov, E.A. Mesoporous Al-HMS and Al-MCM-41 supported Ni-Mo sulfide catalysts for HYD and HDS via in situ hydrogen generation through a WGSR. Catal. Today 2019, 329, 156–166. [Google Scholar] [CrossRef]
- Carta, D.; Montini, T.; Casula, M.F.; Monai, M.; Bullita, S.; Fornasiero, P.; Corrias, A. The water gas shift reaction over Pt–CeO2 nanoparticles confined within mesoporous SBA-16. J. Mater. Chem. A 2017, 5, 20024–20034. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.-V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Xin, Q.; Glisenti, A.; Philippopoulos, C.; Poulakis, E.; Mertens, M.; Nyalosaso, J.; Meynen, V.; Cool, P. Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement. Catalysts 2016, 6, 164. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Domínguez, R.A.; Vargas-Villagrán, H.; Peñaloza-Orta, C.; Saavedra-Rubio, K.; Bokhimi, X.; Klimova, T.E. A facile method to increase metal dispersion and hydrogenation activity of Ni/SBA-15 catalysts. Fuel 2017, 198, 110–122. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.; Yang, W.; He, D. Synergetic effect of Ni and Co in Ni–Co/SBA-15-CD catalysts and their catalytic performance in carbon dioxide reforming of methane to syngas. Catal. Sci. Technol. 2016, 6, 5631–5646. [Google Scholar] [CrossRef]
- Tao, M.; Xin, Z.; Meng, X.; Bian, Z.; Lv, Y. Highly dispersed nickel within mesochannels of SBA-15 for CO methanation with enhanced activity and excellent thermostability. Fuel 2017, 188, 267–276. [Google Scholar] [CrossRef]
- Lu, B.; Ju, Y.; Abe, T.; Kawamoto, K. Grafting Ni particles onto SBA-15, and their enhanced performance for CO2 methanation. RSC Adv. 2015, 5, 56444–56454. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wu, H.; Miyake, T.; He, D. CO2 reforming of methane over Ni/SBA-15 prepared with β-cyclodextrin—Role of β-cyclodextrin in Ni dispersion and performance. Int. J. Hydrogen Energy 2013, 38, 15200–15209. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of Renewable Hydrogen from Glycerol Steam Reforming over Bimetallic Ni-(Cu, Co, Cr) Catalysts Supported on SBA-15 Silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Kawamoto, K. Preparation of monodispersed NiO particles in SBA-15, and its enhanced selectivity for reverse water gas shift reaction. J. Environ. Chem. Eng. 2013, 1, 300–309. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Liu, H.; Li, Y.; Wu, H.; He, D. CO2 reforming of methane to syngas over highly-stable Ni/SBA-15 catalysts prepared by P123-assisted method. Int. J. Hydrogen Energy 2016, 41, 1513–1523. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.; Li, Y.; He, D. Interaction mechanism of Ni(NO3)2·6H2O and P123 in preparing highly-dispersed Ni/SBA-15 catalytic materials. Microporous Mesoporous Mater. 2016, 228, 174–181. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Tao, M.; Xin, Z.; Meng, X.; Lv, Y.; Bian, Z. Impact of double-solvent impregnation on the Ni dispersion of Ni/SBA-15 catalysts and catalytic performance for the syngas methanation reaction. RSC Adv. 2016, 6, 35875–35883. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Cao, K.; Yang, W.; Gao, H.; Wang, Y.; Li, H. Highly dispersed nickel loaded on mesoporous silica: One-spot synthesis strategy and high performance as catalysts for methane reforming with carbon dioxide. Appl. Catal. B Environ. 2012, 125, 324–330. [Google Scholar] [CrossRef]
- He, S.; He, S.; Zhang, L.; Li, X.; Wang, J.; He, D.; Lu, J.; Luo, Y. Hydrogen production by ethanol steam reforming over Ni/SBA-15 mesoporous catalysts: Effect of Au addition. Catal. Today 2015, 258 Pt 1, 162–168. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Shi, Y.; Zhao, B.; Wang, M.; Liu, Q.; Wang, J.; Long, K.; Duan, Y.; Ning, P. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Long, K.; Wang, J.; Zhang, T.; Song, Z.; Lin, Q. A novel promoting effect of chelating ligand on the dispersion of Ni species over Ni/SBA-15 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 14103–14114. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Zhang, T.; Wang, Y.; Wang, J.; Long, K.; Song, Z.; Liu, X.; Ning, P. Facile one-pot synthesis of highly dispersed Ni nanoparticles embedded in HMS for dry reforming of methane. Chem. Eng. J. 2017, 313, 1370–1381. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.; Bian, Z.; Xin, Z. Effect of impregnation solvent on Ni dispersion and catalytic properties of Ni/SBA-15 for CO methanation reaction. Fuel 2016, 165, 289–297. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.; Wei, Q.; Shen, W. Two-Dimensional Pd Nanosheets with Enhanced Catalytic Activity for Selective Hydrogenation of Nitrobenzene to Aniline. Energy Fuels 2021, 35, 4358–4366. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.-W.; Shi, C.; Ma, D. Highly Dispersed Copper over β-Mo2C as an Efficient and Stable Catalyst for the Reverse Water Gas Shift (RWGS) Reaction. ACS Catal. 2017, 7, 912–918. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Delgado, J.J.; Zhang, T. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]




| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| SBA-15 | 690 | 1.12 |
| 1%Ni/SBA-15(P) | 564 | 1.15 |
| 1%Ni/SBA-15 | 581 | 1.13 |
| Catalysts | Metal Loading (wt%) | Temperature (°C) | CO2 Conversion Rate (mol/gcat/h) | CO Selectivity (%) | References |
|---|---|---|---|---|---|
| 1%Ni/SBA-15(P) | 1 | 500 | 3.21 | 96.7 | This work |
| 600 | 5.36 | 98.0 | This work | ||
| Ni/Ce-Zr-O | 1 | 600 | 0.08 | ~75 | [11] |
| Cu/β-Mo2C | 1 | 600 | 1.72 | 99.2 | [39] |
| Pt/mullite | 2 | 500 | 0.39 | ~92.5 | [40] |
| Pt-K/mullite | 2 | 500 | 0.63 | ~100 | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, L. Highly Dispersed and Stable Ni/SBA-15 Catalyst for Reverse Water-Gas Shift Reaction. Crystals 2021, 11, 790. https://doi.org/10.3390/cryst11070790
Liu H, Wang L. Highly Dispersed and Stable Ni/SBA-15 Catalyst for Reverse Water-Gas Shift Reaction. Crystals. 2021; 11(7):790. https://doi.org/10.3390/cryst11070790
Chicago/Turabian StyleLiu, Hui, and Luhui Wang. 2021. "Highly Dispersed and Stable Ni/SBA-15 Catalyst for Reverse Water-Gas Shift Reaction" Crystals 11, no. 7: 790. https://doi.org/10.3390/cryst11070790







