RETRACTED: Preparation and Characterization of Mesoporous Silica from Bagasse Bottom Ash from the Sugar Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bagasse Bottom Ash
2.2. Chemical and Mineralogical Analyses
2.3. Silica Extraction
2.4. Preparation of Mesoporous Silica
3. Results and Discussion
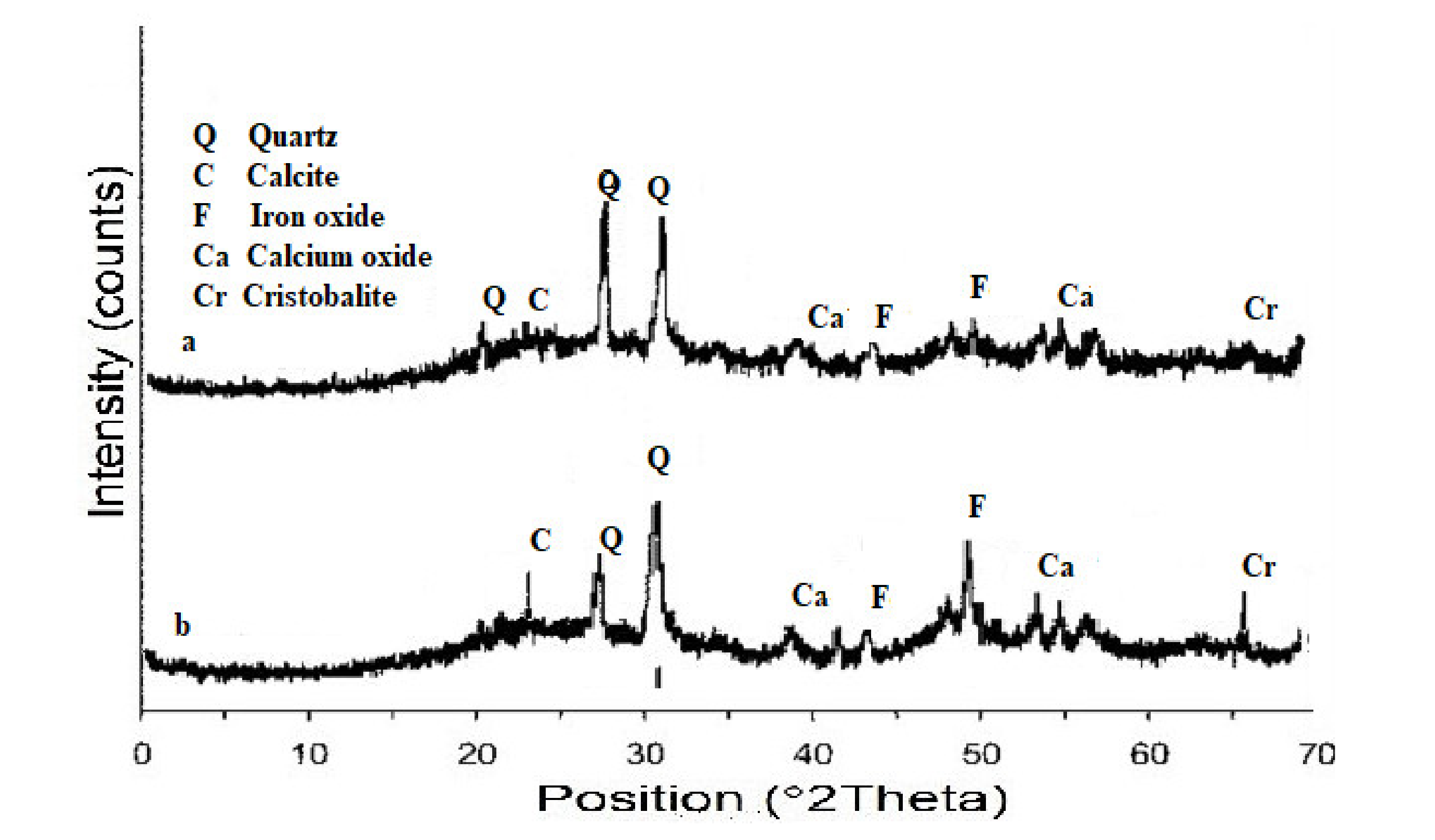
3.1. Characterization of BBA
3.2. Pretreatment of BBA
3.3. Silica Extraction
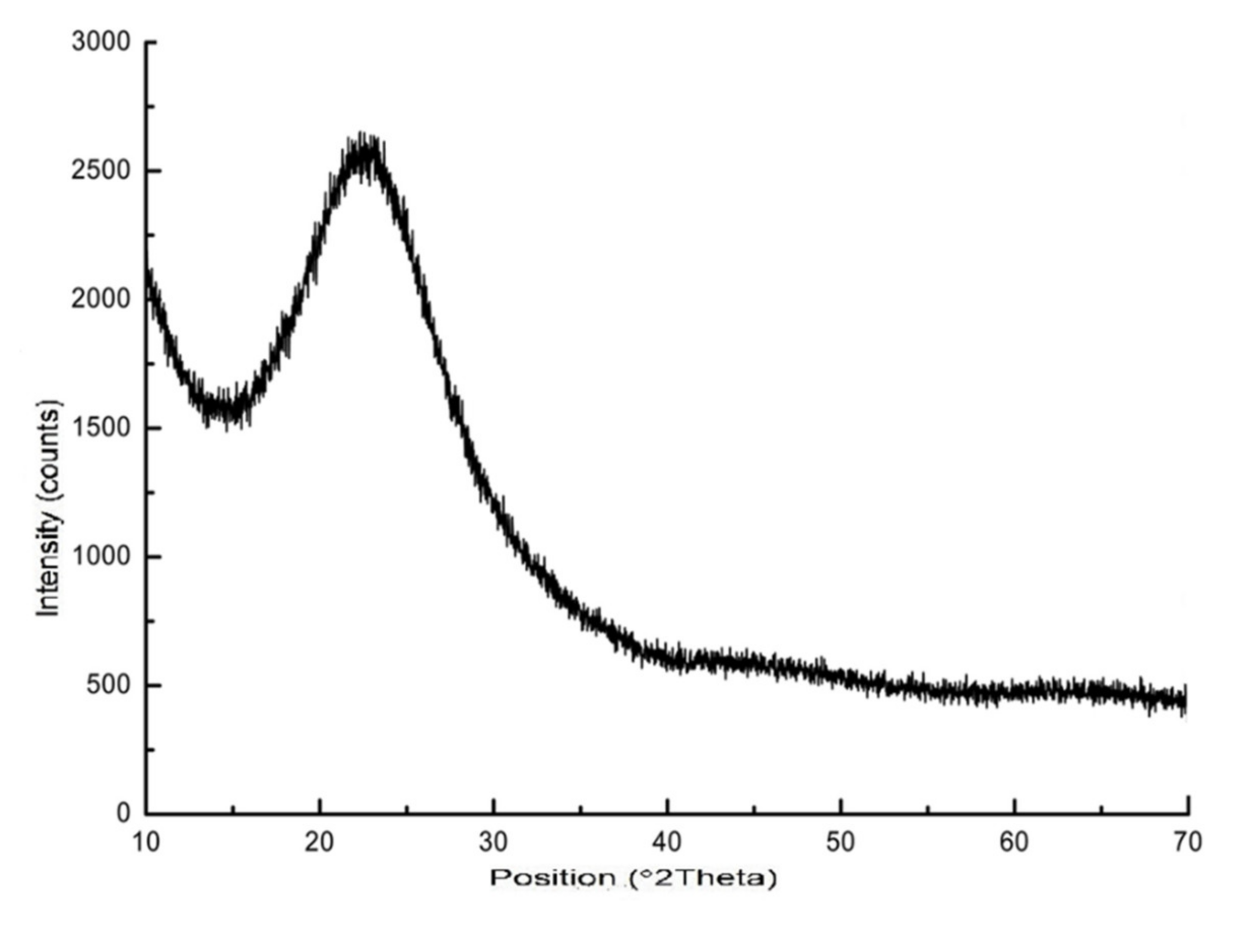
3.4. Mesoporous Silica
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papa, E.; Medri, V.; Amari, S.; Manaud, J.; Benito, P.; Vaccari, A.; Landi, E. Zeolite-geopolymer composite materials: Production and characterization. J. Clean. Prod. 2018, 171, 76–84. [Google Scholar] [CrossRef]
- Prazeres, S.F.; Zapata, F.; Canilho, N.; Pasc, A.; García-Ruiz, C.; Montalvo, G. Probing the confinement of β-galactosidase into meso-macro porous silica by Raman spectroscopy. Microp. Mesop. Mat. 2018, 278, 149–155. [Google Scholar] [CrossRef]
- Affandi, S.; Setyawan, H.; Winardi, S.; Purwanto, A.; Balgis, R. A facile method for production of high-purity silica xerogels from bagasse ash. Adv. Powd. Tech. 2009, 20, 468–472. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Lee, K.J.; Cho, M.; Lim, S.S.; Seo, S.K.; Cho, K.M.; Bang, K.S.; Oh, B.T. Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J. Ind. Eng. Chem. 2015, 29, 298–303. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Li, M.; Fang, Y. Understanding fly-ash formation during fluidized-bed gasification of high-silicon-aluminum coal based on its characteristics. Energy 2018, 150, 142–152. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Ranjbar, N. Synthesis of sodium waterglass from white rice husk ash as an activator to produce metakaolin-based geopolymer cements. J. Build. Eng. 2016, 6, 252–261. [Google Scholar] [CrossRef]
- Nazari, A.; Sanjayan, J. Synthesis of geopolymer from industrial wastes. J. Clean. Prod. 2015, 99, 297–304. [Google Scholar] [CrossRef]
- Amin, N.; Alam, S.; Gul, S. Effect of Thermally activated clay on corrosion and chloride resistivity of cement mortar. J. Clean. Prod. 2016, 111, 155–160. [Google Scholar] [CrossRef]
- Cui, S.; Yu, S.; Lin, B.; Shen, X.; Gu, D. Preparation of SiO2 aerogel from rice husk ash. RSC Adv. 2015, 5, 65818–65826. [Google Scholar] [CrossRef]
- Sagon, S.; Hunt, A.J.; Attard, T.M.; Mengchang, P.; Ngernyen, Y.; Supanchaiyamat, N. Valorisation of waste rice straw for the production of highly effective carbon based adsorbents for dyes removal. J. Clean. Prod. 2018, 172, 1128–1139. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Ju, F.; Han, L.; Chang, L.; Bao, W. Supercritical hydrothermal synthesis of zeolites from coal fly ash for mercury removal from coal derived gas. Fuel Proces. Tech. 2015, 136, 96–105. [Google Scholar] [CrossRef]
- Nazriati, N.; Setyawan, H.; Affandi, S.; Yuwana, M.; Winardi, S. Using bagasse ash a a silica source when preparing silica aerogels via ambient pressure drying. J. Non-Cryst. Solid. 2014, 400, 6–11. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Soe, J.T.; Zhang, S.; Ahn, J.-W.; Park, M.B.; Ahn, W.-S. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: A mini review. Chem. Eng. J. 2017, 317, 821–843. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.C.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of zeolites at low temperatures in fly ash-kaolinite mixtures. Microporous Mesoporous Mater. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Javadian, H.; Ghorbani, F.; Tayebi, H.-a.; Asl, S.M.H. Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab. J. Chem. 2015, 8, 837–849. [Google Scholar] [CrossRef]
- Amin, N.; Alam, S.; Gul, S. Assessment of pozzolanic activity of thermally activated clay and its impact on strength development in cement mortar. RSC Adv. 2015, 5, 6079–6084. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H. Synthesis of MCM-41 from coal fly ash by a green approach: Influence of synthesis pH. J. Hazard. Mater. 2006, 137, 1135–1148. [Google Scholar] [CrossRef]
- Shim, J.; Velmurugan, P.; Oh, B.T. Extraction and physical characterization of amorphous silica made from corn cob ash at variable pH conditions via sol gel processing. J. Ind. Eng. Chem. 2015, 30, 249–253. [Google Scholar] [CrossRef]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302, 110203. [Google Scholar] [CrossRef]
- Costantini, A.; Venezia, V.; Pota, G.; Bifulco, A.; Califano, V.; Sannino, F. Adsorption of Cellulase on Wrinkled Silica Nanoparticles with Enhanced Inter-Wrinkle Distance. Nanomaterials 2020, 10, 1799. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Hu, Y.; Sun, L.; Bai, L.; Jiang, T.; Wang, S. Ordered nanoporous silica as carriers for improved delivery of water-insoluble drugs: A comparative study between three dimensional and twodimensional macroporous silica. Int. J. Nanomed. 2013, 8, 4015–4031. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-CanulL, M.I.; González, L.A. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Okoronkwo, E.A.; Imoisili, P.E.; Olusunle, S.O.O. Extraction and Characterization of Amorphous Silica from Corn Cob Ash by Sol-Gel Method. J. Chem. Mater. Res. 2013, 3, 68–72. [Google Scholar] [CrossRef]
- Amin, N. A multi-directional utilization of different ashes. RSC Adv. 2014, 4, 62769–62788. [Google Scholar] [CrossRef]
- Jay, G.; Ali, N.; Chen, L.; Nguyen, G.H. Physical and mechanical properties of lightweight aerated geopolymer. Constr. Build. Mater. 2015, 79, 236–244. [Google Scholar] [CrossRef]
- Salem, S.; Salem, A. A novel design for clean and economical manufacturing new nano-porous zeolite based adsorbent by alkali cement kiln dust for lead uptake from wastewater. J. Clean. Prod. 2017, 143, 440–451. [Google Scholar] [CrossRef]
- Van, V.T.; Christiane, R.; Bui, D.D.; Ludwig, H.M. Pozzolanic reactivity of mesoporous amorphous rice husk ash in portlandite solution. Constr. Build. Mater. 2014, 59, 111–119. [Google Scholar] [CrossRef]



| SiO2 | CaO | Al2O3 | Fe2O3 | Na2O | MgO | K2O | ZnO | CuO | LOI | |
| BBA | 56.52 | 14.62 | 7.93 | 6.44 | 2.92 | 2.01 | 0.91 | 0.34 | 0.32 | 2.75 |
| TBBA | 54.89 | 9.87 | 7.63 | 3.56 | 2.51 | 1.54 | 0.82 | 0.22 | 0.12 | 16.33 |
| Mesoporous | 99.32 | 0.22 | 0.30 | 0.04 | ||||||
| Silica |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, N.; Gul, S.; Sultana, S.; Alam, S. RETRACTED: Preparation and Characterization of Mesoporous Silica from Bagasse Bottom Ash from the Sugar Industry. Crystals 2021, 11, 938. https://doi.org/10.3390/cryst11080938
Amin N, Gul S, Sultana S, Alam S. RETRACTED: Preparation and Characterization of Mesoporous Silica from Bagasse Bottom Ash from the Sugar Industry. Crystals. 2021; 11(8):938. https://doi.org/10.3390/cryst11080938
Chicago/Turabian StyleAmin, Noorul, Saeed Gul, Sabiha Sultana, and Sultan Alam. 2021. "RETRACTED: Preparation and Characterization of Mesoporous Silica from Bagasse Bottom Ash from the Sugar Industry" Crystals 11, no. 8: 938. https://doi.org/10.3390/cryst11080938
APA StyleAmin, N., Gul, S., Sultana, S., & Alam, S. (2021). RETRACTED: Preparation and Characterization of Mesoporous Silica from Bagasse Bottom Ash from the Sugar Industry. Crystals, 11(8), 938. https://doi.org/10.3390/cryst11080938





