Manufacturing Processes of Microporous Polyolefin Separators for Lithium-Ion Batteries and Correlations between Mechanical and Physical Properties
Abstract
:1. Introduction
2. Manufacturing: Dry and Wet Processes with Uniaxial or Biaxial Stretching
2.1. Dry Processing for Microporous Polypropylene Separators
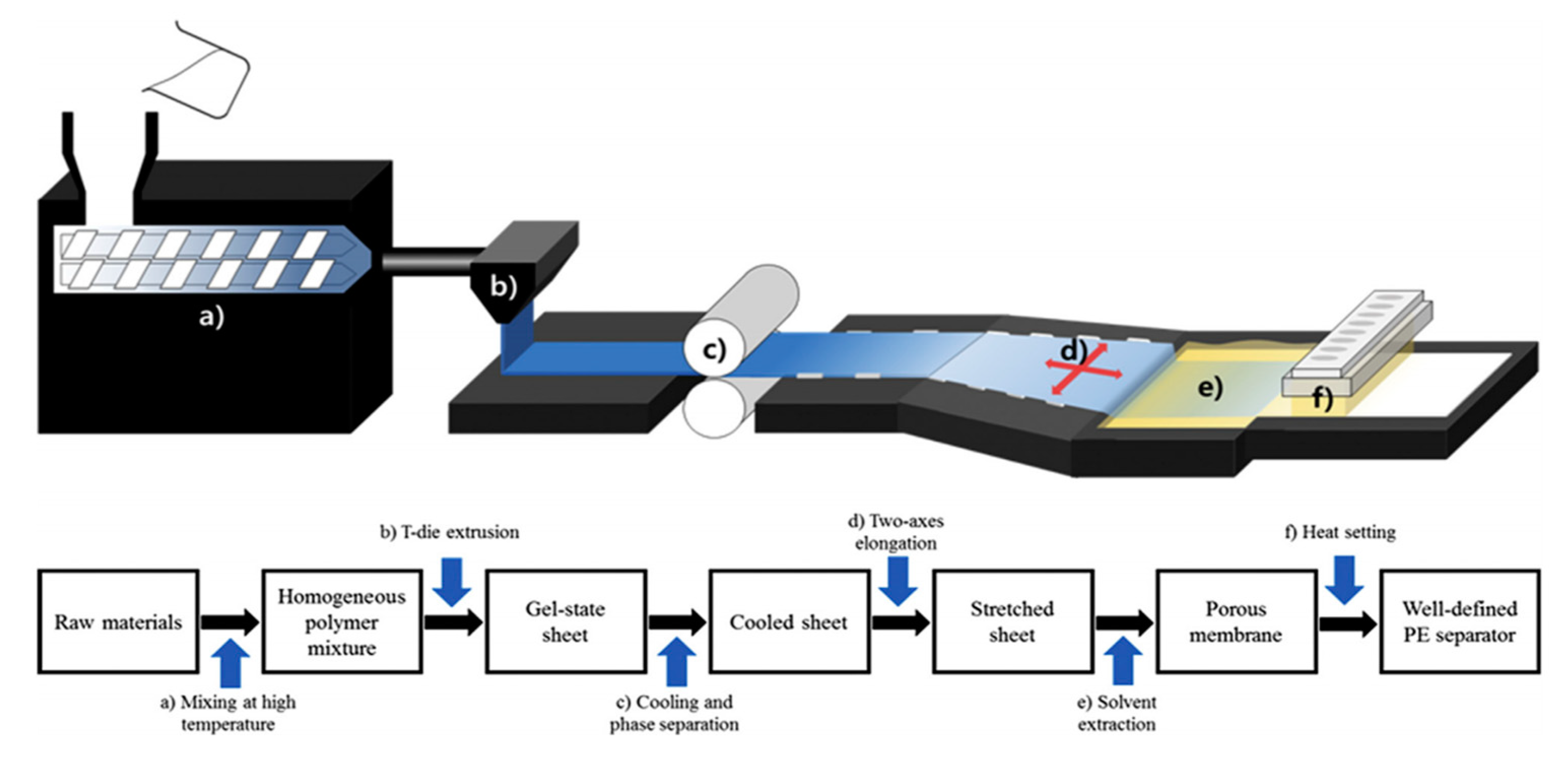
2.2. Wet Process for Microporous Polyethylene Separators
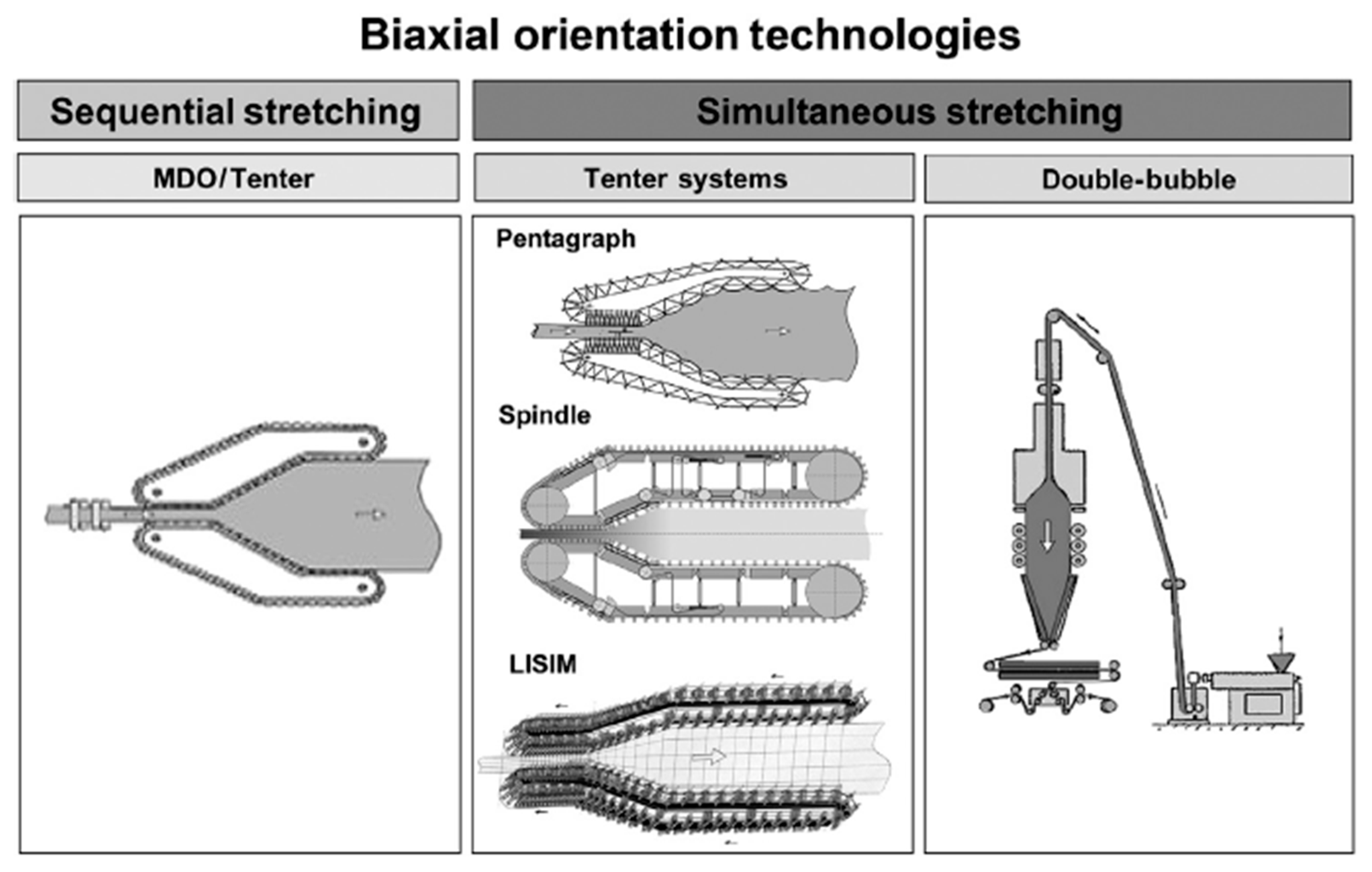
2.3. Biaxial Stretching of Separators: Simultaneous and Sequential Stretching
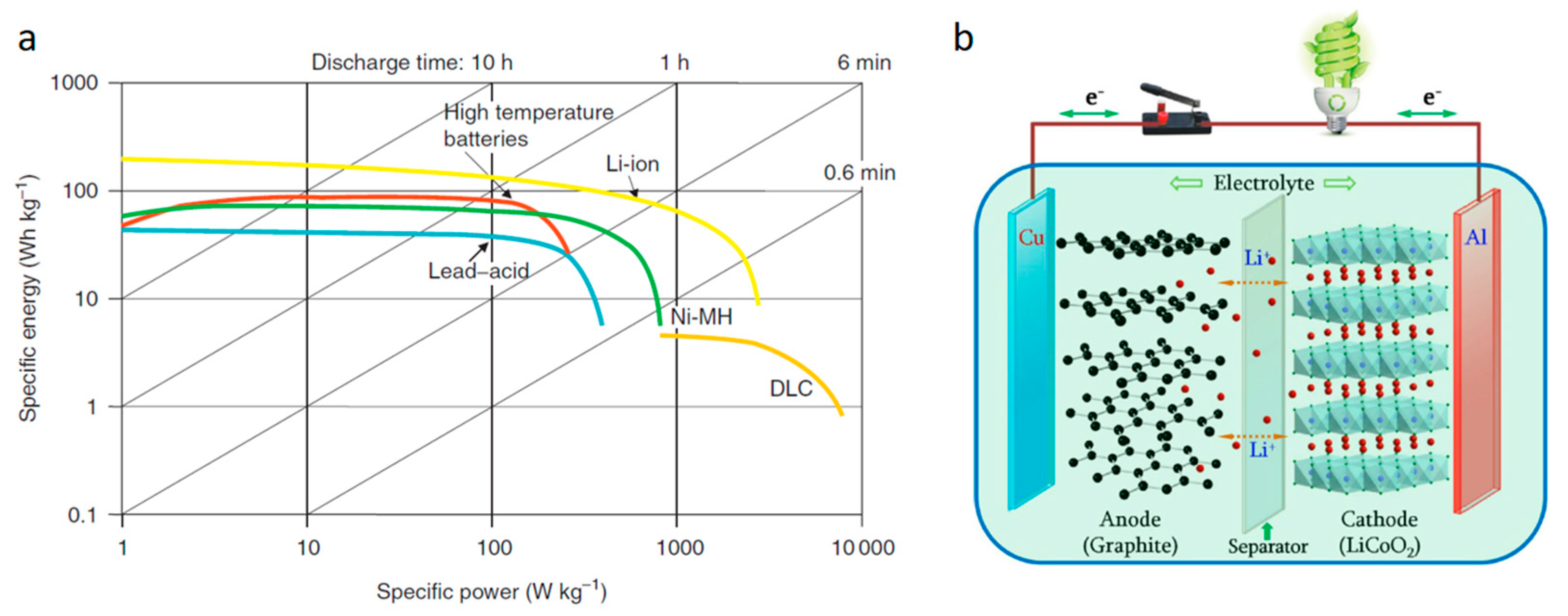
3. Thermal Stability for Safety Features
4. Mechanical Properties
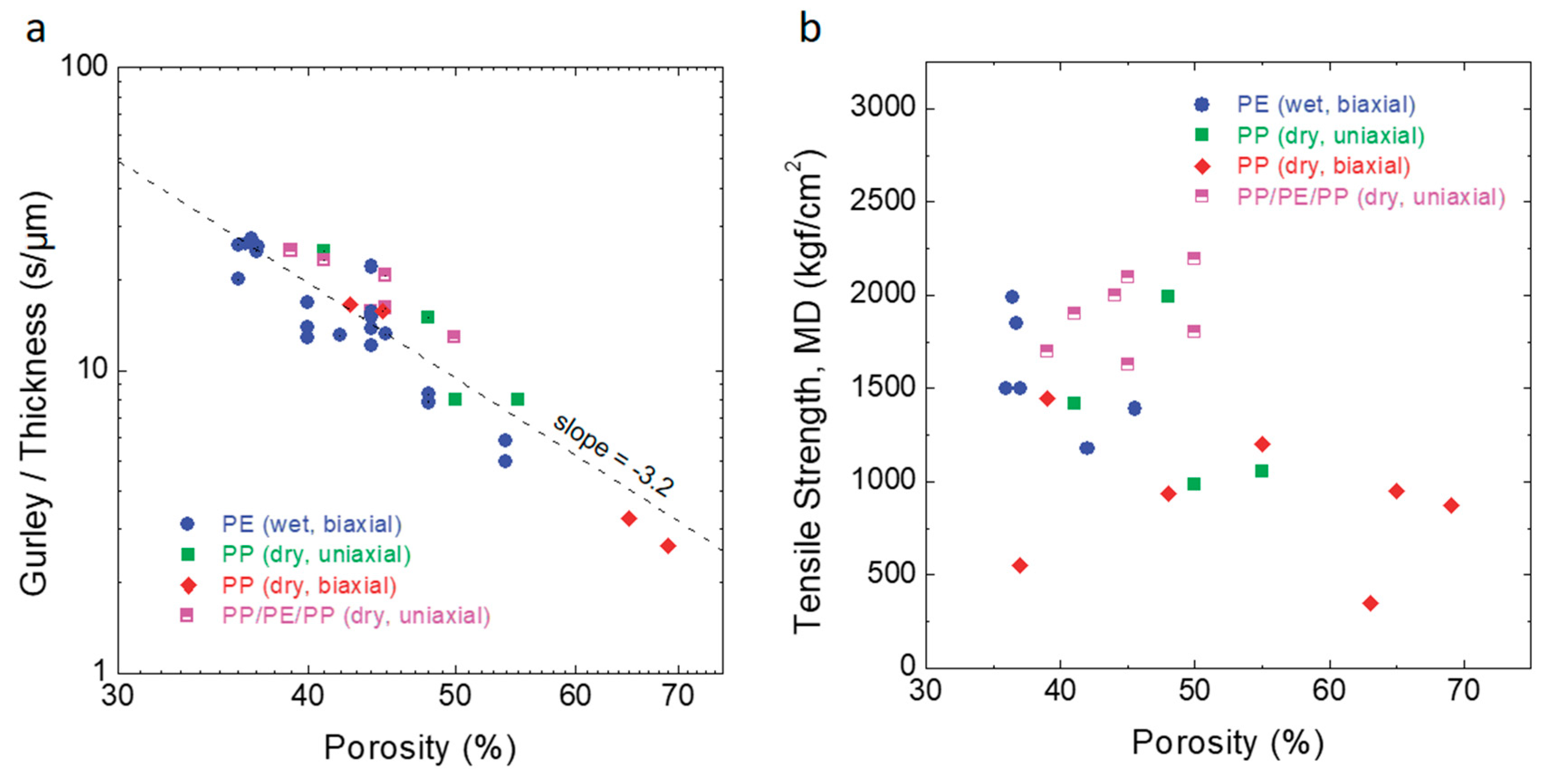
5. Porosity and Air Permeability
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Gutmann, G. Applications—Transportation|Electric Vehicle: Batteries. In Encyclopedia of Electrochemical Power Sources, 1st ed.; Garche, J., Dyer, C., Moseley, P., Ogumi, Z., Rand, D., Scrosati, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 219–235. [Google Scholar]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Zubi, G.; Adhikari, R.S.; Sánchez, N.E.; Acuña-Bravo, W. Lithium-ion battery-packs for solar home systems: Layout, cost and implementation perspectives. J. Energy Storage 2020, 32, 101985. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.-E.; Su, L.-Y.; Huang, S.-S.; Fang, C.-C.; Wu, Y.-S.; Chou, J.; Wu, N.-L. A proof-of-concept graphite anode with a lithium dendrite suppressing polymer coating. J. Power Sources 2018, 406, 63–69. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Zhang, J. Lithium-Ion Batteries Advanced Materials and Technologies, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 231. [Google Scholar]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, H.; Wang, L.; Xia, R.; Nie, S.; Chen, C.; Wang, H. A paper-supported inorganic composite separator for high-safety lithium-ion batteries. J. Membr. Sci. 2018, 553, 10–16. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Li, Y.; Zhang, X. SiO2/polyacrylonitrile membranes via centrifugal spinning as a separator for Li-ion batteries. J. Power Sources 2015, 273, 1114–1119. [Google Scholar] [CrossRef]
- Wang, E.; Wu, H.-P.; Chiu, C.-H.; Chou, P.-H. The Effect of Battery Separator Properties on Thermal Ramp, Overcharge and Short Circuiting of Rechargeable Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A125–A131. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Wu, T.; Wang, K.; Xiang, M.; Fu, Q. Progresses in manufacturing techniques of lithium-ion battery separators in China. Chin. J. Chem. 2018, 37, 1207–1215. [Google Scholar] [CrossRef]
- Kalnaus, S.; Wang, Y.; Turner, J.A. Mechanical behavior and failure mechanisms of Li-ion battery separators. J. Power Sources 2017, 348, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.; Yamaoka, T.; Ide, H.; Kimura, Y. Microvoid formation process during the plastic deformation of β-form polypropylene. Polymer 1994, 35, 3442–3448. [Google Scholar] [CrossRef]
- Wu, T.; Xiang, M.; Cao, Y.; Kang, J.; Yang, F. Pore formation mechanism of β nucleated polypropylene stretched membranes. RSC Adv. 2014, 4, 36689–36701. [Google Scholar] [CrossRef]
- Ding, L.; Xu, R.; Pu, L.; Yang, F.; Wu, T.; Xiang, M. Pore formation and evolution mechanism during biaxial stretching of β-iPP used for lithium-ion batteries separator. Mater. Des. 2019, 179, 107880. [Google Scholar] [CrossRef]
- Shi, G.; Chu, F.; Zhou, G.; Han, Z. Plastic deformation and solid-phase transformation in β-phase polypropylene. Makromol. Chem. 1989, 190, 907–913. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene: III. The equilibrium melting point and the phase diagram. Polymer 1998, 39, 3735–3744. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.; Zhou, M.; Hong, R.; Zhang, J. The α-, β-, and γ-polymorphs of polypropylene–polyethylene random copolymer modified by two kinds of β-nucleating agent. Polym. Bull. 2019, 76, 865–881. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Varga, J. Effects of β-α transformation on the static and dynamic tensile behavior of isotactic polypropylene. J. Appl. Polym. Sci. 1996, 62, 291–300. [Google Scholar] [CrossRef]
- Chena, H.B.; Karger-Kocsis, J.; Wu, J.S.; Varga, J. Fracture toughness of α-and β-phase polypropylene homopolymers and random-and block-copolymers. Polymer 2002, 43, 6505–6514. [Google Scholar] [CrossRef]
- Wei, X.; Haire, C. Biaxially Oriented Microporous Membrane. U.S. Patent 8,795,565B2, 5 August 2014. [Google Scholar]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally-induced phase separation. II. Liquid—liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Zeng, F.; Xu, R.; Ye, L.; Xiong, B.; Kang, J.; Xiang, M.; Li, L.; Sheng, X.; Hao, Z. Effects of Heat Setting on the Morphology and Performance of Polypropylene Separator for Lithium Ion Batteries. Ind. Eng. Chem. Res. 2019, 58, 2217–2224. [Google Scholar] [CrossRef]
- DeMeuse, M.T. Biaxial Stretching of Film, 1st ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–26. [Google Scholar]
- Sheng, L.; Du, Y.; Zhang, H.; Chen, Z.; Pan, J.; Wang, T.; Huang, X.; He, J. Effects of cooling process on the solid–liquid phase separation process in ultra-high-molecular-weight polyethylene/liquid paraffin blends. Polym. Bull. 2020, 77, 165–181. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, C.; Yu, W. Phase separation and structure control in ultra-high molecular weight polyethylene microporous membrane. J. Membr. Sci. 2011, 379, 268–278. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Park, J.; Jeon, H.; Yeon, D.; Kim, B.-H.; Cho, K.Y.; Ryou, M.-H.; Lee, Y.M. In-depth correlation of separator pore structure and electrochemical performance in lithium-ion batteries. J. Power Sources 2016, 325, 732–738. [Google Scholar] [CrossRef]
- Yamazawa, T.; Kimura, Y.; Toda, K.; Fukushima, T.; Ikegaya, M. History and Future Prospects of Plastics Machinery. JSW Tech. Rev. 2018, 20, 31–56. [Google Scholar]
- Breil, J. Oriented Film Technology. In Multilayer Flexible Packaging, 2nd ed.; Wagner, J.R., Ed.; William Andrew Publishing: Oxford, UK, 2016; pp. 153–172. [Google Scholar]
- Ihm, D.W.; Noh, J.G.; Kim, J.Y. Effect of polymer blending and drawing conditions on properties of polyethylene separator prepared for Li-ion secondary battery. J. Power Sources 2002, 109, 388–393. [Google Scholar] [CrossRef]
- Cheng, C.L.; Wan, C.C.; Wang, Y.Y.; Wu, M.S. Thermal shutdown behavior of PVdF-HFP based polymer electrolytes comprising heat sensitive cross-linkable oligomers. J. Power Sources 2005, 144, 238–243. [Google Scholar] [CrossRef]
- Chung, Y.S.; Yoo, S.H.; Kim, C.K. Enhancement of Meltdown Temperature of the Polyethylene Lithium-Ion Battery Separator via Surface Coating with Polymers Having High Thermal Resistance. Ind. Eng. Chem. Res. 2009, 48, 4346–4351. [Google Scholar] [CrossRef]
- Valiotti, N.B.; Nenakhov, S.A.; Zaikov, G.E. The influence of deformation temperature under uniaxial stretching on the parameters of heat shrinkage of high density polyethylene. Eur. Polym. J. 1998, 24, 167–170. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, C.; Ren, M.; Tang, Y.; Liu, L.-Z.; He, B. Evaluation of principal residual stress and its relationship with crystal orientation and mechanical properties of polypropylene films. Polymer 2017, 123, 137–143. [Google Scholar] [CrossRef]
- Bourg, V.; Ienny, P.; Caro-Bretelle, A.S.; Le Moigne, N.; Guillard, V.; Bergeret, A. Modeling of internal residual stress in linear and branched polyethylene films during cast film extrusion: Towards a prediction of heat-shrinkability. J. Mater. Process. Technol. 2019, 271, 599–608. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Shin, W.-K.; Kim, D.-W. High performance ceramic-coated separators prepared with lithium ion-containing SiO2 particles for lithium-ion batteries. J. Power Sources 2013, 226, 54–60. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, S.H.; Kim, D.-W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Song, J.; Ryou, M.-H.; Son, B.; Lee, J.-N.; Lee, D.J.; Lee, Y.M.; Choi, J.W.; Park, J.-K. Co-polyimide-coated polyethylene separators for enhanced thermal stability of lithium ion batteries. Electrochim. Acta 2012, 85, 524–530. [Google Scholar] [CrossRef]
- Lee, D.-W.; Lee, S.-H.; Kim, Y.-N.; Oh, J. Preparation of a high-purity ultrafine α-Al2O3 powder and characterization of an Al2O3-coated PE separator for lithium-ion batteries. Powder Technol. 2017, 320, 125–132. [Google Scholar] [CrossRef]
- Stokes, K.K.; Mason, W.J.; Xiao, K.K.; Zhang, X.; Summey, B.J.; Moran, R.; Poley, J.G.; Stepp, B.R.; Adams, C.W.; Alexander, D.R.; et al. Membranes, Calendered Microporous Membranes, Battery Separators, and Related Methods. U.S. Patent 2017/0084898A1, 23 March 2017. [Google Scholar]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Cannarella, J.; Arnold, C.B. Stress evolution and capacity fade in constrained lithium-ion pouch cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers, 4th ed.; Elsevier: Oxford, UK, 2009; p. 457. [Google Scholar]
- Xu, G.; Ding, L.; Wu, T.; Xiang, M.; Yang, F. Effect of high molecular weight on pore formation and various properties of microporous membrane used for lithium-ion battery separator. J. Polym. Res. 2018, 25, 166. [Google Scholar] [CrossRef]
- Sadeghi, F.; Tabatabaei, S.H.; Ajji, A.; Carreau, P.J. Properties of uniaxially stretched polypropylene films: Effect of drawing temperature and random copolymer content. Can. J. Chem. Eng. 2010, 88, 1091–1098. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-Y.; Song, H.-S. Effects of melt-extension and annealing on row-nucleated lamellar crystalline structure of HDPE films. J. Appl. Polym. Sci. 2007, 103, 3326–3333. [Google Scholar] [CrossRef]
- Knoche, T.; Lund, R.; Prymak, O.; Epple, M.; Ulbricht, M. Effect of annealing temperature on pore formation in preparation of advanced polyethylene battery separator membranes. Mater. Today Commun. 2016, 8, 23–30. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, S.; Wang, J.; Kang, J.; Xiang, M.; Yang, F. Impact of different die draw ratio on crystalline and oriented properties of polypropylene cast films and annealed films. J. Polym. Res. 2018, 25, 142. [Google Scholar] [CrossRef]
- Changbin, C.; Caihong, L.; Qi, C.; Haibin, M.; Ruijie, X. Influence of annealing time on the structure and properties of high-density polyethylene microporous membrane. J. Plast. Film Sheet. 2015, 31, 78–95. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.; Jeong, H.; Kang, Y.; Jo, C. A Review of Functional Separators for Lithium Metal Battery Applications. Materials 2020, 13, 4625. [Google Scholar] [CrossRef]
- Kong, X.; Plett, G.L.; Trimboli, M.S.; Zhang, Z.; Qiao, D.; Zhao, T.; Zheng, Y. Pseudo-two-dimensional model and impedance diagnosis of micro internal short circuit in lithium-ion cells. J. Energy Storage 2020, 27, 101085. [Google Scholar] [CrossRef]
- Li, R.; Gao, P. Nanoporous UHMWPE Membrane Separators for Safer and High-Power-Density Rechargeable Batteries. Glob. Chall. 2017, 1, 1700020. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Microporous membranes obtained from PP/HDPE multilayer films by stretching. J. Membr. Sci. 2009, 345, 148–159. [Google Scholar] [CrossRef]
- Technical Data from Celgard, LLC. Available online: https://www.celgard.com/literature/ (accessed on 29 April 2021).
- Yamada, K.; Ito, Y. Separator Materials for Li Ion Batteries. Nippon Gomu Kyokaishi 2019, 92, 422–429. [Google Scholar] [CrossRef]
- Jung, B.; Lee, B.; Jeong, Y.-C.; Lee, J.; Yang, S.R.; Kim, H.; Park, M. Thermally stable non-aqueous ceramic-coated separators with enhanced nail penetration performance. J. Power Sources 2019, 427, 271–282. [Google Scholar] [CrossRef]
- Yang, C.; Tong, H.; Luo, C.; Yuan, S.; Chen, G.; Yang, Y. Boehmite particle coating modified microporous polyethylene membrane: A promising separator for lithium ion batteries. J. Power Sources 2017, 348, 80–86. [Google Scholar] [CrossRef]
- Martinsen, M.J. Material Behavior Characterization of a Thin Film Polymer Used in Lithium-Ion Batteries. Master’s Thesis, The University of Wisconsin-Milwaukee, Milwaukee, WI, USA, December 2012. [Google Scholar]
- Kim, K.J.; Kim, J.-H.; Park, M.-S.; Kwon, H.K.; Kim, H.; Kim, Y.-J. Enhancement of electrochemical and thermal properties of polyethylene separators coated with polyvinylidene fluoride–hexafluoropropylene co-polymer for Li-ion batteries. J. Power Sources 2012, 198, 298–302. [Google Scholar] [CrossRef]
- Kannan, D.R.R.; Terala, P.K.; Moss, P.L.; Weatherspoon, M.H. Analysis of the separator thickness and porosity on the performance of lithium-ion batteries. Int. J. Electrochem. 2018, 2018, 1925708. [Google Scholar]
- Djian, D.; Alloin, F.; Martinet, S.; Lignier, H.; Sanchez, J.Y. Lithium-ion batteries with high charge rate capacity: Influence of the porous separator. J. Power Sources 2007, 172, 416–421. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Koponen, A.; Kataja, M.; Timonen, J. Permeability and effective porosity of porous media. Phys. Rev. E 1997, 56, 3319–3325. [Google Scholar] [CrossRef]
- Srisutthiyakorn, N.; Mavko, G.M. What is the role of tortuosity in the Kozeny-Carman equation? Interpretation 2017, 5, SB57–SB67. [Google Scholar] [CrossRef]
- Technical Data from Shanghai Energy New Materials Technology Co., Ltd. (SEMCORP). Available online: http://en.semcorpglobal.com/products.aspx?TypeId=11&FId=t3:11:3 (accessed on 29 April 2021).
- Liu, J.; Mo, Y.; Wang, S.; Ren, S.; Han, D.; Xiao, M.; Sun, L.; Meng, Y. Ultrastrong and Heat-Resistant Poly(ether ether ketone) Separator for Dendrite-Proof and Heat-Resistant Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3886–3895. [Google Scholar] [CrossRef]
- Shi, J.; Xia, Y.; Yuan, Z.; Hu, H.; Li, X.; Zhang, H.; Liu, Z. Porous membrane with high curvature, three-dimensional heat-resistance skeleton: A new and practical separator candidate for high safety lithium ion battery. Sci. Rep. 2015, 5, 8255. [Google Scholar] [CrossRef] [PubMed]
- Technical Data from Entek Membranes, LLC. Available online: https://entek.com/lithium/products/ (accessed on 27 June 2021).
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ding, J.; Hu, W.; Paek, E.; Mitlin, D. Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar] [CrossRef] [PubMed]
- Won, J.H.; Kim, M.K.; Jeong, H.M. Porous polymer thin film encapsulated sulfur nanoparticles on graphene via partial evaporation for high-performance lithium–sulfur batteries. Appl. Surf. Sci. 2021, 547, 149199. [Google Scholar] [CrossRef]
- Won, J.H.; Mun, S.C.; Kim, G.H.; Jeong, H.M.; Kang, J.K. Generic Strategy to Synthesize High-Tap Density Anode and Cathode Structures with Stratified Graphene Pliable Pockets via Monomeric Polymerization and Evaporation, and Their Utilization to Enable Ultrahigh Performance in Hybrid Energy Storages. Small 2020, 16, 2001756. [Google Scholar] [CrossRef] [PubMed]



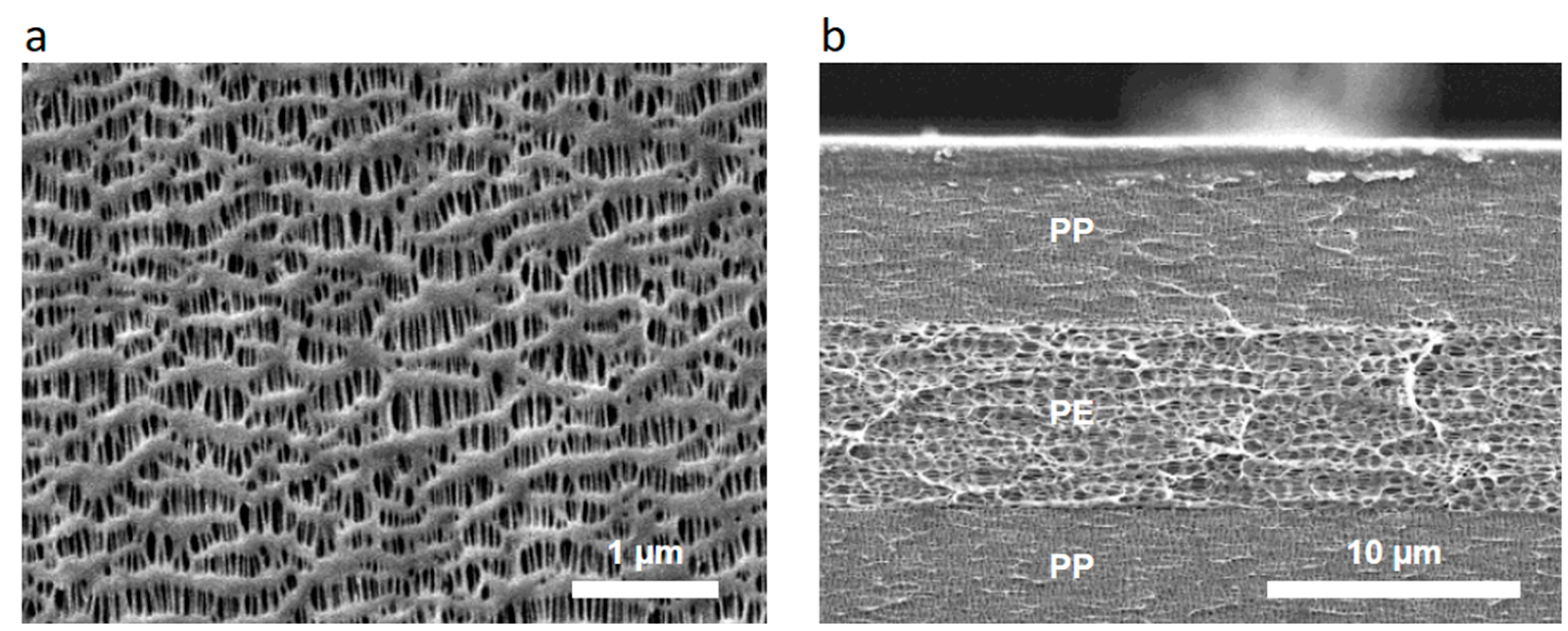

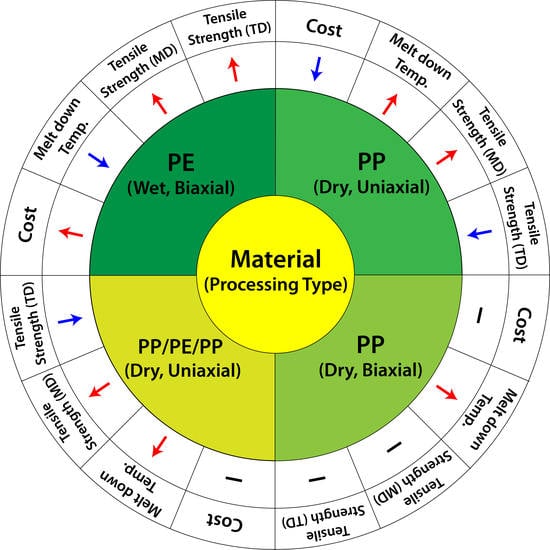
| Material | PE | PP | PP/PE/PP | PP |
|---|---|---|---|---|
| Processing Type | Wet, Biaxial | Dry, Uniaxial | Dry, Uniaxial | Dry, Biaxial |
| Manufacturing cost | Expensive | Cheap | Moderate | Moderate |
| Simplicity of Manufacturing | Complicated | Simple | Moderate | Complicated |
| Anistotropy of Micropores | Low | High | High | Low |
| Shut down function | O | X | O | X |
| Melt down temperature | Low | High | High | High |
| Tensile Strength (MD) | Strong | Strong | Strong | Moderate |
| Tensile Strength (TD) | Strong | Weak | Weak | Moderate |
| Resistance to Puncture | Moderate | Weak | Weak | Weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.C.; Won, J.H. Manufacturing Processes of Microporous Polyolefin Separators for Lithium-Ion Batteries and Correlations between Mechanical and Physical Properties. Crystals 2021, 11, 1013. https://doi.org/10.3390/cryst11091013
Mun SC, Won JH. Manufacturing Processes of Microporous Polyolefin Separators for Lithium-Ion Batteries and Correlations between Mechanical and Physical Properties. Crystals. 2021; 11(9):1013. https://doi.org/10.3390/cryst11091013
Chicago/Turabian StyleMun, Sung Cik, and Jong Ho Won. 2021. "Manufacturing Processes of Microporous Polyolefin Separators for Lithium-Ion Batteries and Correlations between Mechanical and Physical Properties" Crystals 11, no. 9: 1013. https://doi.org/10.3390/cryst11091013
APA StyleMun, S. C., & Won, J. H. (2021). Manufacturing Processes of Microporous Polyolefin Separators for Lithium-Ion Batteries and Correlations between Mechanical and Physical Properties. Crystals, 11(9), 1013. https://doi.org/10.3390/cryst11091013