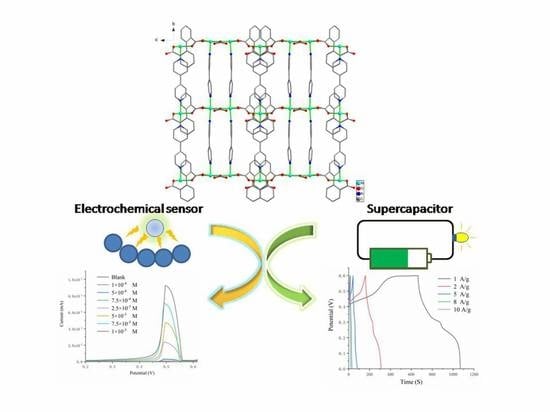
Electrochemical Detection of Sarcosine and Supercapacitor Based on a New Ni–Metal Organic Framework Electrode Material
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Ni-1
2.3. Single-Crystal Structure Determination
2.4. Fabrication of Working Electrode
2.5. Electrochemical Measurements
3. Results and Discussion
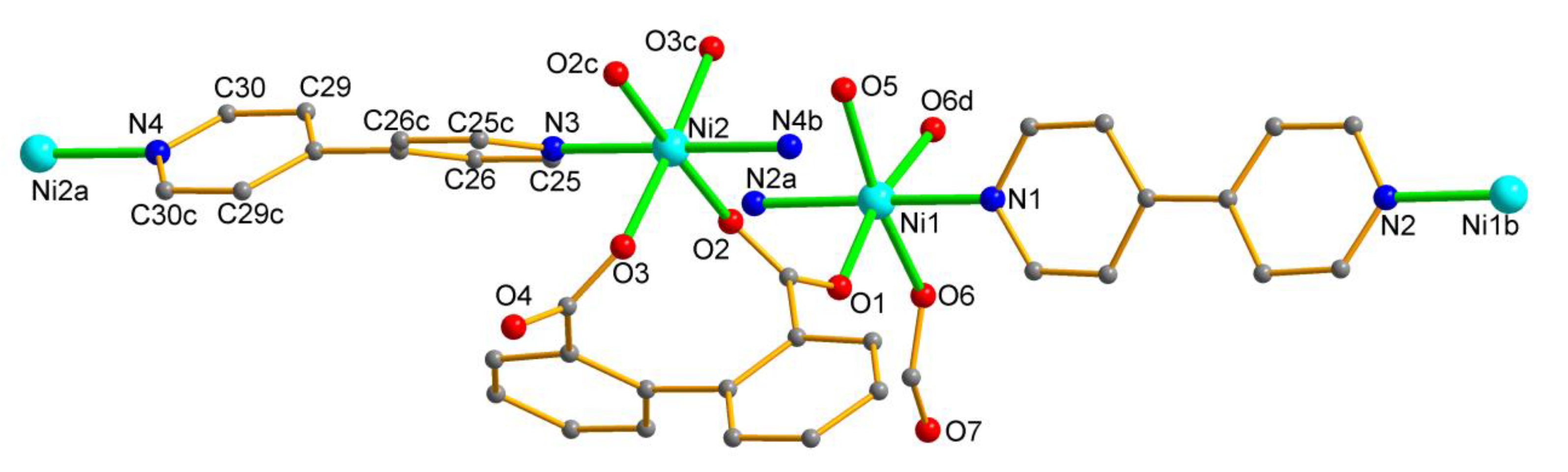
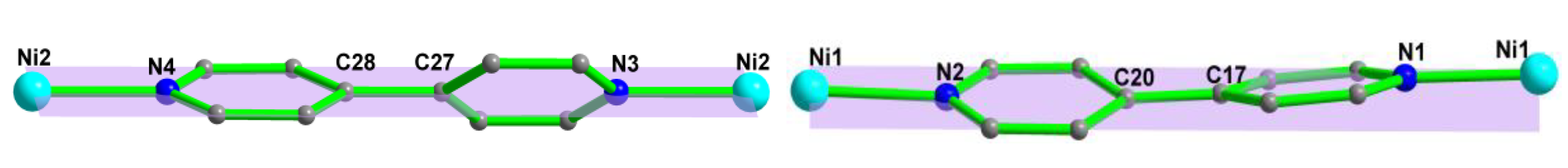
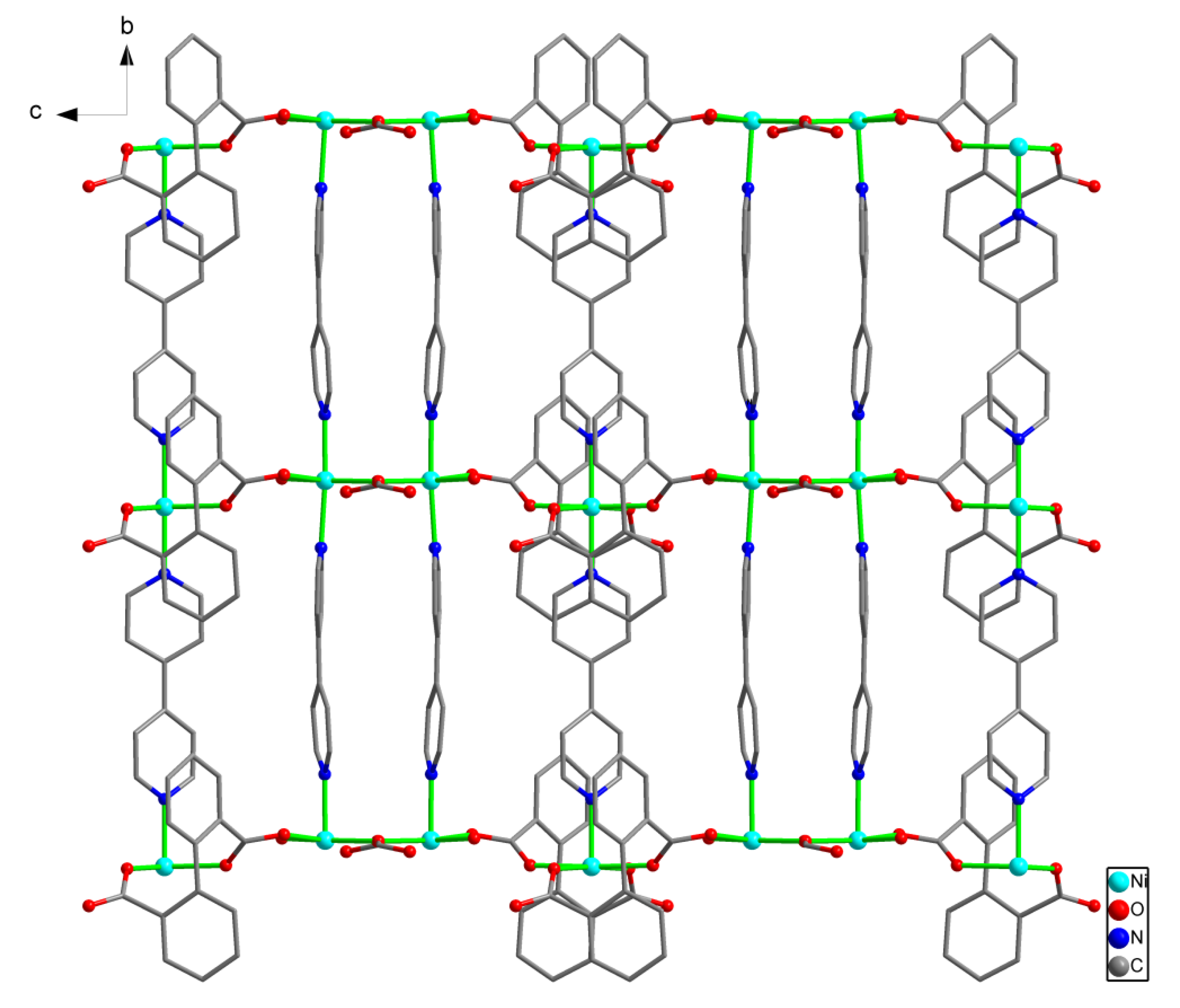
3.1. Structure Description
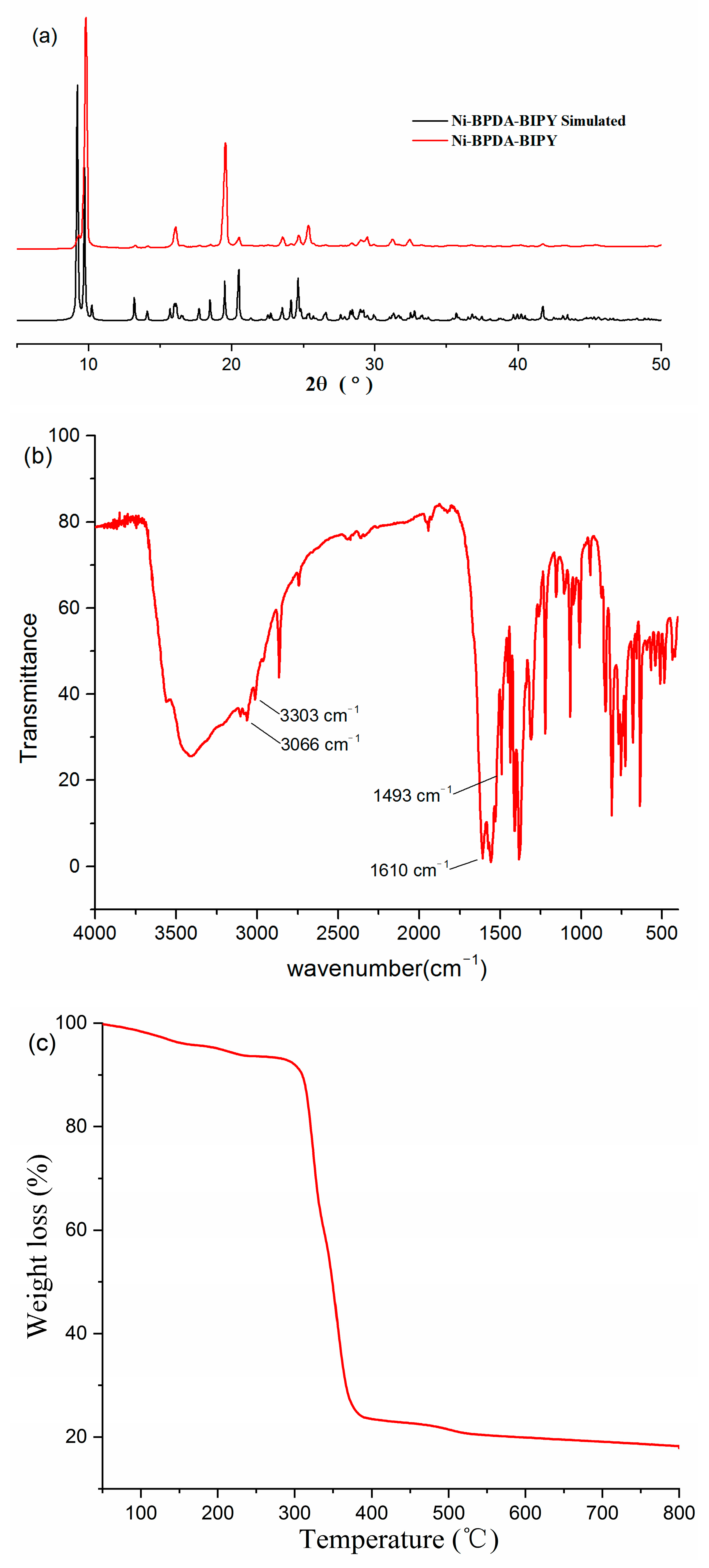
3.2. Purity and Thermal Stability
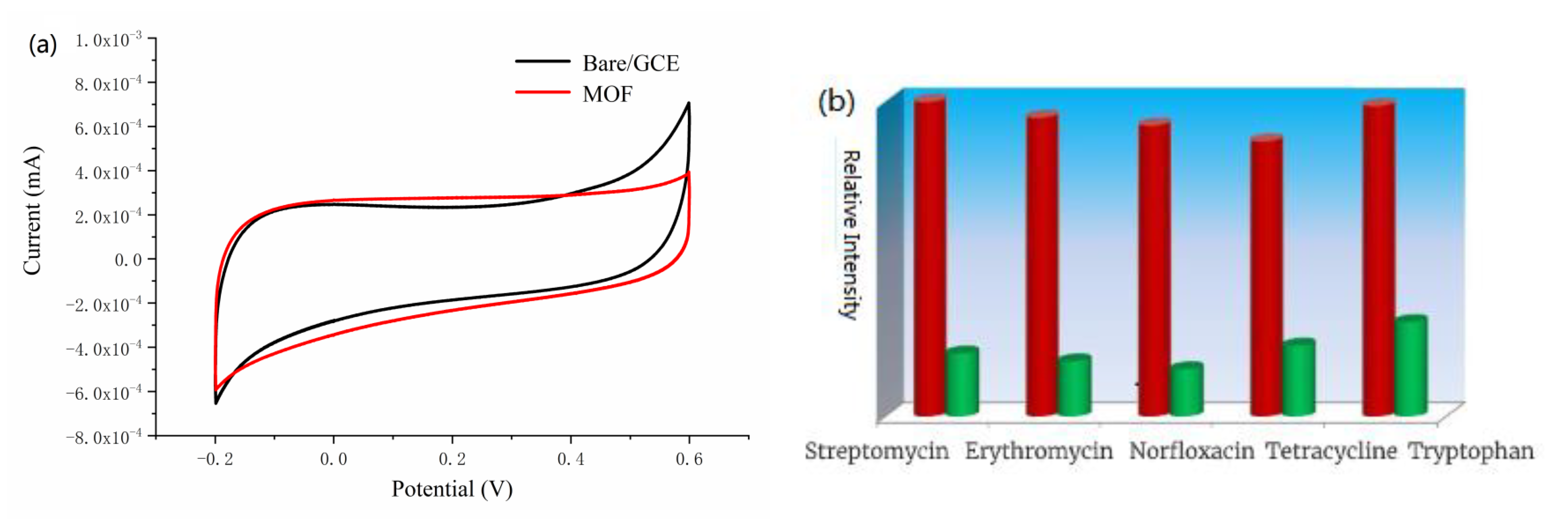
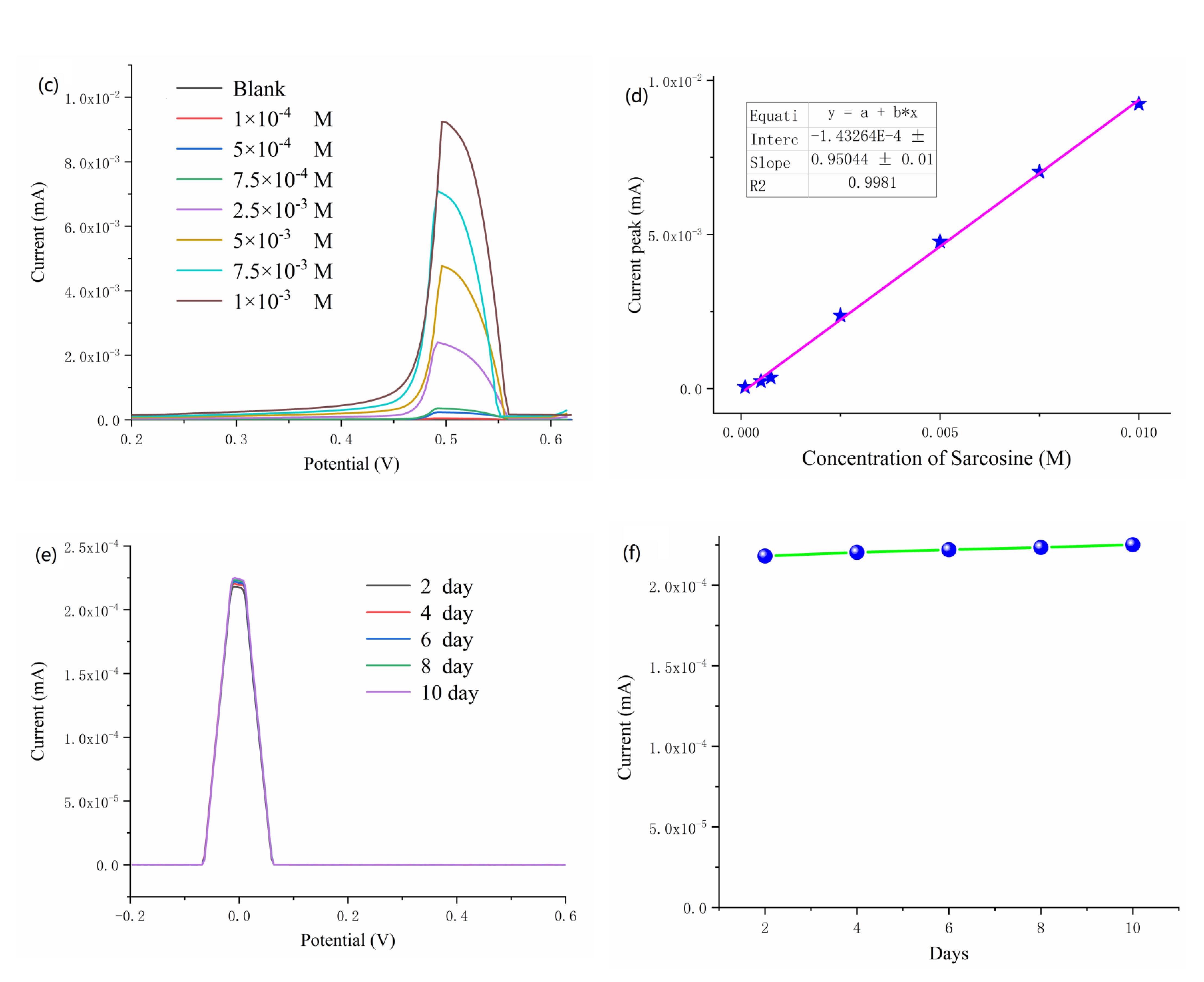
3.3. Investigation of Electrochemical Properties
3.4. Selectivity and Stability of the Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, K.; Zeng, Y.X.; Liu, S.; Zeng, C.H.; Tong, Y.X.; Zheng, Z.K.; Zhu, T.S.; Lu, X.H. Valence and Surface Modulated Vanadium Oxide Nanowires as New High-Energy and Durable Negative Electrode for Flexible Asymmetric Supercapacitors. Energy Storage Mater. 2019, 22, 410–417. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Tang, J.; Tan, H.; Wang, J.; Kaneti, Y.V.; Bando, Y.; Wang, T.; He, J.; Yamauchi, Y. MOF nanoleaves as new sacrificial templates for the fabrication of nanoporous Co–Nx/C electrocatalysts for oxygen reduction. Nanoscale Horiz. 2019, 4, 1006–1013. [Google Scholar] [CrossRef]
- Young, C.; Kim, J.; Kaneti, Y.V.; Yamauchi, Y. One-Step Synthetic Strategy of Hybrid Materials from Bimetallic Metal–Organic Frameworks for Supercapacitor Applications. ACS Appl. Energy Mater. 2018, 1, 2007–2015. [Google Scholar]
- Zhang, S.; Xi, W.; Yang, Q.; Kaneti, Y.V.; Xu, X.; Alshehri, S.M.; Ahamad, T.; Hossain, M.S.A.; Na, J.; Tang, J.; et al. Core-shell motif construction: Highly graphitic nitrogen-doped porous carbon electrocatalysts using MOF-derived carbon@COF heterostructures as sacrificial templates. Chem. Eng. J. 2020, 396, 125154. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Sankar, A.B.; Rohita, D.S.; Rao, T.N.; Karthik, M. Conversion of Biomass Waste into High Performance Supercapacitor Electrodes for Real-Time Supercapacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.Z.; Shen, S.H.; Zhong, Y.; Liu, S.; Xia, X.H.; Tong, Y.X.; Lu, X.H. Enhancing the Capacitive Storage Performance of Carbon Fiber Textile by Surface and Structural Modulation for Advanced Flexible Asymmetric Supercapacitors. Adv. Funct. Mater. 2019, 29, 1806329. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.Y.; Liu, H.C.; Zhang, S.; Yang, J.L.; Zhou, J.; Yu, J.L.; Ji, M.W.; Zhu, C.Z.; Xu, J. Tear resistant Tyvek/Ag/poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate (PEDOT:PSS)/carbon nanotubes electrodes for flexible high-performance supercapacitors. Chem. Eng. J. 2020, 2020, 127665. [Google Scholar] [CrossRef]
- Soram, B.S.; Dai, J.Y.; Thangjam, I.S.; Kim, N.H.; Lee, J.H. One-step electrodeposited MoS2@Ni-mesh electrode for flexible and transparent asymmetric solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 24040–24052. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yan, C.; Wang, G.G.; Li, F.; Shang, Y.; Zhang, H.Y.; Han, J.C.; Yang, H.Y. Self-templated formation of (NiCo)9S8yolk–shelled spheres for high-performance hybrid supercapacitors. Nanoscale 2020, 12, 23497–23505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wang, J.M.; Sui, Y.W.; Wei, F.X.; Qi, J.Q.; Meng, Q.K.; He, Y.Z.; Zhuang, D.D. Hierarchical Nickel–Cobalt Phosphide/Phosphate/Carbon Nanosheets for High-Performance Supercapacitors. ACS Appl. Nano Mater. 2020, 3, 11945–11954. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Ji, S.Y.; Xu, H.Y.; Zhao, W.; Xu, J.J.; Chen, H.Y. Bidirectional Electrochemiluminescence Color Switch: An Application in Detecting Multimarkers of Prostate Cancer. Anal. Chem. 2018, 90, 3570–3575. [Google Scholar] [CrossRef]
- Mbage, B.; Li, Y.M.; Si, H.P.; Zhang, X.Y.; Li, Y.; Wang, X.H.; Salah, A.; Zhang, K.Z. Fabrication of folate functionalized polyoxometalate nanoparticle to simultaneously detect H2O2 and sarcosine in colorimetry. Sens. Actuators B 2020, 304, 127429. [Google Scholar] [CrossRef]
- Yang, Q.G.; Li, N.; Li, Q.; Chen, S.Q.; Wang, H.L.; Yang, H.P. Amperometric sarcosine biosensor based on hollow magnetic Pt–Fe3O4@C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef]
- Hu, J.; Wei, W.W.; Ke, S.M.; Zeng, X.R.; Lin, P. A novel and sensitive sarcosine biosensor based on organic electrochemical transistor. Electrochim. Acta 2019, 307, 100–106. [Google Scholar] [CrossRef]
- Roy, A.; Chen, Y.P.; Qiu, J.T.T.; Maikap, S. Sarcosine Prostate Cancer Biomarker Detection by Controlling Oxygen inNiOx Membrane on Vertical Silicon Nanowires in Electrolyte–Insulator–Nanowire Structure. Anal. Chem. 2020, 92, 8064–8071. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.E.; Fox, S.D.; Issaq, H.J.; Xu, X.; Chu, L.W.; Veenstra, T.D.; Hsing, A.W. A Reproducible and High-Throughput HPLC/MS Method To Separate Sarcosine from α- and β-Alanine and To Quantify Sarcosine in Human Serum and Urine. Anal. Chem. 2011, 83, 5735–5740. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Cheng, X.L.; Wang, C.; Ma, Y.F. Quantitative Determination of Sarcosine and Related Compounds in Urinary Samples by Liquid Chromatography with Tandem Mass Spectrometry. Anal. Chem. 2010, 82, 9022–9027. [Google Scholar] [CrossRef]
- Yin, C.; Zhuang, Q.; Xiao, Q.; Wang, Y.; Xie, J. Electropolymerization of poly(methylene blue) on flower-like nickel-based MOFs used for ratiometric electrochemical sensing of total polyphenolic content in chrysanthemum tea. Anal. Methods 2021, 13, 1154–1163. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, X.; Chi, K.N.; Yang, T.; Yang, Y.H. Bifunctional MOFs-Based Ratiometric Electrochemical Sensor for Multiplex Heavy Metal Ions. ACS Appl. Mater. Interfaces 2020, 12, 30770–30778. [Google Scholar] [CrossRef] [PubMed]
- Gumilar, G.; Kaneti, Y.V.; Henzie, J.; Chatterjee, S.; Na, J.; Yuliarto, B.; Nugraha, N.; Patah, A.; Bhaumik, A.; Yamauchi, Y. General synthesis of hierarchical sheet/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) metal–organic frameworks for electrochemical non-enzymatic glucose sensing. Chem. Sci. 2020, 11, 3644–3655. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.F.; Qian, S.H.; Wang, X.B.; Cui, X.L.; Chen, B.L.; Xing, H.B. Energy-efficient separation alternatives: Metal–organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal–organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Fan, P.; Tu, X.X.; Min, H.; Yu, X.Y.; Li, X.F.; Zeng, J.L.; Zhang, S.W.; Cheng, P. A Bifunctional Luminescent Metal–Organic Framework for the Sensing of Paraquat and Fe3+Ions in Water. Chem. Asian J. 2019, 14, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, Z.Q.; Huang, Y.; Chen, F.; Zeng, C.H.; Zhong, S.L.; Ng, S.W. Highly luminescent Ln-MOFs based on 1,3-adamantanediacetic acid as bifunctional sensor. Sens. Actuators B Chem. 2018, 257, 705–713. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Yan, L.; Peng, R.; Huang, M.J.; Guo, X.X.; Li, Y.; Wu, P.Y. Multifunctional LuminescentEu(III)-Based Metal–Organic Framework for Sensing Methanol and Detection and Adsorption of Fe(III) Ions in Aqueous Solution. Inorgan. Chem. 2016, 55, 12660–12668. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Drake, H.; Li, J.L.; Pang, J.D.; Wang, Y.; Yuan, S.; Wang, Q.; Cai, P.Y.; Qin, J.S.; Zhou, H.C. Flexible and Hierarchical Metal–Organic Framework Composites for High-Performance Catalysis. Angew. Chem. Int. Ed. 2018, 57, 8916–8920. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Uemura, Y.; Chun, W.J.; Satter, S.S.; Nakajima, K.; Manaka, Y.; Motokura, K. Controllable Factors of Supported Ir Complex Catalysis for Aromatic C–H Borylation. ACS Catal. 2020, 10, 14552–14559. [Google Scholar] [CrossRef]
- Karmakar, A.; Pombeiro, A.J.L. Recent advances in amide functionalized metal organic frameworks for heterogeneous catalytic applications. Coord. Chem. Rev. 2019, 395, 86–129. [Google Scholar] [CrossRef]
- Fei, H.H.; Shin, J.W.; Meng, Y.S.; Adelhardt, M.; Sutter, J.; Meyer, K.; Cohen, S.M. Reusable Oxidation Catalysis Using Metal-Monocatecholato Species in a Robust Metal–Organic Framework. J. Am. Chem. Soc. 2014, 136, 4965–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh, M.A.; Eshkevari, B.M.; Tavakoli, M.; Zamani, F. Metal–organic frameworks: Advanced tools for multicomponent reactions. Green Chem. 2020, 22, 7265–7300. [Google Scholar] [CrossRef]
- Bao, Z.B.; Chang, G.G.; Xing, H.B.; Krishna, R.; Ren, Q.L.; Chen, B.L. Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci. 2016, 9, 3612–3641. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Shahrak, M.N.; Ehsani, A.; Kiaei, Z.; Torkzaban, H.; Ershadi, M.; Eshkalak, S.K.; Asl, V.H.; Chinnappan, A.; et al. A review on thefield patents and recent developments over the application of metal organic frameworks (MOFs) in supercapacitors. Coord. Chem. Rev. 2020, 422, 213441. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, C.K.; Nayak, G.C.; Nayek, H.P. A Cobalt(II) Metal-organic Framework Featuring Supercapacitor Application. J. Solid State Chem. 2020, 282, 121093. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yoon, S.J.; Shrestha, N.K.; Lee, S.-H.; Ahn, H.; Han, S.-H. Unusual energy storage and charge retention in Co-based metal–organic-frameworks. Microporous Mesoporous Mater. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- He, H.; Wang, G.; Shen, B.; Wang, Y.; Lu, Z.; Guo, S.; Zhang, J.; Yang, L.; Jiang, Q.; Xiao, Z. Three isostructural Zn/Ni nitro-containing metal-organic frameworks for supercapacitor. J. Solid State Chem. 2020, 288, 121375. [Google Scholar] [CrossRef]

| Compound | Ni-1 |
|---|---|
| Chemical Formula | C60H54N6Ni3O18 |
| Formula weight | 1323.16 |
| Crystal system | Monoclinic |
| Space group | C2 |
| a (Å) | 22.501(2) |
| b (Å) | 11.2879(12) |
| c (Å) | 13.4015(12) |
| α (°) | 90 |
| β (°) | 126.099(10) |
| γ (°) | 90 |
| V (Å3) | 2750.3(5) |
| Z | 2 |
| Dc (g/cm3) | 1.598 |
| μ (mm−1) | 1.101 |
| T (K) | 293(2) |
| Wavelength (Å) | 0.71073 |
| F(000) | 1368.0 |
| Crystal size (mm) | 0.20 × 0.19 × 0.12 |
| θ range(°) | 7.22 to 59.298 |
| Index ranges | −28 ≤ h ≤ 31 |
| −15 ≤ k ≤ 15 | |
| −18 ≤ l ≤ 16 | |
| Reflections collected | 11,900 |
| Independent reflections | 11,900 [Rint = 0.0759, Rsigma = 0.1150] |
| Parameters | 396 |
| Goodness-of-fit on F2 | 1.056 |
| R1 indices [I > 2σ(I)] | 0.0769 |
| wR2 indices [I > 2σ(I)] | 0.1609 |
| R1 indices [all data] | 0.1005 |
| wR2 indices [all data] | 0.1772 |
| Peak and hole (e Å-3) | 1.37/−0.95 |
| Ni1- O1 = 2.029(7) | Ni2- O3 = 2.078(8) |
| Ni1- O7 = 2.048(6) | Ni2- O3 2 = 2.078(8) |
| Ni1- N2 = 2.090(12) | Ni2- N3 = 2.098(15) |
| Ni1- O5 1 = 2.091(6) | Ni2- O2 2 = 2.118(7) |
| Ni1- N1 = 2.113(12) | Ni2- O2 = 2.118(7) |
| Ni1- O5 = 2.120(6) | Ni2- N4 = 2.127(15) |
| O1-Ni1-O7 98.1(3) | O3-Ni2-O3 2 176.6(5) |
| O1-Ni1-N2 84.6(3) | O3-Ni2-N3 88.3(3 |
| O7-Ni1-N2 87.3(4) | O3 2-Ni2-N3 88.3(3) |
| O1-Ni1-O5 1 93.2(3) | O3-Ni2-O2 2 99.7(3) |
| O7-Ni1-O5 1 168.4(3 | O3 2-Ni2-O2 2 80.4(3) |
| N2-Ni1-O5 1 91.2(4) | N3-Ni2-O2 2 91.0(2) |
| O1-Ni1-N1 92.0(4) | O3-Ni2-O2 80.4(3) |
| O7-Ni1-N1 90.8(4) | O3 2-Ni2-O2 99.7(3) |
| N2-Ni1-N1 175.9(3) | N3-Ni2-O2 91.0(2) |
| O5 1-Ni1-N1 91.3(4) | O2 2-Ni2-O2 178.0(5) |
| O1-Ni1-O5 168.3(3) | O3-Ni2-N4 91.7(3) |
| O7-Ni1-O5 92.6(3) | O3 2-Ni2-N4 91.7(3) |
| N2-Ni1-O5 91.1(4) | N3-Ni2-N4 180.0 |
| O5 1-Ni1-O5 76.0(3) | O2 2-Ni2-N4 89.0(2) |
| N1-Ni1-O5 92.6(4) | O2-Ni2-N4 89.0(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Wang, Y.; Zhang, C.; Wu, Y.; Zhang, B.; Zhou, C.; Yang, H. Electrochemical Detection of Sarcosine and Supercapacitor Based on a New Ni–Metal Organic Framework Electrode Material. Crystals 2021, 11, 1036. https://doi.org/10.3390/cryst11091036
Lin S, Wang Y, Zhang C, Wu Y, Zhang B, Zhou C, Yang H. Electrochemical Detection of Sarcosine and Supercapacitor Based on a New Ni–Metal Organic Framework Electrode Material. Crystals. 2021; 11(9):1036. https://doi.org/10.3390/cryst11091036
Chicago/Turabian StyleLin, Shaomin, Yi Wang, Chenyang Zhang, Yunying Wu, Bodong Zhang, Chunjuan Zhou, and Huan Yang. 2021. "Electrochemical Detection of Sarcosine and Supercapacitor Based on a New Ni–Metal Organic Framework Electrode Material" Crystals 11, no. 9: 1036. https://doi.org/10.3390/cryst11091036
APA StyleLin, S., Wang, Y., Zhang, C., Wu, Y., Zhang, B., Zhou, C., & Yang, H. (2021). Electrochemical Detection of Sarcosine and Supercapacitor Based on a New Ni–Metal Organic Framework Electrode Material. Crystals, 11(9), 1036. https://doi.org/10.3390/cryst11091036