Synthesis and Structural Studies of Two New Anthracene Derivatives
Abstract
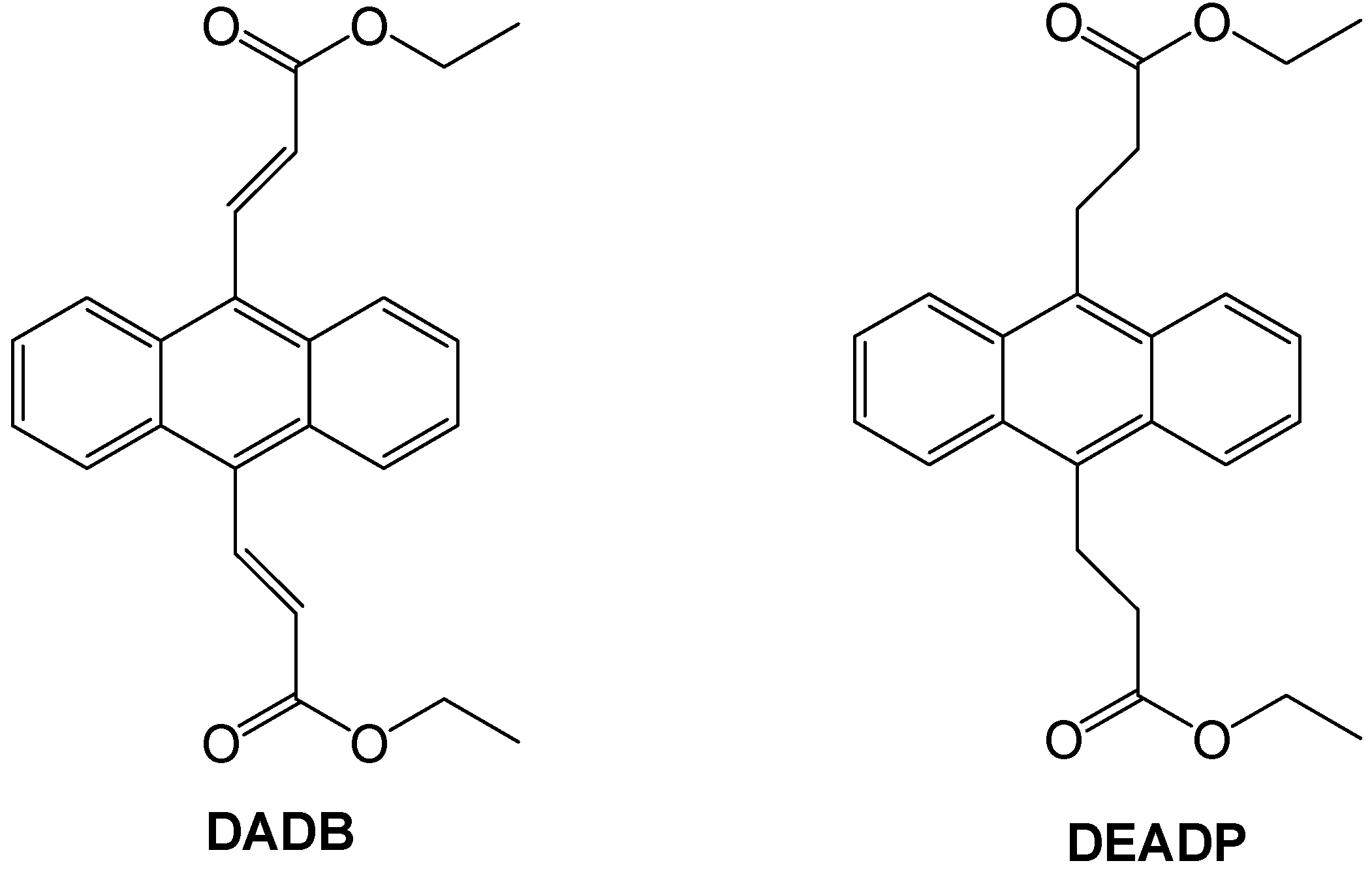
:1. Introduction
2. Experimental and Computational Procedures
2.1. Synthesis and Spectroscopic Analysis
2.2. Thermogravimetric Analysis
2.3. Scanning Electronic Microscopy
2.4. X-ray Crystallography
2.5. Hirshfeld Surface Analysis
2.6. Molecular Modeling Analysis
3. Results and Discussion
3.1. Spectroscopy Analysis
3.2. Thermogravimetric Analysis
3.3. Scanning Electronic Microscopy
3.4. Solid State Studies
3.5. Molecular Modeling Analysis
3.6. Supramolecular Arrangement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, H.D. Unimolecular photochemistry of anthracenes. Chem. Rev. 1993, 93, 145–172. [Google Scholar] [CrossRef]
- Bouas-Laurent, H.; Castellan, A.; Desvergne, J.P.; Lapouyade, R. Photodimerization of anthracenes in fluid solution: Strutural aspects. Chem. Soc. Rev. 2000, 29, 43–55. [Google Scholar] [CrossRef]
- Tomlinson, W.J.; Chandross, E.A.; Fork, R.L.; Pryde, C.A.; Lamola, A.A. Photodimerization of anthracenes in fluid solution: Structural aspects. Appl. Opt. 1972, 11, 533. [Google Scholar] [CrossRef]
- Zehm, D.; Fudickar, W.; Hans, M.; Schilde, U.; Kelling, A.; Linker, T. 9,10-Diarylanthracenes as Molecular Switches: Syntheses, Properties, Isomerisations and Their Reactions with Singlet Oxygen. Chem. Eur. J. 2008, 14, 11429–11441. [Google Scholar] [CrossRef] [PubMed]
- Pierlot, C.; Aubry, J.M.; Briviba, K.; Sies, H.; Mascio, P. Singlet Oxygen, UV-A, and Ozone; Academic Press Inc.: San Diego, CA, USA, 2000. [Google Scholar]
- Martinez, G.R.; Medeiros, M.H.G.; Mascio, P. Utilizacão de endoperoxidos de derivados de naftaleno como fontes químicas de oxigenio singlete em sistemas biológicos. Quim. Nov. 2000, 23, 686. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, H.H.; Wiberg, K.B.; Larsen, D.L.; Parr, J. Photooxidation of methylnaphthalenes. J. Org. Chem. 2005, 70, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, H.H.; Larsen, D.L. Formation of 1,4-endoperoxide from the dye-sensitized photooxygenation of alkyl naphthalenes. J. Chem. Soc. Chem. Commun. 1972, 5, 253–254. [Google Scholar] [CrossRef]
- Pierlot, C.; Aubry, J.M.; Briviba, K.; Sies, H.; Mascio, P. Naphthalene endoperoxides as generators of singlet oxygen in biological media. Methods Enzym. 2000, 319, 3–20. [Google Scholar]
- Pierlot, C.; Hajjam, S.; Barthelemy, C.; Aubry, J.-M. Water-soluble naphthalene derivatives as singlet oxygen (1O2, 1Δg) carriers for biological media. J. Photochem. Photobiol. 1996, 36, 31–39. [Google Scholar] [CrossRef]
- Dewilde, A.; Pellieux, C.; Pierlot, C.; Wattre, P.; Aubry, J.-M. Inactivation of intracellular and 3,3′-(1,4-Naphthylidene)dipropionate. Monomol and dimol photoemission and the effects of 1,4-diazabicyclo[2.2.2]octane. Biol. Chem. 1998, 379, 1377. [Google Scholar] [PubMed]
- Klotz, O.; Pellieux, C.; Briviba, K.; Pierlot, C.; Aubry, J.-M.; Sies, H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur. J. Biochem. 1999, 260, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Severino, D.; Prado, F.M.; Angeli, J.P.F.; Motta, F.D.; Baptista, M.S.; Medeiros, M.H.G.; Mascio, P. Di Singlet molecular oxygen trapping by the fluorescent probe diethyl-3,3′-(9,10-anthracenediyl)bisacrylate synthesized by the Heck reaction. Photochem. Photobiol. Sci. 2011, 10, 1546–1555. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Al, E. Heck reaction synthesis of anthracene and naphthalene derivatives as traps and clean chemical sources of singlet molecular oxygen in biological systems. Photochem. Photobiol. Sci. 2020, 19, 1590–1602. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in retrospect and prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, revision E. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhang, G.; Musgrave, C.B. Comparison of DFT methods for molecular orbital eigenvalue calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef]
- Náray-Szabó, G.; Ferenczy, G.G. Molecular Electrostatics. Chem. Rev. 1995, 95, 829–847. [Google Scholar] [CrossRef]
- Grant, G.H.; Richards, W.G. Computational Chemistry, 1st ed.; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Van Duijneveldt, F.B.; van Duijneveldt-van de Rijdt, J.G.C.M.; van Lenthe, J.H. State of the Art in Counterpoise Theory. Chem. Rev. 1994, 94, 1873–1885. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Matta, C.F.M.; Boyd, R.J. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007; ISBN 9783527307487. [Google Scholar]
- Matta, C.F.; Bader, R.F.W. Atoms-in-molecules study of the genetically encoded amino acids. III. Bond and atomic properties and their correlations with experiment including mutation-induced changes in protein stability and genetic coding. Proteins Struct. Funct. Bioinform. 2003, 52, 360–399. [Google Scholar] [CrossRef]
- Allen, F.H.; Watson, D.; Brammer, L.; Orpen, A.G.; Taylor, R. Typical interatomic distances: Organic compounds. Int. Tables Crystallogr. 2006, C, 790–811. [Google Scholar] [CrossRef]
- Duarte, H.A. Chemical reactivity indexes from density functional theory: Formalism and perspectives. Quim. Nova 2001, 24, 501–508. [Google Scholar] [CrossRef] [Green Version]















| Empirical Formula | C24H22O4 (DADB) | C24H26O4 (DEADP) | ||
|---|---|---|---|---|
| Formula weight | 374.42 | 378.46 | ||
| Temperature | 296(2) K | 298(2) K | ||
| Wavelength | 1.54178 Å | 1.54178 Å | ||
| Crystal system, space group | Monoclinic; P 21/c | Monoclinic; C 2/c | ||
| a = 13.0556(3) Å | α = 90° | a = 28.6843(10) Å | α = 90° | |
| Unit cell dimensions | b = 4.03270(10) Å | β = 90.7920(10)° | b = 4.9458(2) Å | β = 113.062(2)° |
| c = 18.0384(4) Å | γ = 90° | c = 15.8870(6) Å | γ = 90° | |
| Volume | 949.62(4) Å3 | 2073.71(14) Å3 | ||
| Z, Density (calculated) | 2; 1.309 Mg/m3 | 1.212 Mg/m3 | ||
| Absorption coefficient, F(000) | 0.714 mm−1; 396 | 0.654 mm−1; 808 | ||
| Crystal size | 0.071 × 0.138 × 0.372 mm3 | 0.470 × 0.202 × 0.144 mm3 | ||
| Theta range for data collection | 3.385 to 68.159°. | 3.349 to 66.783°. | ||
| Index ranges | −15 ≤ h ≤ 15, −3 ≤ k ≤ 4, −21 ≤ l ≤ 21 | −32 ≤ h ≤ 33. −5 ≤ k ≤ 3. −18 ≤ l ≤ 17 | ||
| Reflections collected; Independent reflections | 5976; 1686 [R(int) = 0.0232] | 6387; 1724 [R(int) = 0.0275] | ||
| Completeness to theta | 98.1% | 92.20% | ||
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | ||
| Data/restraints/parameters | 1686/0/129 | 1724/3/141 | ||
| Goodness-of-fit on F2 | 1.068 | 1.077 | ||
| Final R indices [I > 2sigma(I)] | R1 = 0.0338, wR2 = 0.0958 | R1 = 0.0450. wR2 = 0.1423 | ||
| R indices (all data) | R1 = 0.0384, wR2 = 0.0992 | R1 = 0.0568. wR2 = 0.1562 | ||
| Extinction coefficient | 0.0028(6) | 0.0006(3) | ||
| Largest diff. peak and hole | 0.147 and −0.133 e·Å−3 | 0.235 and −0.206 e·Å−3 | ||
| Molecular Parameters | Dadb (kcal·mol−1) | Deadp (kcal·mol−1) |
|---|---|---|
| ΔEHOMO-LUMO | 116.04 | 121.91 |
| Electronegativity () | 157.31 | 152.40 |
| Chemical Potential () | −99.29 | −91.44 |
| Chemical Hardness ( | 58.02 | 61.00 |
| Global Electrophilicity ( | 61.86 | 65.44 |
| Donor () | ED () | Acceptor () | ED () | (a.u.) | (a.u.) | ||
|---|---|---|---|---|---|---|---|
| Frontal Interaction | |||||||
| Unit 1 to 2 | (C11i–H) | 1.9855 | (C11–H) | 0.0211 | 0.10 | 1.08 | 0.009 |
| (O2i) | 1.9773 | (O1–C11) | 0.0359 | 0.46 | 1.11 | 0.020 | |
| (O2i) | (C12–H) | 0.0046 | 0.10 | 1.28 | 0.010 | ||
| (O2i) | 1.8649 | (O1–C11) | 0.0359 | 0.13 | 0.67 | 0.008 | |
| Unit 2 to 1 | (C11–H) | 1.9852 | (C11i–H) | 0.0205 | 0.07 | 1.05 | 0.008 |
| Axial Interaction | |||||||
| Unit 1 to 2 | (C3i–C4i) | 1.7752 | (C1i–C2i) | 0.4056 | 0.13 | 0.37 | 0.007 |
| (C1–C5) | 0.3934 | 0.19 | 0.38 | 0.008 | |||
| (C2i–C5) | 1.5062 | π*(C5i–C2) | 0.4979 | 0.06 | 0.33 | 0.004 | |
| (C1–C5) | 0.3934 | 0.18 | 0.34 | 0.007 | |||
| (C8i–H) | 1.9686 | (C6i–C7i) | 0.2097 | 0.13 | 0.67 | 0.009 | |
| (C6i–C7i) | 1.7806 | (C6i–C7i) | 0.2097 | 0.15 | 0.39 | 0.007 | |
| (C3–C4) | 0.2028 | 0.20 | 0.40 | 0.008 | |||
| (C11i–H) | 1.9853 | (C10i–H) | 0.0180 | 0.05 | 1.28 | 0.007 | |
| (C12i–H) | 1.9865 | (C11i–H) | 0.0210 | 0.06 | 1.04 | 0.007 | |
| (C1–C2) | 1.6016 | (C3–C4) | 0.2028 | 0.23 | 0.37 | 0.009 | |
| (C6–C7) | 1.7848 | (C8–H) | 0.0225 | 0.18 | 0.83 | 0.011 | |
| (C6–C7) | 0.2057 | 0.20 | 0.40 | 0.008 | |||
| (C9–H) | 1.9741 | (C3–H) | 0.0155 | 0.06 | 1.12 | 0.007 | |
| (C10–O2) | 1.9800 | (C11–H) | 0.0214 | 0.32 | 0.95 | 0.16 | |
| (C11–H) | 1.9857 | (C12–H) | 0.0096 | 0.07 | 1.07 | 0.008 | |
| (O1i) | 1.8133 | (C8i–C9i) | 0.0158 | 0.07 | 0.48 | 0.005 | |
| (C10i–O2i) | 0.2290 | 0.07 | 0.45 | 0.005 | |||
| (O1) | 1.8125 | (C12–H) | 0.0101 | 0.18 | 0.88 | 0.012 | |
| Unit 2 to 1 | (C1i–O2i) | 1.6016 | (C3i–O4i) | 0.2029 | 0.23 | 0.37 | 0.009 |
| (C5i–O2) | 1.5061 | (C1i–O5i) | 0.3933 | 0.18 | 0.34 | 0.007 | |
| (C2i–O5) | 0.4979 | 0.06 | 0.33 | 0.004 | |||
| (C6i–C7i) | 1.7848 | (C8i–H) | 0.0225 | 0.18 | 0.83 | 0.011 | |
| (C6i–C7i) | 0.2056 | 0.20 | 0.40 | 0.008 | |||
| (C9i–H) | 1.9741 | (C3i–H) | 0.0155 | 0.06 | 1.12 | 0.007 | |
| (C10i–O1i) | 1.9800 | (C11i–H) | 0.0214 | 0.32 | 0.95 | 0.016 | |
| (C11i–H) | 1.9857 | (C12i–H) | 0.0096 | 0.07 | 1.07 | 0.008 | |
| (C3–C4) | 1.7753 | (C1i–C5i) | 0.3933 | 0.19 | 0.38 | 0.008 | |
| (C1–C2) | 0.4056 | 0.13 | 0.37 | 0.007 | |||
| (C8–H) | 1.9686 | (C6–C7) | 0.2098 | 0.13 | 0.67 | 0.009 | |
| (C6–C7) | 1.7805 | (C3i–C4i) | 0.2029 | 0.20 | 0.40 | 0.008 | |
| π*(C6–C7) | 0.2098 | 0.15 | 0.39 | 0.007 | |||
| (C11–H) | 1.9852 | (C10–O2) | 0.0180 | 0.05 | 1.28 | 0.007 | |
| (C12–H) | 1.9865 | (C11–H) | 0.0210 | 0.06 | 1.04 | 0.007 | |
| (O1i) | 1.8125 | (C12i–H) | 0.0101 | 0.18 | 0.88 | 0.012 | |
| (O1i) | 1.8133 | (C8–C9) | 0.0568 | 0.07 | 0.48 | 0.005 | |
| (C10–O2) | 0.2291 | 0.07 | 0.45 | 0.005 | |||
| Donor () | ED () | Acceptor () | ED () | (a.u.) | (a.u.) | ||
|---|---|---|---|---|---|---|---|
| Frontal Interaction | |||||||
| Unit 1 to 2 | (C3–C4) | 1.7854 | (C3–H) | 0.0156 | 0.08 | 0.85 | 0.008 |
| (C4–H) | 0.0163 | 0.24 | 0.84 | 0.013 | |||
| σ(C9i–H) | 1.96446 | (C4–H) | 0.0163 | 0.06 | 1.10 | 0.007 | |
| (C1i–C7i) | 1.6120 | π*(C3–C4) | 0.2058 | 0.06 | 0.01 | 0.001 | |
| (C3–C4) | 1.7854 | (C3–H) | 0.0156 | 0.07 | 0.45 | 0.015 | |
| (C4–H) | 0.0163 | 0.06 | 0.45 | 0.014 | |||
| Unit 2 to 1 | σ(C3–H) | 1.9769 | π*(C3–C4) | 0.0162 | 0.18 | 0.67 | 0.010 |
| *(C5–C6) | 0.2054 | 0.07 | 0.67 | 0.006 | |||
| σ(C4–H) | 1.9770 | *(C3–C4) | 0.0162 | 0.08 | 0.66 | 0.007 | |
| (C10i–O1i) | 1.9931 | (C5–H) | 0.0174 | 0.23 | 1.01 | 0.014 | |
| (O1i) | 1.9769 | (C5–H) | 0.0174 | 0.36 | 1.33 | 0.019 | |
| (O1i) | 1.8664 | (C5–H) | 0.0174 | 0.42 | 0.89 | 0.018 | |
| Axial Interaction | |||||||
| Unit 1 to 2 | π(C3– C4) | 1.7794 | (C5–C6) | 0.2098 | 0.22 | 0.39 | 0.008 |
| π(C5– C6) | 1.7882 | (C8–H) | 0.0169 | 0.13 | 0.80 | 0.010 | |
| (C1i–C7i) | 1.6098 | π*(C1i–C2i) | 0.5102 | 0.09 | 0.34 | 0.005 | |
| π*(C3i–C4i) | 0.2083 | 0.35 | 0.37 | 0.011 | |||
| π(C5i– C6i) | 1.7858 | π*(C3i–C4i) | 0.2083 | 0.12 | 0.40 | 0.006 | |
| π(C10i– O1i) | 1.9928 | (C8i–C9i) | 0.0158 | 0.05 | 0.92 | 0.006 | |
| (C9i–H) | 0.0136 | 0.13 | 0.97 | 0.010 | |||
| (C2) | 1.0084 | π*(C1–C7) | 0.3825 | 0.35 | 0.18 | 0.009 | |
| (C8–C9) | 0.0162 | 0.09 | 0.55 | 0.009 | |||
| (O2) | 1.9776 | (C8i–C9i) | 0.0158 | 0.10 | 1.23 | 0.010 | |
| Unit 2 to 1 | (C1–C7) | 1.6099 | (C2) | 1.0084 | 0.13 | 0.18 | 0.005 |
| π*(C3–C4) | 0.2082 | 0.35 | 0.37 | 0.011 | |||
| (C5–C6) | 1.7858 | π*(C3–C4) | 0.2082 | 0.12 | 0.40 | 0.006 | |
| (C8–H) | 1.9758 | π*(C1–C7) | 0.0302 | 0.06 | 0.64 | 0.006 | |
| (C8–H) | 1.9762 | (C2) | 1.0084 | 0.05 | 0.46 | 0.006 | |
| (C10–O1) | 1.9928 | (C8–C9) | 0.0158 | 0.05 | 0.92 | 0.006 | |
| (C9–H) | 0.0136 | 0.13 | 0.97 | 0.010 | |||
| (C1i–C2i) | 1.5094 | π*(C1i–C7i) | 0.3826 | 0.06 | 0.35 | 0.004 | |
| (C8i–C9i) | 0.0162 | 0.10 | 0.71 | 0.009 | |||
| (C3i–C4i) | 1.7793 | (C5i–C6i) | 0.2098 | 0.22 | 0.39 | 0.008 | |
| (C5i–C6i) | 1.7882 | (C8i–H) | 0.0169 | 0.13 | 0.80 | 0.010 | |
| (O1) | 1.9776 | (C8–C9) | 0.0158 | 0.10 | 1.23 | 0.010 | |
| Interaction | Electronic Density, (a.u.) | Laplacian, (a.u.) |
|---|---|---|
| Frontal Interaction | ||
| (CO)–O C11 | 0.023 | 0.037 |
| C11–H H–C11 | 0.027 | 0.062 |
| Axial Interaction | ||
| C10–O2 H–C11 | 0.021 | 0.035 |
| O1 H–C12 | 0.025 | 0.057 |
| C8–H C6 | 0.029 | 0.037 |
| C9–H H–C3 | 0.042 | 0.005 |
| C2 C3 | 0.030 | 0.025 |
| Interaction | Electronic Density, (a.u.) | Laplacian, (a.u.) |
|---|---|---|
| Frontal Interaction | ||
| C8–H H–CAR | 0.017 | 0.034 |
| CAR–H H–CAR | 0.022 | 0.016 |
| CAR–H O1 = C10 | 0.033 | 0.031 |
| Axial Interaction | ||
| C11–H H–C12 | 0.016 | 0.014 |
| C10 = O1 H–C8 | 0.021 | 0.045 |
| C8–H CAR | 0.023 | 0.015 |
| CAR CAR | 0.024 | 0.008 |
| CAR H–C8 | 0.013 | 0.011 |
| C9–H O1 = C10 | 0.020 | 0.037 |
| C12–H H–C11 | 0.015 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.F.; Oliveira, M.S.; Aguiar, A.S.N.; Custodio, J.M.F.; Di Mascio, P.; Sabino, J.R.; Verde, G.V.; Souza, J.C.P.d.; Santin, L.G.; Camargo, A.J.; et al. Synthesis and Structural Studies of Two New Anthracene Derivatives. Crystals 2021, 11, 934. https://doi.org/10.3390/cryst11080934
Costa RF, Oliveira MS, Aguiar ASN, Custodio JMF, Di Mascio P, Sabino JR, Verde GV, Souza JCPd, Santin LG, Camargo AJ, et al. Synthesis and Structural Studies of Two New Anthracene Derivatives. Crystals. 2021; 11(8):934. https://doi.org/10.3390/cryst11080934
Chicago/Turabian StyleCosta, Rogério F., Marilene S. Oliveira, Antônio S. N. Aguiar, Jean M. F. Custodio, Paolo Di Mascio, José R. Sabino, Giuliana V. Verde, João Carlos Perbone de Souza, Lauriane G. Santin, Ademir J. Camargo, and et al. 2021. "Synthesis and Structural Studies of Two New Anthracene Derivatives" Crystals 11, no. 8: 934. https://doi.org/10.3390/cryst11080934
APA StyleCosta, R. F., Oliveira, M. S., Aguiar, A. S. N., Custodio, J. M. F., Di Mascio, P., Sabino, J. R., Verde, G. V., Souza, J. C. P. d., Santin, L. G., Camargo, A. J., Barbosa, I. C., Oliveira, S. S., & Napolitano, H. B. (2021). Synthesis and Structural Studies of Two New Anthracene Derivatives. Crystals, 11(8), 934. https://doi.org/10.3390/cryst11080934








