Mesomorphic Behaviour and DFT Insight of Arylidene Schiff Base Liquid Crystals and Their Pyridine Impact Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of N-arylidene-4-alkylbenzenamine (3.a–3.h)
2.2. Computational Method
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Mesomorphic Behaviour
3.3. DFT Calculations
3.4. Frontier Molecular Orbitals (FMOs)
3.5. Molecular Electrostatic Potential (MEP)
3.6. Aromaticity, π–π Stacking and LOL-π
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geelhaar, T.; Griesar, K.; Reckmann, B. 125 years of liquid crystals—A scientific revolution in the home. Angew. Chem. Int. Ed. 2013, 52, 8798–8809. [Google Scholar] [CrossRef]
- Lin, B.; Chen, S.; Chen, H.; Lee, J.; Wu, S. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light Sci. 2018, 7, 17168. [Google Scholar]
- Bisoyi, H.K.; Li, Q. Light-driven liquid crystalline materials: From photo-induced phase transitions and property modulations to applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Han, M.J.; Wei, D.; Kim, Y.H.; Ahn, H.; Shin, T.J.; Clark, N.A.; Walba, D.M.; Yoon, D.K. Highly oriented liquid crystal semiconductor for organic field-effect transistors. ACS Cent. Sci. 2018, 4, 1495–1502. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, Y.; Yu, H.; Zhou, Z.; Turiv, T.; Lavrentovich, O.D.; Wei, Q.H. Low f-Number Diffraction-Limited Pancharatnam–Berry Microlenses Enabled by Plasmonic Photopatterning of Liquid Crystal Polymers. Adv. Mater. 2019, 31, 1808028. [Google Scholar] [CrossRef]
- Ula, S.W.; Traugutt, N.A.; Volpe, R.H.; Patel, R.R.; Yu, K.; Yakacki, C.M. Liquid crystal elastomers: An introduction and review of emerging technologies. Liq. Cryst. Rev. 2018, 6, 78–107. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Xiao, Y.Y.; Tong, X.; Zhao, Y. Selective Decrosslinking in Liquid Crystal Polymer Actuators for Optical Reconfiguration of Origami and Light-Fueled Locomotion. Angew. Chem. Int. Ed. 2019, 58, 5332–5337. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Shanks, K.; Ghosh, A.; Tahir, A.; Sundaram, S.; Mallick, T.K. Temperature regulation of concentrating photovoltaic window using argon gas and polymer dispersed liquid crystal films. Renew. Energy 2021, 164, 96–108. [Google Scholar] [CrossRef]
- Zhang, C.; Nakano, K.; Nakamura, M.; Araoka, F.; Tajima, K.; Miyajima, D. Noncentrosymmetric Columnar Liquid Crystals with the Bulk Photovoltaic Effect for Organic Photodetectors. J. Am. Chem. Soc. 2020, 142, 3326–3330. [Google Scholar] [CrossRef]
- Siddiqi, H.M.; Kun Lin, Y.; Hussain, Z.; Majeed, N. Bovine serum albumin protein-based liquid crystal biosensors for optical detection of toxic heavy metals in water. Sensors 2020, 20, 298. [Google Scholar]
- Lee, M.-J.; Lee, W. Liquid crystal-based capacitive, electro-optical and dielectric biosensors for protein quantitation. Liq. Cryst. 2020, 47, 1145–1153. [Google Scholar] [CrossRef]
- Wilkinson, T. Applications of liquid crystals in telecommunications. Handb. Liq. Cryst. 2014, 8, 1–28. [Google Scholar] [CrossRef]
- Yilmaz Canli, N.; Ocak, H.; Okutan, M.; Karanlık, G.; Bilgin Eran, B. Comparative dielectric parameters and conductivity mechanisms of pyridine-based rod-like liquid crystals. Phase Transit. 2020, 93, 784–792. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Zhong, X.; Hu, J.; Shi, F.; Mi, H. Preparation and catalytic performance of alginate-based Schiff Base. Carbohydr. Polym. 2019, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, J.E.; Reinig, K.M.; Barnes, C.L.; Kelley, S.P.; Jurisson, S.S. Technetium and rhenium Schiff base compounds for nuclear medicine: Syntheses of rhenium analogues to 99mTc-furifosmin. Inorg. Chem. 2018, 57, 12920–12933. [Google Scholar] [CrossRef]
- Ansari, K.; Chauhan, D.S.; Quraishi, M.; Mazumder, M.A.; Singh, A. Chitosan Schiff base: An environmentally benign biological macromolecule as a new corrosion inhibitor for oil & gas industries. Int. J. Biol. Macromol. 2020, 144, 305–315. [Google Scholar] [PubMed]
- Guo, S.; Liu, G.; Fan, C.; Pu, S. A new diarylethene-derived probe for colorimetric sensing of Cu (II) and fluorometric sensing of Cu (II) and Zn (II): Photochromism and high selectivity. Sens. Actuators B Chem. 2018, 266, 603–613. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Hagar, M.; Ahmed, H.A.; Naoum, M.M.; Sobaih, H.A.; Almshaly, J.S.; Haddad, M.M.; Alhaisoni, R.A.; Alsobhi, T.A. Binary liquid crystal mixtures based on schiff base derivatives with oriented lateral substituents. Crystals 2020, 10, 319. [Google Scholar] [CrossRef]
- Ahmed, H.; Mansour, E.; Hagar, M. Mesomorphic study and DFT simulation of calamitic Schiff base liquid crystals with electronically different terminal groups and their binary mixtures. Liq. Cryst. 2020, 47, 2292–2304. [Google Scholar] [CrossRef]
- Nakum, K.J.; Katariya, K.D.; Jadeja, R.; Prajapati, A. Schiff base of 4-n-alkoxy-2-hydroxy benzaldehyde with 4-amino acetophenone and their Cu (II) complexes: Synthesis, characterization and mesomorphic behavior. Mol. Cryst. Liq. Cryst. 2019, 690, 1–13. [Google Scholar] [CrossRef]
- Nakum, K.J.; Katariya, K.D.; Jadeja, R.N. Synthesis, characterization, and mesomorphic properties of some new Schiff base homologues series and their Cu (II) complexes. Mol. Cryst. Liq. Cryst. 2020, 708, 1–13. [Google Scholar] [CrossRef]
- Cîrcu, V.; Mocanu, A.S.; Roşu, C.; Manaila-Maximean, D.; Dumitraşcu, F. Thermal behaviour and electro-optical properties of a series of liquid crystals based on palladium complexes with mixed ligands: Schiff bases and N-benzoyl thioureas. J. Therm. Anal. Calorim. 2012, 107, 877–886. [Google Scholar] [CrossRef]
- Roşu, C.; Manaila-Maximean, D.; Cîrcu, V.; Molard, Y.; Roisnel, T. Differential negative resistance in the current–voltage characteristics of a new palladium (II) metallomesogen. Liq. Cryst. 2011, 38, 757–765. [Google Scholar] [CrossRef]
- Patel, R.V.; Panchal, J.G.; Rana, V.; Menon, S.K. Liquid crystals based on calix [4] arene Schiff bases. J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 285–295. [Google Scholar] [CrossRef]
- Vardar, D.; Ocak, H.; Akdaş Kılıç, H.; Jeannin, O.; Camerel, F.; Eran, B.B. Synthesis and characterization of new pyridine-based chiral calamitic liquid crystals. Liq. Cryst. 2020, 850–861. [Google Scholar] [CrossRef]
- Nafee, S.S.; Ahmed, H.; Hagar, M. Theoretical, experimental and optical study of new thiophene-based liquid crystals and their positional isomers. Liq. Cryst. 2020, 47, 1291–1302. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bodurtha, K.J.; Heeder, N.J.; Godfrin, M.P.; Tripathi, A.; Hurt, R.H.; Shukla, A.; Bose, A. Massive electrical conductivity enhancement of multilayer graphene/polystyrene composites using a nonconductive filler. ACS Appl. Mater. Interfaces 2014, 6, 16472–16475. [Google Scholar] [CrossRef]
- Fouad, F.S.; Ness, T.; Wang, K.; Ruth, C.E.; Britton, S.; Twieg, R.J. Biphenylyl-1, 2, 4-oxadiazole based liquid crystals–synthesis, mesomorphism, effect of lateral monofluorination. Liq. Cryst. 2019, 46, 2281–2290. [Google Scholar] [CrossRef]
- Ibraheem, T.K.; Karam, N.H.; Al-Dujaili, A.H. Synthesis and characterization of symmetrical liquid crystalline compounds based on oxazole and thaizole rings. Mol. Cryst. Liq. Cryst. 2020, 710, 1–12. [Google Scholar] [CrossRef]
- Devadiga, D.; Ahipa, T. Heterodimeric hydrogen-bonded mesogens comprising pyridine moiety: A review. Liq. Cryst. Rev. 2020, 8, 5–28. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2020, 47, 114–124. [Google Scholar] [CrossRef]
- Vardar, D.; Akdaş Kılıç, H.; Ocak, H.; Jeannin, O.; Camerel, F.; Eran, B.B. Pyridine-based chiral smectogens: Effects of polar end groups on liquid crystal properties. Liq. Cryst. 2020, 1–10. [Google Scholar] [CrossRef]
- Petrov, V.F. Nitrogen-containing fused heterocycles as the structural fragments in calamitic liquid crystals. Liq. Cryst. 2001, 28, 217–240. [Google Scholar] [CrossRef]
- Nash, J.; Gray, G. Studies of some heterocyclic mesogens. Mol. Cryst. Liq. Cryst. 1974, 25, 299–321. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, C.H.; Cheng, P.S.; Tsai, W.L. Aromatic heterocycles used as terminal groups of calamitic liquid crystals. J. Chin. Chem. Soc. 2012, 59, 81–86. [Google Scholar] [CrossRef]
- Ong, L.-K.; Ha, S.-T.; Yeap, G.-Y.; Lin, H.-C. Heterocyclic pyridine-based liquid crystals: Synthesis and mesomorphic properties. Liq. Cryst. 2018, 45, 1574–1584. [Google Scholar] [CrossRef]
- Muniprasad, M.; Srinivasulu, M.; Chalapathi, P.; Potukuchi, D. Induction of liquid crystalline phases and influence of chain length of fatty acids in linear hydrogen-bonded liquid crystal complexes. Mol. Cryst. Liq. Cryst. 2012, 557, 102–117. [Google Scholar] [CrossRef]
- Srinivasu, C.; Pisipati, V.G.; Prabhu, C.R.; Murty, P.N.; Lakshiminarayana, S. Phase transitions in p-(phenyl benzylidene)-p1-alkylaniline compounds: A dilatometric study. Z. Für Nat. A 2007, 62, 75–83. [Google Scholar] [CrossRef]
- Bhagavath, P.; Mahabaleshwara, S.; Bhat, S.G.; Potukuchi, D.; Shetty, P.; Maddasani, S. Influence of polar substituents and flexible chain length on mesomorphism in non-mesogenic linear hydrogen bonded complexes. J. Mol. Liq. 2021, 336, 116313. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Paterson, D.A.; Abberley, J.P.; Harrison, W.T.; Storey, J.M.; Imrie, C.T. Cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 44, 127–146. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Scrocco, E.; Tomasi, J. Electronic molecular structure, reactivity and intermolecular forces: An euristic interpretation by means of electrostatic molecular potentials. In Advances in quantum chemistry; Elsevier: Amsterdam, The Netherlands, 1978; Volume 11, pp. 115–193. [Google Scholar]
- Politzer, P.; Murray, J.S. Relationships between dissociation energies and electrostatic potentials of C-NO2 bonds: Applications to impact sensitivities. J. Mol. Struct. 1996, 376, 419–424. [Google Scholar] [CrossRef]
- Marks, T.J.; Ratner, M.A. Design, synthesis, and properties of molecule-based assemblies with large second-order optical nonlinearities. Angew. Chem. Int. Ed. Engl. 1995, 34, 155–173. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Giambiagi, M.; de Giambiagi, M.S.; Mundim, K.C. Definition of a multicenter bond index. Struct. Chem. 1990, 1, 423–427. [Google Scholar] [CrossRef]
- Gonthier, J.F.; Steinmann, S.N.; Roch, L.; Ruggi, A.; Luisier, N.; Severin, K.; Corminboeuf, C. π-Depletion as a criterion to predict π-stacking ability. Chem. Commun. 2012, 48, 9239–9241. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. A simple method of identifying π orbitals for non-planar systems and a protocol of studying π electronic structure. Theor. Chem. Acc. 2020, 139, 1–12. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

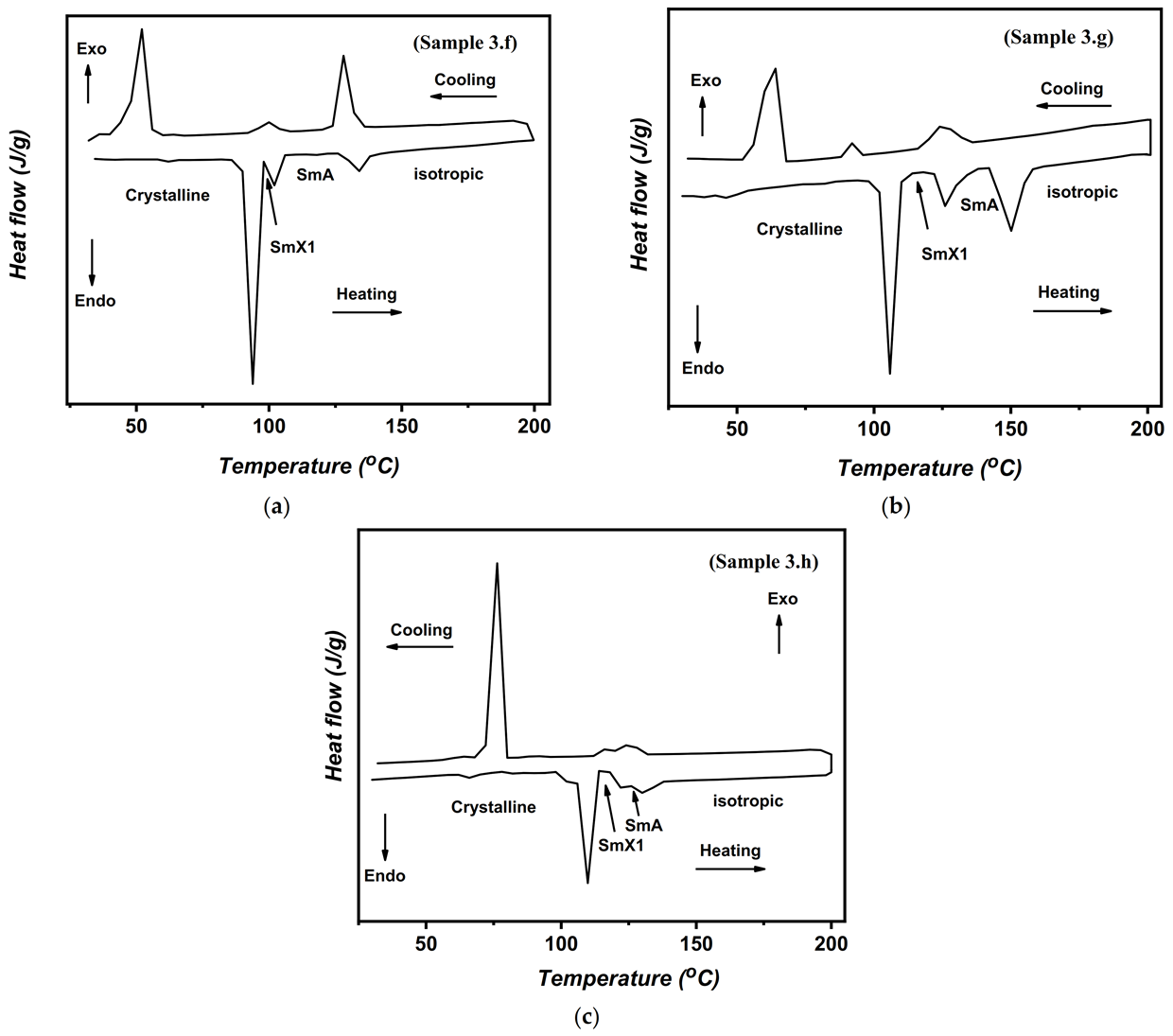






| Compound | °C | kJ/mol | °C | kJ/mol | °C | kJ/mol | °C | kJ/mol | °C | kJ/mol | °C | kJ/mol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCr-I | ΔHCr-I | TCr-SmX1 | ΔHCr-SmX1 | TSmX1-SmA | ΔHSm A-SmX1 | TSmA-I | ΔHSm A-I | TSmA-N | ΔHSmA-N | TN-I | ΔHN-I | |
| 3.b [40] | 63.4 | 11.54 | ||||||||||
| 3.c [39] | 95.1 | 28.09 | 130.0 | 3.03 | 132.2 | 1.77 | 134.5 | 0.46 | ||||
| 3.d [39] | 105.4 | 53.10 | 121.5 | 5.09 | 128.6 | 5.68 | ||||||
| 3.e [39] | 109.0 | 46.62 | 116.9 | 3.78 | 124.6 | 3.71 | ||||||
| 3.f | 93.9 | 26.78 | 102.0 | 4.16 | 134.0 | 3.62 | ||||||
| 3.g | 105.8 | 38.23 | 126.0 | 3.07 | 150.1 | 6.95 | ||||||
| 3.h | 109.9 | 40.62 | 122.1 | 4.05 | 130.0 | 6.52 |
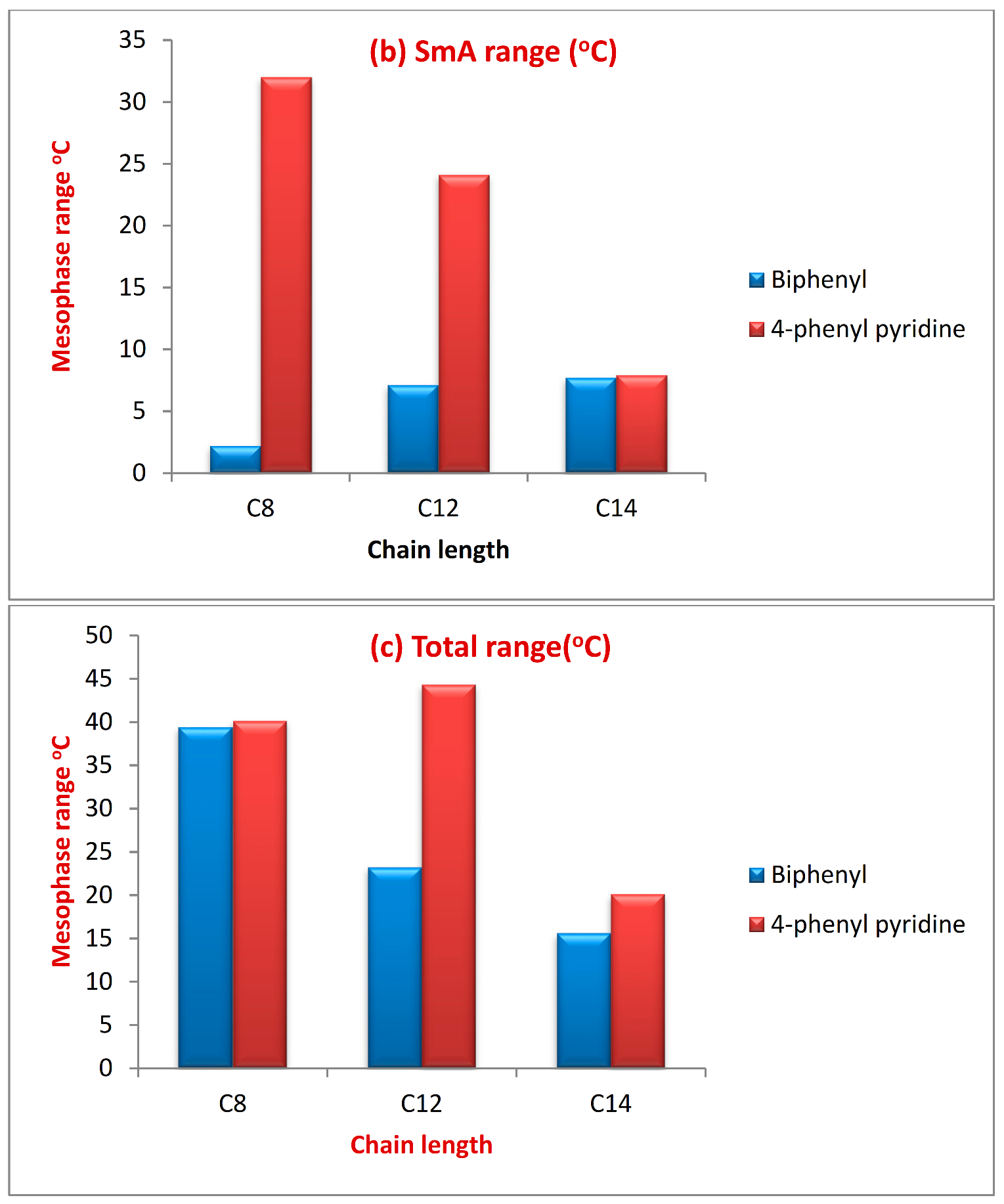
| Alkyl Chain Length | SmX1 Range (°C) | SmA Range (°C) | Total Range (°C) | |||
|---|---|---|---|---|---|---|
| Biphenyl | 4-Phenylpyridine | Biphenyl | 4-Phenylpyridine | Biphenyl | 4-phenylpyridine | |
| C8 | 34.9 | 8.1 | 2.2 | 32.0 | 39.4 | 40.1 |
| C12 | 16.1 | 20.2 | 7.1 | 24.1 | 23.2 | 44.3 |
| C14 | 7.9 | 12.2 | 7.7 | 7.9 | 15.6 | 20.1 |
| Compounds | HOMO | LUMO | ΔE |
|---|---|---|---|
| 3.a | −5.92 | −1.75 | 4.18 |
| 3.b | −6.22 | −2.18 | 4.05 |
| 3.e | −5.81 | −1.92 | 3.89 |
| 3.h | −6.01 | −2.14 | 3.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, M.A.; Alazmi, M.; Katariya, K.D.; El Kilany, Y.; El Ashry, E.S.H.; Jaremko, M.; Hagar, M.; Mohammady, S.Z. Mesomorphic Behaviour and DFT Insight of Arylidene Schiff Base Liquid Crystals and Their Pyridine Impact Investigation. Crystals 2021, 11, 978. https://doi.org/10.3390/cryst11080978
Zakaria MA, Alazmi M, Katariya KD, El Kilany Y, El Ashry ESH, Jaremko M, Hagar M, Mohammady SZ. Mesomorphic Behaviour and DFT Insight of Arylidene Schiff Base Liquid Crystals and Their Pyridine Impact Investigation. Crystals. 2021; 11(8):978. https://doi.org/10.3390/cryst11080978
Chicago/Turabian StyleZakaria, Mohamed A., Mohammed Alazmi, Kanubhai D. Katariya, Yeldez El Kilany, El Sayed H. El Ashry, Mariusz Jaremko, Mohamed Hagar, and Sayed Z. Mohammady. 2021. "Mesomorphic Behaviour and DFT Insight of Arylidene Schiff Base Liquid Crystals and Their Pyridine Impact Investigation" Crystals 11, no. 8: 978. https://doi.org/10.3390/cryst11080978






