Crystal Structure and Magnetism of Potassium-Intercalated 2,7-Dimethylnaphthalene
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
3.1. Magnetic Property of Potassium-Intercalated 2,7-DMN
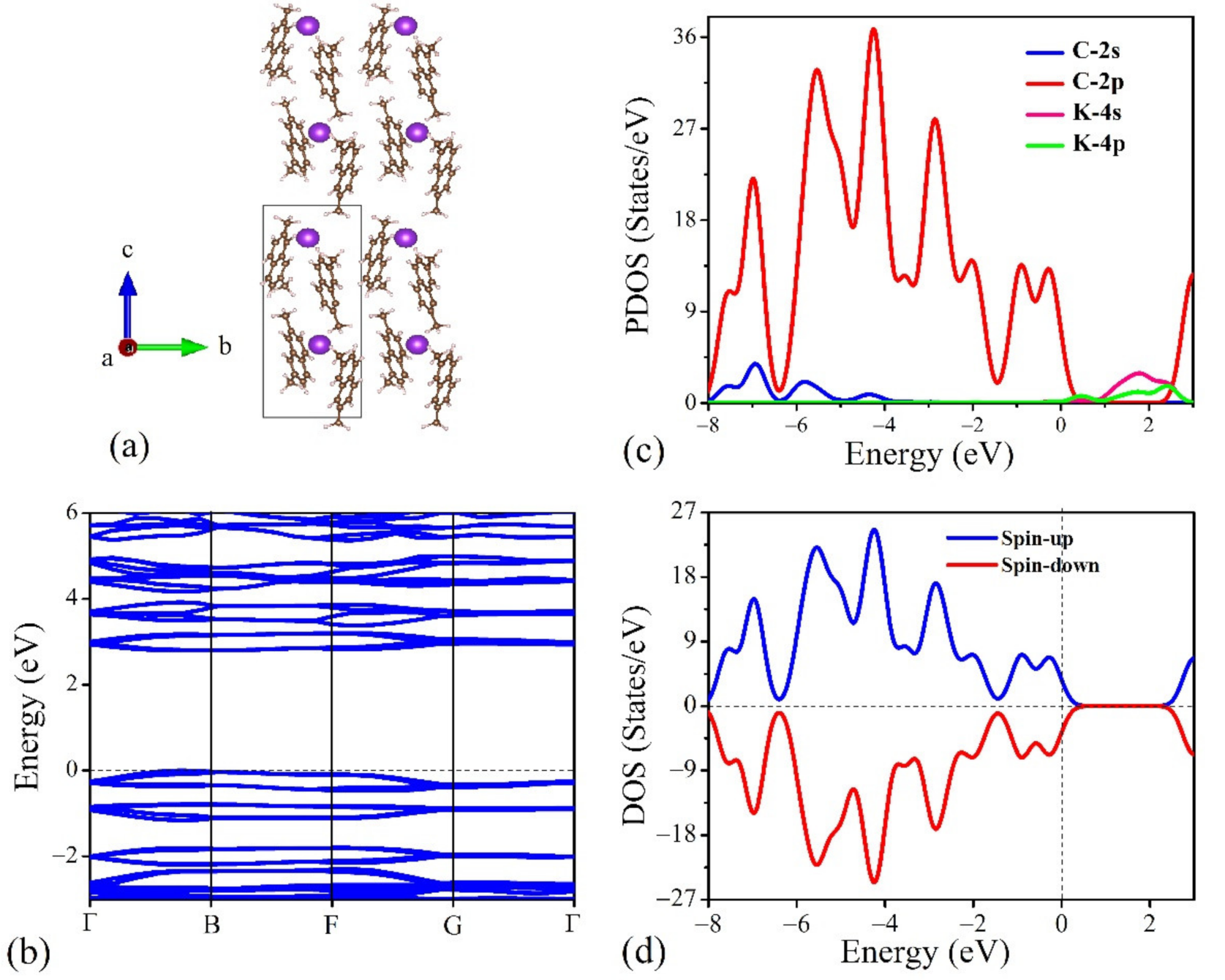
3.2. Crystal and Electronic Structures of Potassium-Intercalated 2,7-DMN
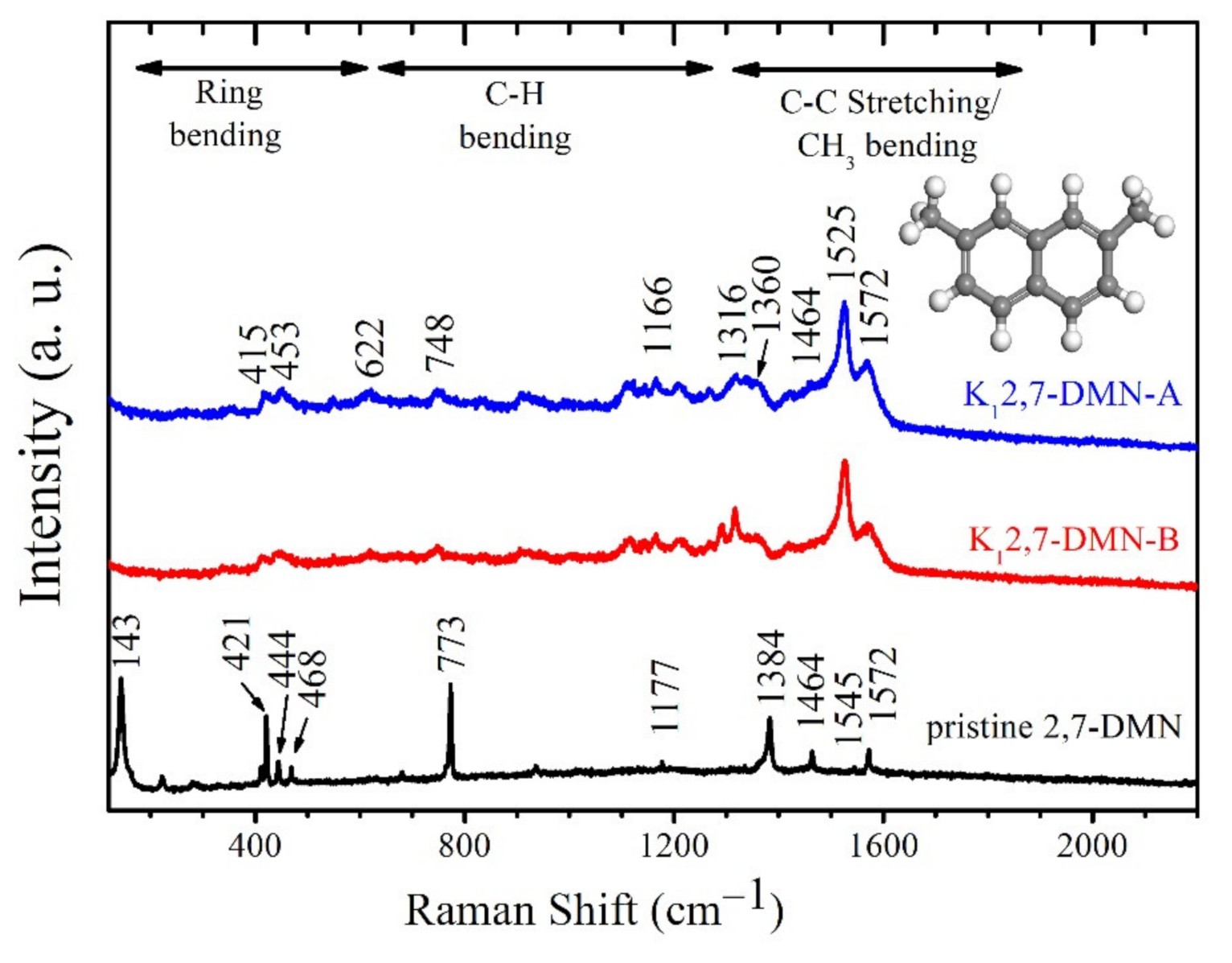
3.3. Raman Spectroscopy of Pristine and Potassium-Intercalated 2,7-DMN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radovic, L.R. Probing the ‘elephant’: On the essential difference between graphenes and polycyclic aromatic hydrocarbons. Carbon 2021, 171, 798–805. [Google Scholar] [CrossRef]
- Wang, N.; Zhi, Y.; Wei, Y.; Zhang, W.; Liu, Z. Molecular elucidating of an unusual growth mechanism for polycyclic aromatic hydrocarbons in confined space. Nat. Commun. 2020, 11, 1079. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.; Jin, J.; Guo, C.; Chen, D.J. Magnetic magnesium oxide composites for rapid removal of polycyclic aromatic hydrocarbons and cadmium ions from water. Environ. Chem. 2020, 17, 479–487. [Google Scholar] [CrossRef]
- Mu, Q.; Shiraiwa, M.; Octaviani, M.; Ma, N.; Ding, A.; Su, H.; Lammel, G.; PoSchl, U.; Heng, Y. Temperature effect on phase state and reactivity controls atmospheric multiphase chemistry and transport of PAHs. Sci. Adv. 2018, 4, eaap7314. [Google Scholar] [CrossRef] [Green Version]
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Human health risk assessment of ground water contaminated with petroleum PAHs using Monte Carlo simulations: A case study of an Indian metropolitan city. J. Environ. Manag. 2018, 205, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, G.R.; Aklilu, Y.A.; Landis, M.S.; Hsu, Y.M. Impacts of a large boreal wildfire on ground level atmospheric concentrations of PAHs, VOCs and ozone. Atmos. Environ. 2018, 178, 19–30. [Google Scholar] [CrossRef]
- Maigorzata, R.; Kinga, K.; Lekhanath, K.; Gabriela, B.N.; Marta, S.; Mariusz, M.; Katarzyna, C.; Halina, P.; Bozena, Z.; Jacek, N. Homogeneity study of candidate reference material (contaminated soil) based on determination of selected metals, pcbs and pahs. Measurement 2018, 128, 1–12. [Google Scholar]
- Shi, W.; Guo, Y.; Liu, Y. When flexible organic field-effect transistors meet biomimetics: A prospective view of the internet of things. Adv. Mater. 2019, 32, 1901493. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Huh, J.S.; Kim, J.J.; Park, J. Highly efficient deep-blue fluorescence OLEDs with excellent charge balance based on phenanthro[9,10-d]oxazole-anthracene derivatives. J. Mater. Chem. C 2020, 8, 11168–11176. [Google Scholar] [CrossRef]
- Yagui, J.; Angel, F.A. Benzodithiophene-based small molecules for vacuum-processed organic photovoltaic devices. Opt. Mater. 2020, 109, 110354. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Suzuki, Y.; Yamanari, Y.; Mitamura, H.; Kambe, T.; Ikeda, R.; Okamoto, H.; Fujiwara, A.; Yamaji, M.; Kawasaki, N. Superconductivity in alkali-metal-doped picene. Nature 2010, 464, 76–79. [Google Scholar] [CrossRef]
- Fu, M.A.; Wang, R.S.; Yang, H.; Zhang, P.Y.; Zhang, C.F.; Chen, X.J.; Gao, Y.; Huang, Z.B. π-electron weak ferromagnetism in potassium-intercalated 9-phenylanthracene. Carbon 2021, 173, 587–593. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yuan, Z.; Zhang, J.; Yusenko, K.V.; Jin, C. Structure and magnetic property of potassium intercalated pentacene: Observation of superconducting phase in KxC22H14. J. Phys. Condens. Matter. 2016, 28, 484001. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.H.; Huang, Z.B.; Lin, H.Q. Antiferromagnetism in Potassium-Doped Polycyclic Aromatic Hydrocarbons. IEEE Trans. Magn. 2014, 50, 1700103. [Google Scholar] [CrossRef]
- Takabayashi, Y.; Menelaou, M.; Tamura, H.; Takemori, N.; Prassides, K. π-Electron S = 1/2 Quantum Spin-Liquid State in an Ionic Polyaromatic Hydrocarbon. Nat. Chem. 2017, 9, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Štefančič, A.; Klupp, G.; Knaflič, T.; Yufit, D.S.; Tavčar, G.; Potočnik, A.; Beeby, A.; Arčon, D. Triphenylide-based molecular solid—A new candidate for a quantum spin-liquid compound. J. Phys. Chem. C 2017, 121, 14864–14871. [Google Scholar] [CrossRef] [Green Version]
- Phan, Q.; Heguri, S.; Tanabe, Y.; Shimotani, H.; Nakano, T.; Nozue, Y.; Tanigaki, K. Tuning of the ground state in electron doped anthracene. Dalton Trans. 2014, 43, 10040–10045. [Google Scholar] [CrossRef]
- Heguri, S.; Kobayashi, M.; Tanigaki, K. Questioning the existence of superconducting potassium doped phases for aromatic hydrocarbons. Phys. Rev. B 2015, 92, 014502. [Google Scholar] [CrossRef]
- Romero, F.D.; Pitcher, M.J.; Hiley, C.I.; Whitehead, G.; Kar, S.; Ganin, D.; Antypov, A.Y.; Collins, C.; Dyer, M.S.; Klupp, G. Redox-controlled potassium intercalation into two polyaromatic hydrocarbon solids. Nat. Chem. 2017, 9, 644–652. [Google Scholar] [CrossRef]
- Zhong, G.H.; Yang, D.Y.; Zhang, K.; Wang, R.S.; Zhang, C.; Lin, H.Q.; Chen, X.J. Superconductivity and Phase Stability of Potassium-Doped Biphenyl. Phys. Chem. Chem. Phys. 2018, 20, 25217–25223. [Google Scholar] [CrossRef]
- Wang, R.S.; Gao, Y.; Huang, Z.B.; Chen, X.J. Superconductivity above 120 Kelvin in a Chain Link Molecule. arXiv 2017, arXiv:1703.06641. [Google Scholar]
- Yan, J.F.; Zhong, G.H.; Wang, R.S.; Zhang, K.; Lin, H.Q.; Chen, X.J. Superconductivity and Phase Stability of Potassium-Intercalated p-Quaterphenyl. J. Phys. Chem. Lett. 2018, 10, 40–47. [Google Scholar] [CrossRef]
- Huang, G.; Zhong, G.H.; Wang, R.S.; Han, J.X.; Lin, H.Q.; Chen, X.J. Superconductivity and phase stability of potassium-doped p-quinquephenyl. Carbon 2019, 143, 837–843. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, R.H.; Gui, Z.; Xie, Y.L.; Yan, Y.J.; Ying, J.J.; Luo, X.G.; Chen, X.H. Superconductivity at 5 K in Alkali-Metal-Doped Phenanthrene. Nat. Commun. 2012, 2, 507. [Google Scholar]
- Wang, X.F.; Yan, Y.J.; Gui, Z.; Liu, R.H.; Chen, X.H. Superconductivity in A 1.5 phenanthrene (A = Sr, Ba). Phys. Rev. B 2011, 84, 214523. [Google Scholar]
- Teranishi, K.; He, X.; Sakai, Y.; Izumi, M.; Goto, H.; Eguchi, R.; Takabayashi, Y.; Kambe, T.; Kubozono, Y. Observation of zero resistivity in K-doped picene. Phys. Rev. B 2013, 87, 060505. [Google Scholar]
- Kubozono, Y.; Mitamura, H.; Lee, X.; He, X.; Yamanari, Y.; Takahashi, Y.; Suzuki, Y.; Kaji, Y.; Eguchi, R.; Akaike, K. Metal-intercalated aromatic hydrocarbons: A new class of carbon-based superconductors. Phys. Chem. Chem. Phys. 2011, 13, 16476–16493. [Google Scholar]
- Xue, M.; Cao, T.; Wang, D.; Wu, Y.; Yang, H.; Dong, X.; He, J.; Li, F.; Chen, G.F. Superconductivity above 30 K in Alkali-Metal-Doped Hydrocarbon. Sci. Rep. 2012, 2, 389. [Google Scholar]
- Marik, A.; Kumar, C.; Mohakud, S. Compensated Ferrimagnetism and Half Metallic Behavior in Potassium(K) Intercalated Naphthalene. Phys. Status Solidi B 2020, 257, 2000398. [Google Scholar]
- Peng, D.; Wang, R.S.; Chen, X.J. Superconductivity in Sodium Potassium Alloy Doped 2,2′-Bipyridine from Near Room Temperature Synthesis. J. Phys. Chem. C 2020, 124, 906–912. [Google Scholar] [CrossRef]
- Wang, R.S.; Cheng, J.; Wu, X.L.; Yang, H.; Chen, X.J.; Gao, Y.; Huang, Z.B. Superconductivity at 3.5 K and/or 7.2 K in Potassium-Doped Triphenylbismuth. J. Chem. Phys. 2018, 149, 144502. [Google Scholar] [CrossRef]
- Wang, R.S.; Yang, H.; Cheng, J.; Wu, X.L.; Fu, M.A.; Chen, X.J.; Gao, Y.; Huang, Z.B. Discovery of Superconductivity in Potassium-Doped Tri-p-Tolylbismuthine. J. Phys. Chem. C 2019, 123, 19105–19111. [Google Scholar] [CrossRef]
- Wang, R.S.; Chen, L.C.; Yang, H.; Fu, M.A.; Cheng, J.; Wu, X.L.; Gao, Y.; Huang, Z.B.; Chen, X.J. Superconductivity in an Organometallic Compound. Phys. Chem. Chem. Phys. 2019, 21, 25976–25981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentí, R.; Winter, S.M. Polycyclic aromatic hydrocarbons: Synthesis successes. Nat. Chem. 2017, 9, 608–609. [Google Scholar] [CrossRef]
- Spisak, S.N.; Rogachev, A.Y.; Zabula, A.V.; Filatov, A.S.; Clérac, R.; Petrukhina, M.A. Tuning the separation and coupling of corannulene trianion-radicals through sizable alkali metal belts. Chem. Sci. 2017, 8, 3137–3145. [Google Scholar] [CrossRef] [Green Version]
- Guijarro, A.; Verges, J.A. Prediction of a metallic phase for Cs3Pentacene compound. Mater. Res. Express 2018, 5, 066554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Whitehead, G.; Manning, T.D.; Stewart, D.; Hiley, C.; Pitcher, M.J.; Jansat, S.; Prassides, K.; Rosseinsky, M.J. The reactivity of solid rubrene with potassium: Competition between intercalation and molecular decomposition. J. Am. Chem. Soc. 2018, 140, 18162–18172. [Google Scholar] [CrossRef] [Green Version]
- Yoon, T.; Park, I.; Nguyen, T.P.; Kim, D.Y.; Choi, H.C. Discovery of sodium-doped triphenylene superconductor through searching the organic material database. Chem. Mater. 2020, 32, 3358–3364. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, R.S.; Yang, H.; Zhang, J.; Fu, M.A.; Fang, S.C.; Chen, X.J.; Gao, Y.; Huang, Z.B. Structural and Magnetic Properties of Potassium-Doped 2,3-DiMethylnaphthalene. Crystals 2021, 11, 608. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, M.; Chidiwa, T.; Tanimoto, Y. Magnetic Orientation under Gravity: Biphenyl and Naphthalene Crystals. J. Phys. Chem. B 2000, 104, 8075–8079. [Google Scholar] [CrossRef]
- Lapidus, S.; McConnell, A.; Stephens, P.; Miller, J. Structure and magnetic ordering of a 2-D MnII(TCNE)I(OH2) (TCNE = tetracyanoethylene) organic-based magnet (Tc = 171 K). Chem. Commun. 2011, 47, 7602–7604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3Cu2(C2O4)3(CH3OH)2: An organic-inorganic hybrid antiferromagnetic semiconductor. Chem. Commun. 2012, 48, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.J.; Ramakrishna, Y.; Padmarao, C.V.; Rao, B.V. Vibrational spectroscopic study of 2,6- and 2,7-dimethylnaphthalenes by Density functional theory. Int. J. Adv. Res. Sci. Technol. 2012, 1, 135–159. [Google Scholar]
- Kambe, T.; He, X.; Takahashi, Y.; Yamanari, Y.; Teranishi, K.; Mitamura, H.; Shibasaki, S.; Tomita, K.; Eguchi, R.; Goto, H.; et al. Synthesis and physical properties of metal-doped picene solids. Phys. Rev. B 2012, 86, 214507. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.W.; Zhong, G.H.; Zhang, J.; Zhao, X.M.; Zhang, C.; Lin, H.Q.; Chen, X.J. Constraint on the potassium content for the superconductivity of potassium-intercalated phenanthrene. J. Chem. Phys. 2014, 140, 114301. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G. Intercalation compounds of graphite. Adv. Phys. 1981, 30, 139–326. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, K.A.; Eklund, P.C.; Dresselhaus, G.; Dresselhaus, M.S. Raman-scattering study of the electron-phonon interaction in M3C60 (M = K, Rb). Phys. Rev. B 1993, 48, 8412–8417. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Kubozono, Y.; Eguchi, R.; Goto, H.; Hamao, S.; Kambe, T.; Terao, T.; Nishiyama, S.; Zheng, L.; Miao, X.; Okamoto, H. Recent progress on carbon-based superconductors. J. Phys. Condens. Matter. 2016, 28, 334001. [Google Scholar] [CrossRef]
- Fleming, R.M.; Ramirez, A.P.; Rosseinsky, M.J.; Murphy, D.W.; Haddon, R.C.; Zahurak, R.M.; Makhija, A.V. Relation of structure and superconducting transition temperature in A3C60. Nature 1991, 352, 787–788. [Google Scholar] [CrossRef]
- Zhong, G.H.; Zhang, C.; Wu, G.F.; Huang, Z.B.; Chen, X.J.; Lin, H.Q. First-principles investigations on the magnetic property in tripotassium doped picene. J. Appl. Phys. 2013, 113, 76. [Google Scholar] [CrossRef]


| Sample | χM0 (emu Oe−1 mol−1) | θ (K) | C (emu K Oe−1 mol−1) | M (μB mol−1) |
|---|---|---|---|---|
| K12,7-DMN-A | 0.27 × 10−4 | 0.098 | 28.7 × 10−4 | 0.15 |
| K12,7-DMN-B | 0.99 × 10−4 | 0.234 | 9.6 × 10−4 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.-L.; Wang, R.-S.; Yang, H.; Fu, M.-A.; Lv, H.; Yu, H.-Q.; Chen, X.-J.; Gao, Y.; Huang, Z.-B. Crystal Structure and Magnetism of Potassium-Intercalated 2,7-Dimethylnaphthalene. Crystals 2021, 11, 803. https://doi.org/10.3390/cryst11070803
Wu X-L, Wang R-S, Yang H, Fu M-A, Lv H, Yu H-Q, Chen X-J, Gao Y, Huang Z-B. Crystal Structure and Magnetism of Potassium-Intercalated 2,7-Dimethylnaphthalene. Crystals. 2021; 11(7):803. https://doi.org/10.3390/cryst11070803
Chicago/Turabian StyleWu, Xiao-Lin, Ren-Shu Wang, Hui Yang, Ming-An Fu, Hao Lv, Hua-Qing Yu, Xiao-Jia Chen, Yun Gao, and Zhong-Bing Huang. 2021. "Crystal Structure and Magnetism of Potassium-Intercalated 2,7-Dimethylnaphthalene" Crystals 11, no. 7: 803. https://doi.org/10.3390/cryst11070803






