Microstructure Evolution Mechanism of Geopolymers with Exposure to High-Temperature Environment
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Concluding Remarks
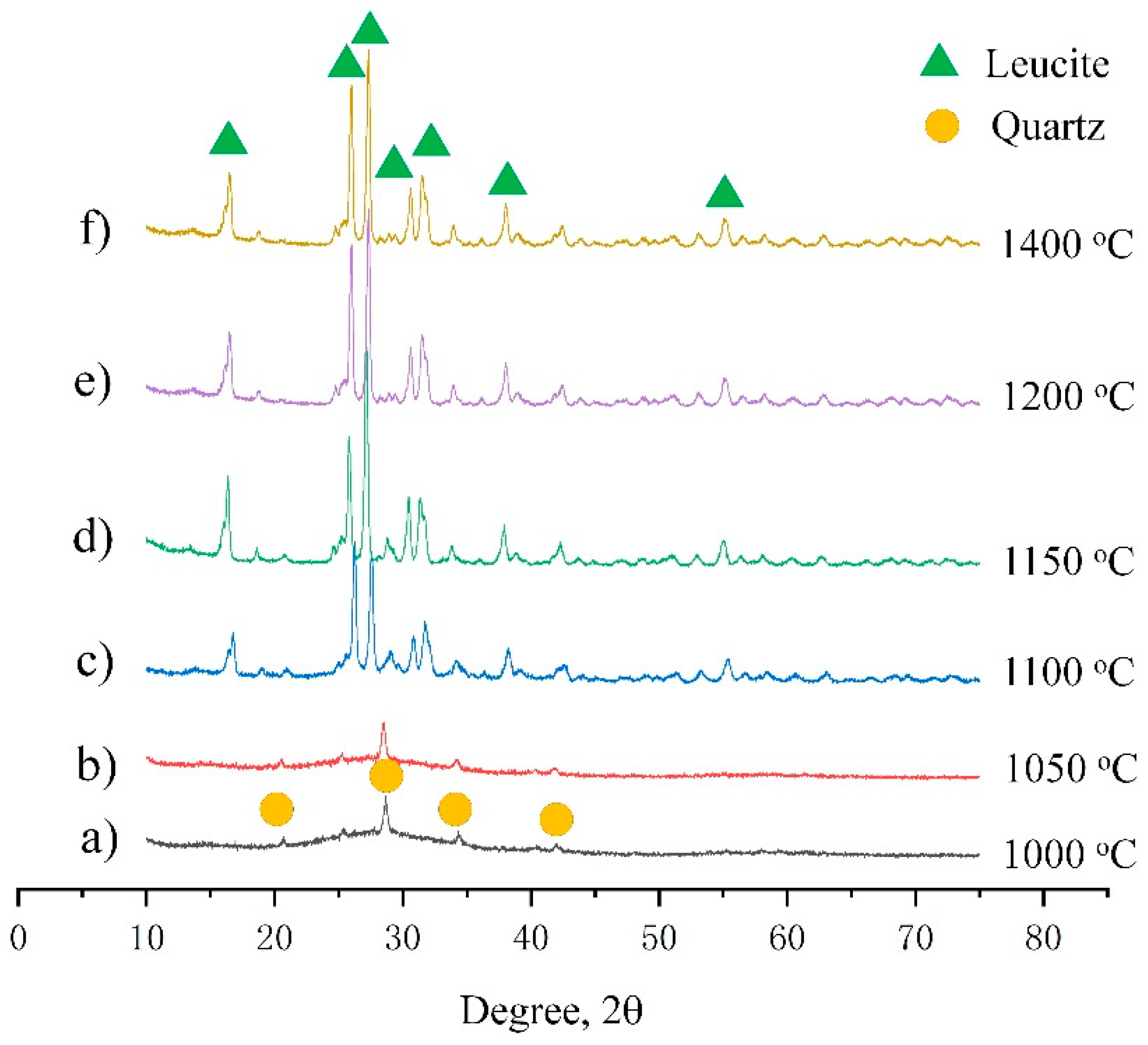
- The XRD results show that the crystallization did not occur until the heating temperature reached 1100 °C.
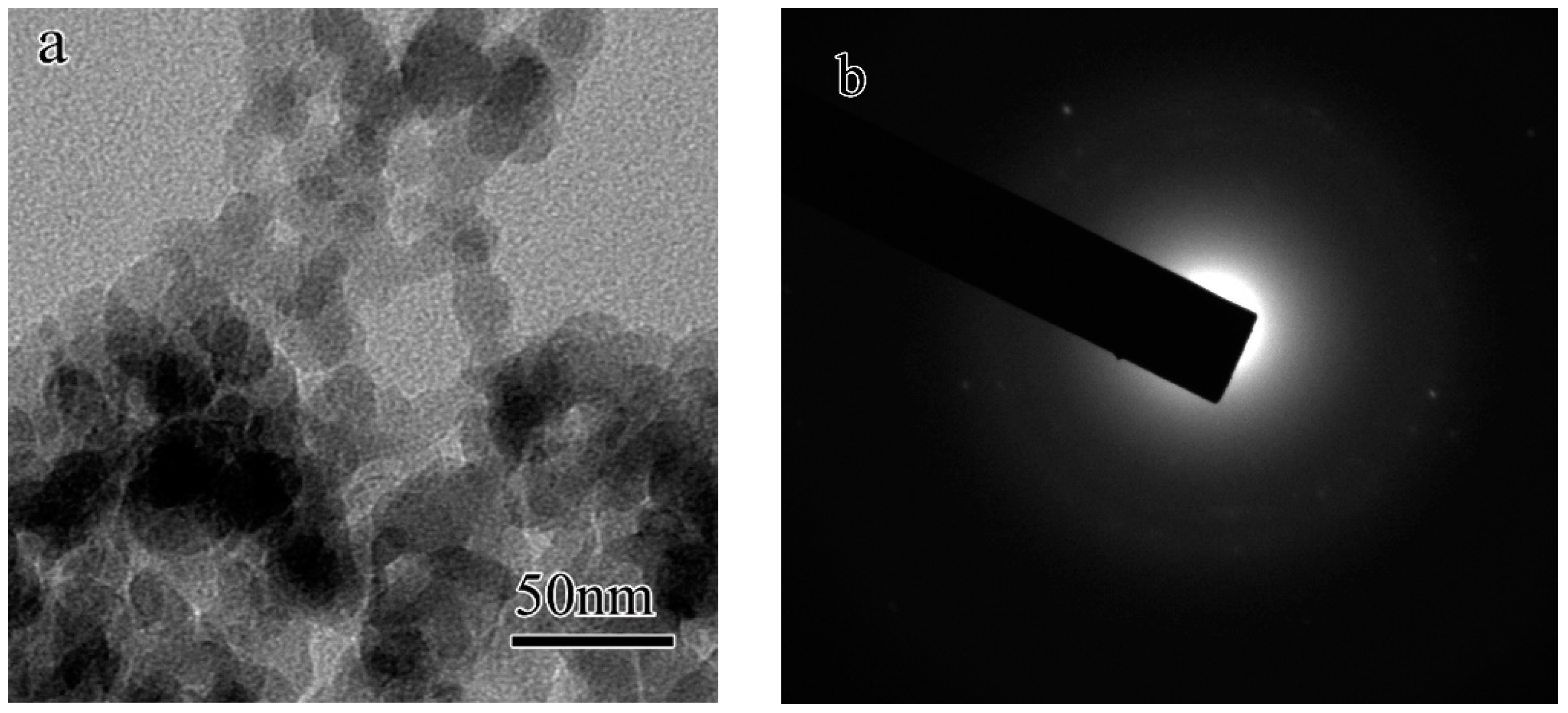
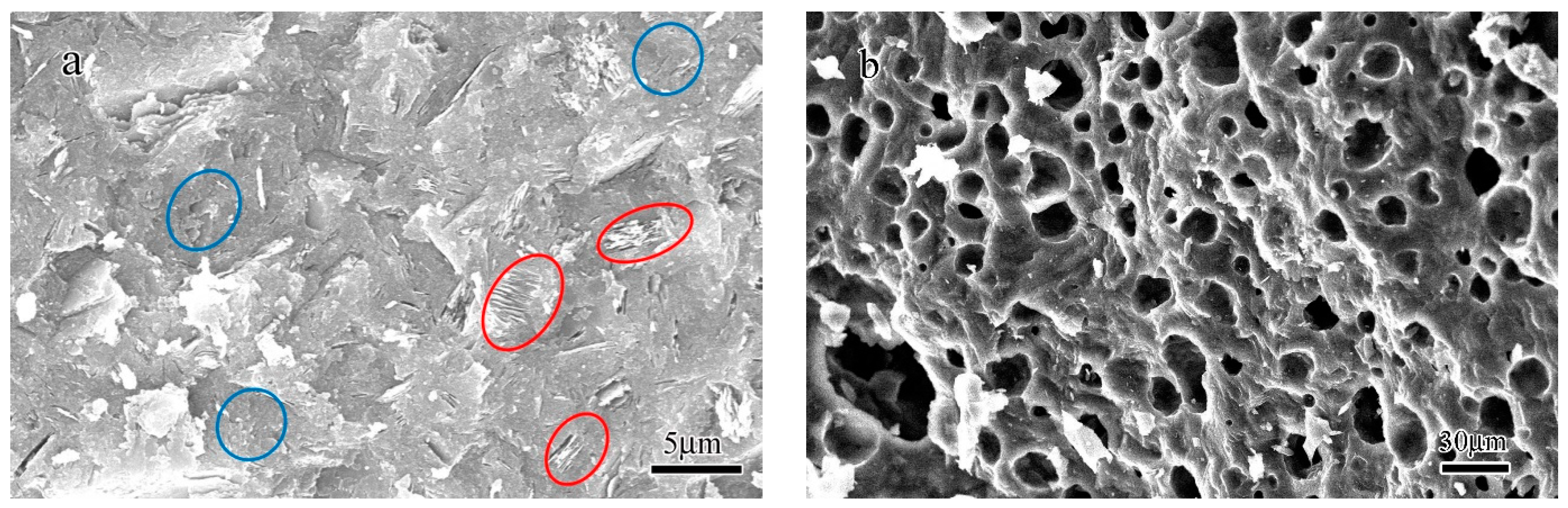
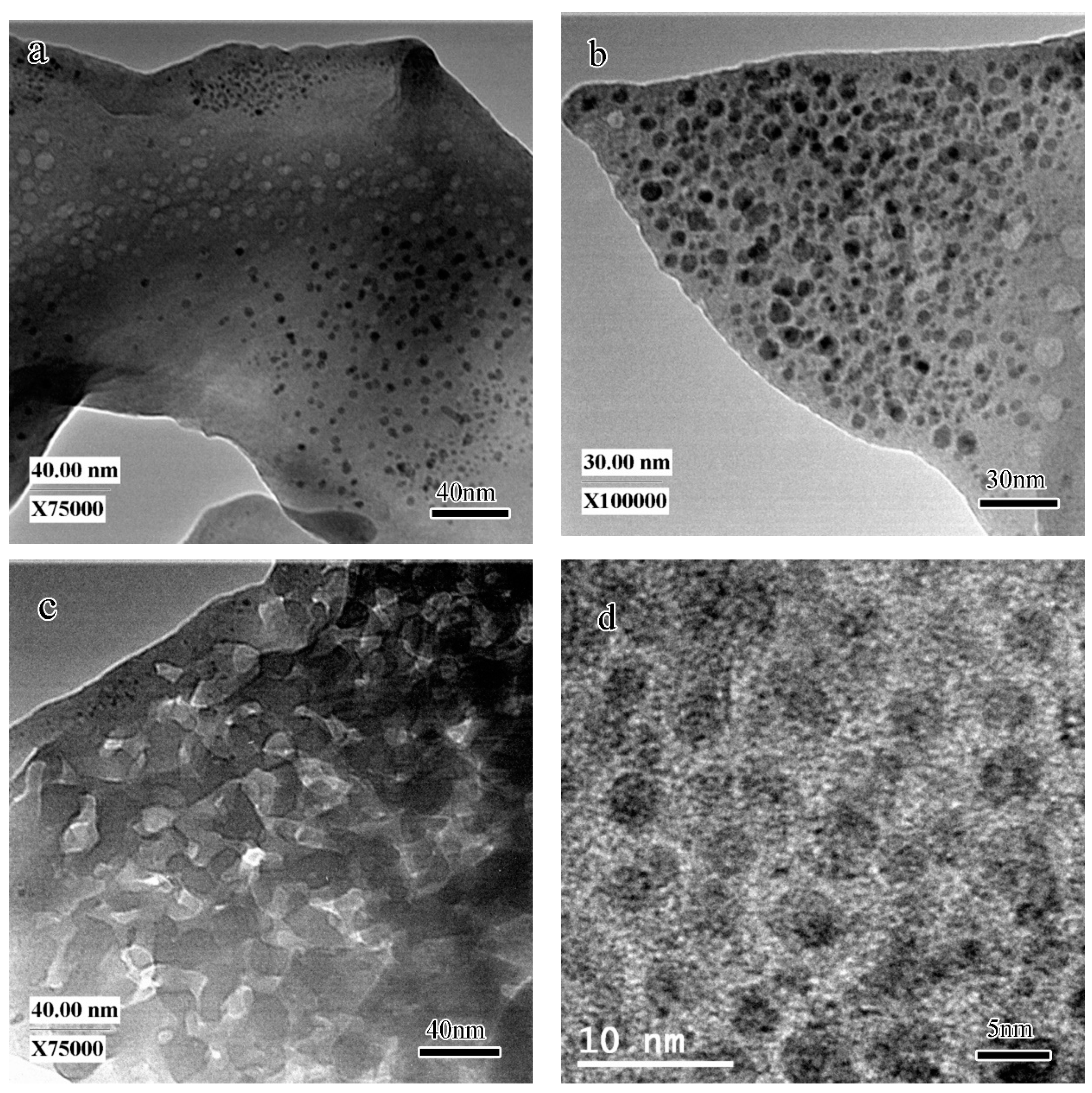
- The SEM observation indicated that the microstructure of the fracture surface of the geopolymer before heating was composed of non-reacted metakaolin with a typical layered structure and reacted amorphous binder phase. The TEM result indicates that the unheated geopolymer was composed of an amorphous hydration product phase, nano-sized pores with size varying from 5 to 30 nm and agglomeration of nanocrystals with an average size of 2–3 nm.
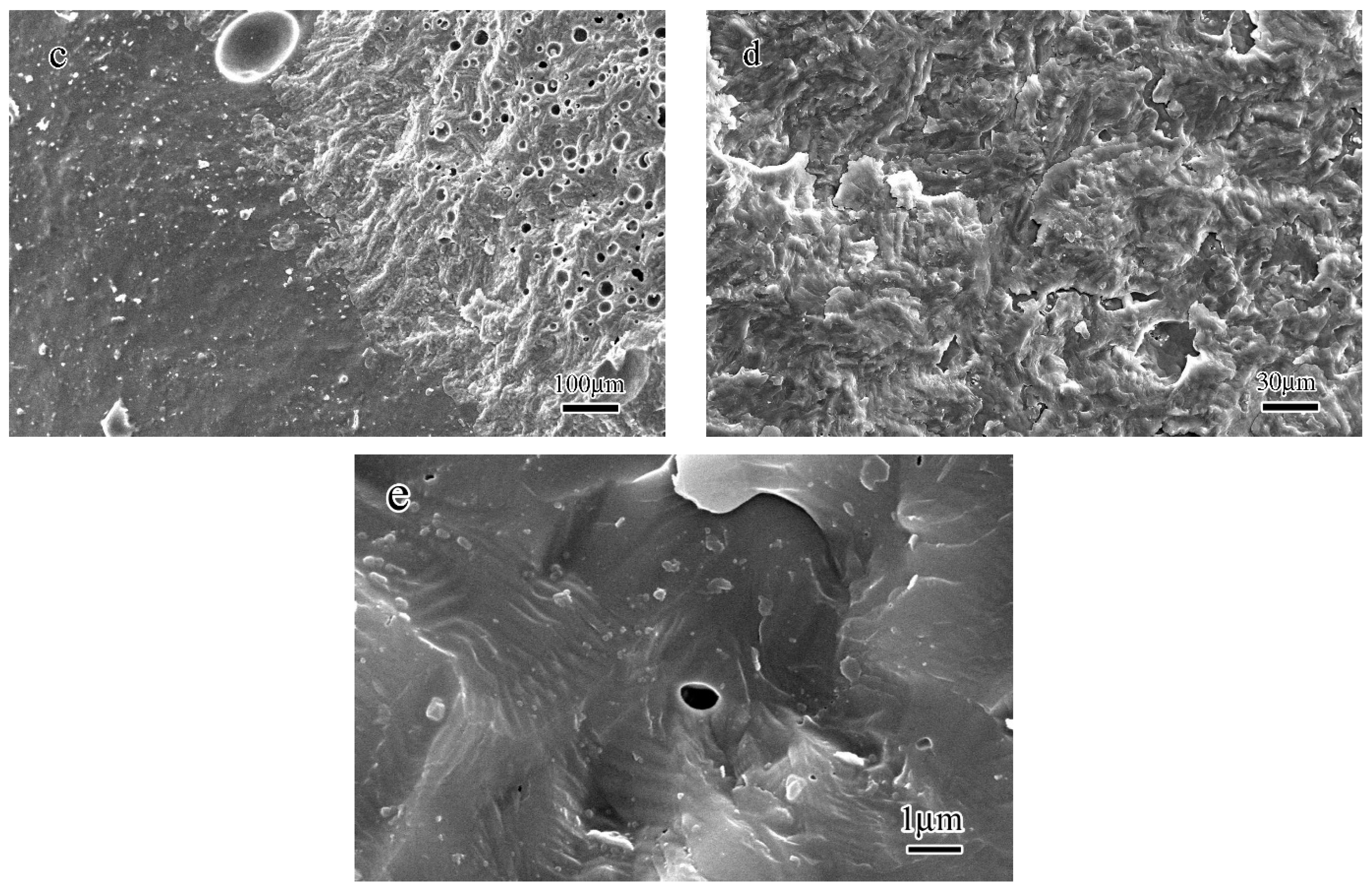
- After the geopolymer was heated to 1000 °C, the microstructure of the geopolymer started to become porous with an average pore size in the range of 5 to 30 μm. When the heating temperature reached 1100 °C, the pores closed along with the leucite crystallization process and the sintering effect and a distinctive interface between the densified microstructure and porous structure could be observed. When the heating temperature reached 1200 °C, most of the pores closed. The TEM results demonstrate that the components of the geopolymer after being heated to 1400 °C were composed of an amorphous glassy phase and crystallized leucite phase.
- The crystallized leucite grains originated from the nano-sized crystal nuclei with an average size of 2–3 nm. The TEM-EDS results indicate that the chemical composition of the glassy phase was complicated. It varied from area to area because of the movement and uneven distribution of K.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Zhang, J.; Li, S.; Lin, C.; Gao, Y.; Liu, C. Feasibility of preparing red mud-based cementitious materials: Synergistic utilization of industrial solid waste, waste heat, and tail gas. J. Clean. Prod. 2021, 285, 124896. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Grumezescu, A.M.; Vrabec, M.; Šmuc, N.R.; Šturm, S.; Ow-Yang, C.; Gulgun, M.A.; Bundur, Z.B.; Ciuca, I.; Vasile, B.S. End-of-life materials used as supplementary cementitious materials in the concrete industry. Materials 2020, 13, 1954. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.S.; Yang, J.; Mo, K.H.; Abdalla, J.A.; Hawileh, R.A.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2020, 267, 128900. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wu, Y.; Xu, F.; Song, X.; Ye, J.; Duan, P.; Zhang, Z. Sulfate resistance of hybrid fiber reinforced metakaolin geopolymer composites. Compos. Part B Eng. 2020, 183, 107689. [Google Scholar] [CrossRef]
- Panda, R.; Sagarika, P.; Mohanty, A.M. Geopolymer Concrete: An Emerging Green Material for Modern Construction. Int. J. Eng. Adv. Technol. 2020, 9, 4046–4050. [Google Scholar]
- Bocullo, V.; Vitola, L.; Vaiciukyniene, D.; Kantautas, A.; Bajare, D. The influence of the SiO2/Na2O ratio on the low calcium alkali activated binder based on fly ash. Mater. Chem. Phys. 2021, 258, 123846. [Google Scholar] [CrossRef]
- Campbell, D.H.; Folk, R.L. Ancient Egyptian Pyramids—Concrete or Rock. Concr. Int. 1991, 13, 28–30. [Google Scholar]
- Zivica, V.; Palou, M.T.; Bágeľ, T.I.Ľ. High strength metahalloysite based geopolymer. Compos. Part B Eng. 2014, 57, 155–165. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; Robayo-Salazar, R.A.; de Gutiérrez, R.M. A novel geopolymer application: Coatings to protect reinforced concrete against corrosion. Appl. Clay Sci. 2017, 135, 437–446. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Van Riessen, A. Fly ash based geopolymer thin coatings on metal substrates and its thermal evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef]
- Sakkas, K.; Sofianos, A.; Nomikos, P.; Panias, D. Behaviour of passive fire protection K-geopolymer under successive severe fire incidents. Materials 2015, 8, 6096–6104. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168, 108586. [Google Scholar] [CrossRef]
- Bakri, A.M.; Liyana, J.; Kamarudin, H.; Bnhussain, M.; Ruzaidi, C.M.; Rafiza, A.R.; Izzat, A.M. Study on refractory materials application using geopolymer processing. Adv. Sci. Lett. 2013, 19, 221–223. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Vickers, L.; Van Riessen, A.; Rickard, W.D. Fire-Resistant Geopolymers: Role of Fibers and Fillers to Enhance Thermal Properties; Springer: Singapore, 2015. [Google Scholar]
- Samal, S.; Thanh, N.P.; Petríková, I.; Marvalová, B.; Vallons, K.A.; Lomov, S.V. Correlation of microstructure and mechanical properties of various fabric reinforced geo-polymer composites after exposure to elevated temperature. Ceram. Int. 2015, 41, 12115–12129. [Google Scholar] [CrossRef]
- Samal, S. Effect of high temperature on the microstructural evolution of fiber-reinforced geopolymer composite. Heliyon 2019, 5, e01779. [Google Scholar] [CrossRef] [Green Version]
- Xie, N.; Bell, J.L.; Kriven, W.M. Fabrication of structural leucite glass–ceramics from potassium-based geopolymer precursors. J. Am. Ceram. Soc. 2010, 93, 2644–2649. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Lin, T.; Wang, M.; Zhou, Y. Effects of high-temperature heat treatment on the mechanical properties of unidirectional carbon fiber reinforced geopolymer composites. Ceram. Int. 2010, 36, 1447–1453. [Google Scholar] [CrossRef]
- Geopolymers: Structures, Processing, Properties and Industrial Applications; Provis, J.L.; Van Deventer, J.S.J. (Eds.) Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- He, P.; Jia, D.; Wang, S. Microstructure and integrity of leucite ceramic derived from potassium-based geopolymer precursor. J. Eur. Ceram. Soc. 2013, 33, 689–698. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Wang, M.; Zhou, Y. Thermal evolution and crystallization kinetics of potassium-based geopolymer. Ceram. Int. 2011, 37, 59–63. [Google Scholar] [CrossRef]


| Chemical Elements | Area A | Area B | Area C | Area D | Area E |
|---|---|---|---|---|---|
| K | 19.7 | 25.1 | 20.0 | 18.2 | 26.5 |
| Al | 21.7 | 17.5 | 17.5 | 17.1 | 17.2 |
| Si | 28.9 | 33.9 | 34.8 | 35.3 | 38.2 |
| O | 29.7 | 23.5 | 27.7 | 29.4 | 18.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Cui, N.; Xian, Y.; Liu, J.; Xing, C.; Xie, N.; Wang, D. Microstructure Evolution Mechanism of Geopolymers with Exposure to High-Temperature Environment. Crystals 2021, 11, 1062. https://doi.org/10.3390/cryst11091062
Lu Y, Cui N, Xian Y, Liu J, Xing C, Xie N, Wang D. Microstructure Evolution Mechanism of Geopolymers with Exposure to High-Temperature Environment. Crystals. 2021; 11(9):1062. https://doi.org/10.3390/cryst11091062
Chicago/Turabian StyleLu, Yuanen, Na Cui, Yougong Xian, Jiaqing Liu, Chao Xing, Ning Xie, and Dawei Wang. 2021. "Microstructure Evolution Mechanism of Geopolymers with Exposure to High-Temperature Environment" Crystals 11, no. 9: 1062. https://doi.org/10.3390/cryst11091062
APA StyleLu, Y., Cui, N., Xian, Y., Liu, J., Xing, C., Xie, N., & Wang, D. (2021). Microstructure Evolution Mechanism of Geopolymers with Exposure to High-Temperature Environment. Crystals, 11(9), 1062. https://doi.org/10.3390/cryst11091062








