Microstructure and Elements Concentration of Inconel 713LC during Laser Powder Bed Fusion through a Modified Cellular Automaton Model
Abstract
:1. Introduction
2. Computational Method
2.1. Finite Element Model
2.2. Microstructure Model
- Nucleation occurs in the cell;
- The cell is captured by a solid cell.
2.3. Laser Processing
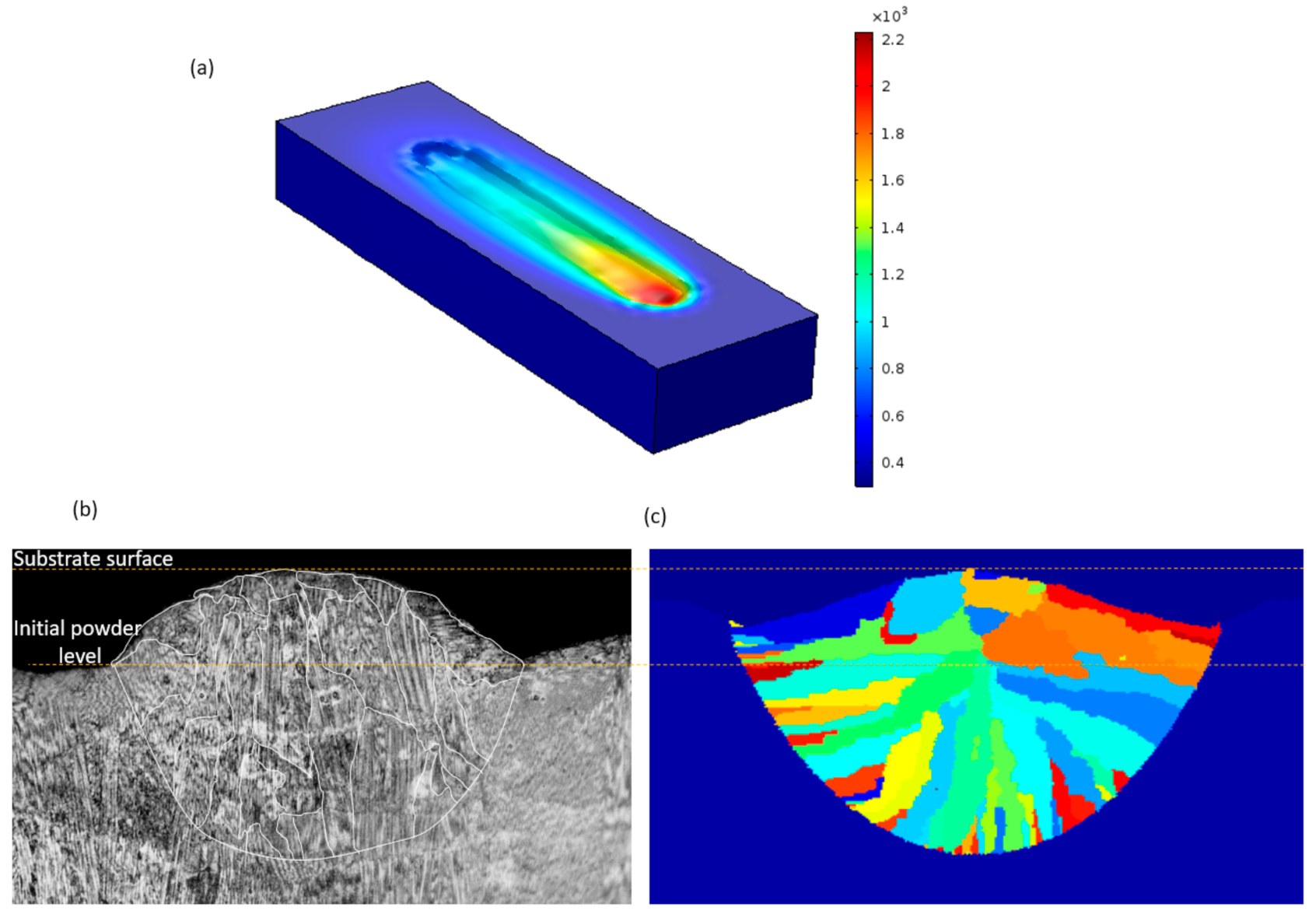
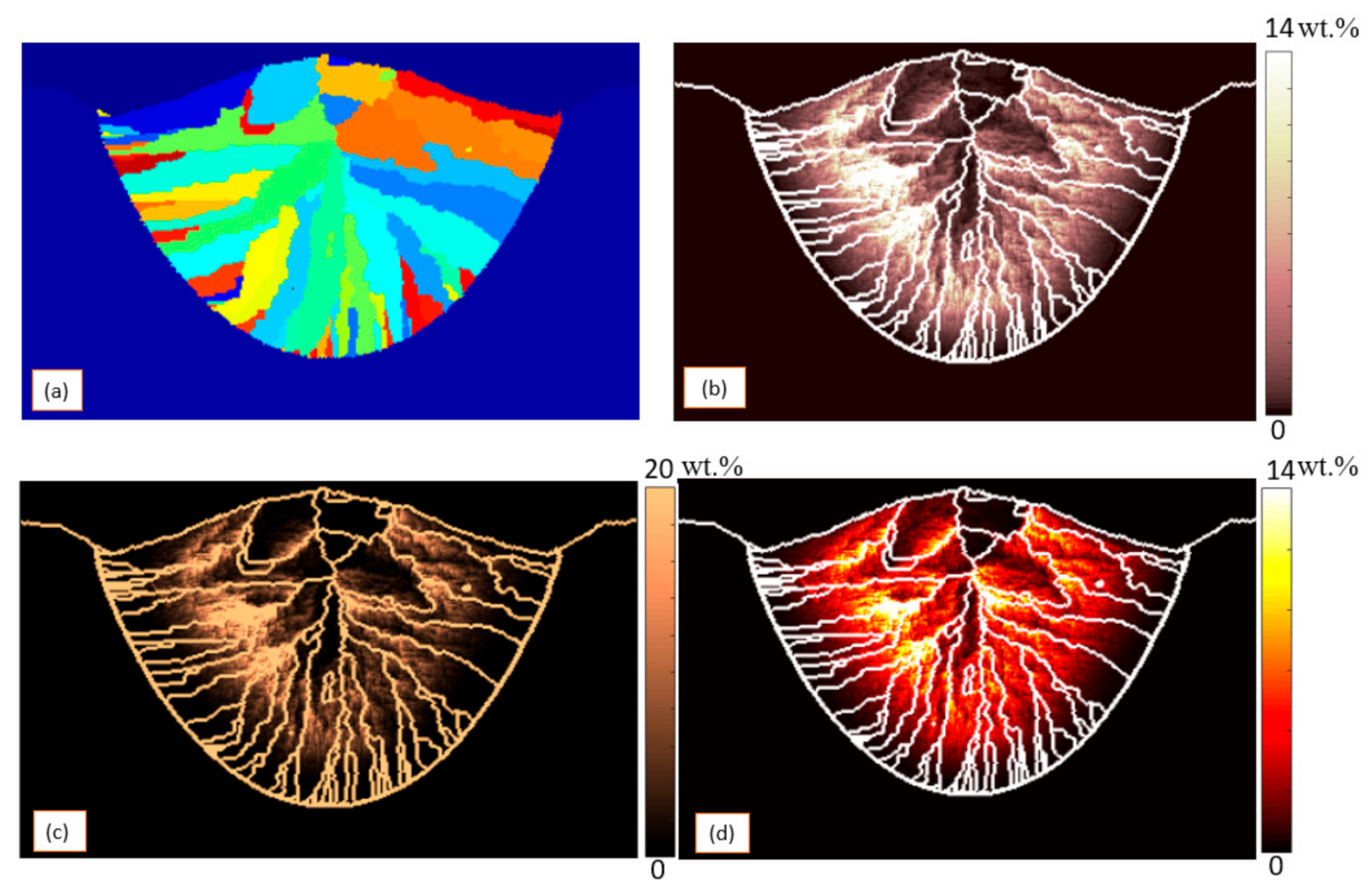
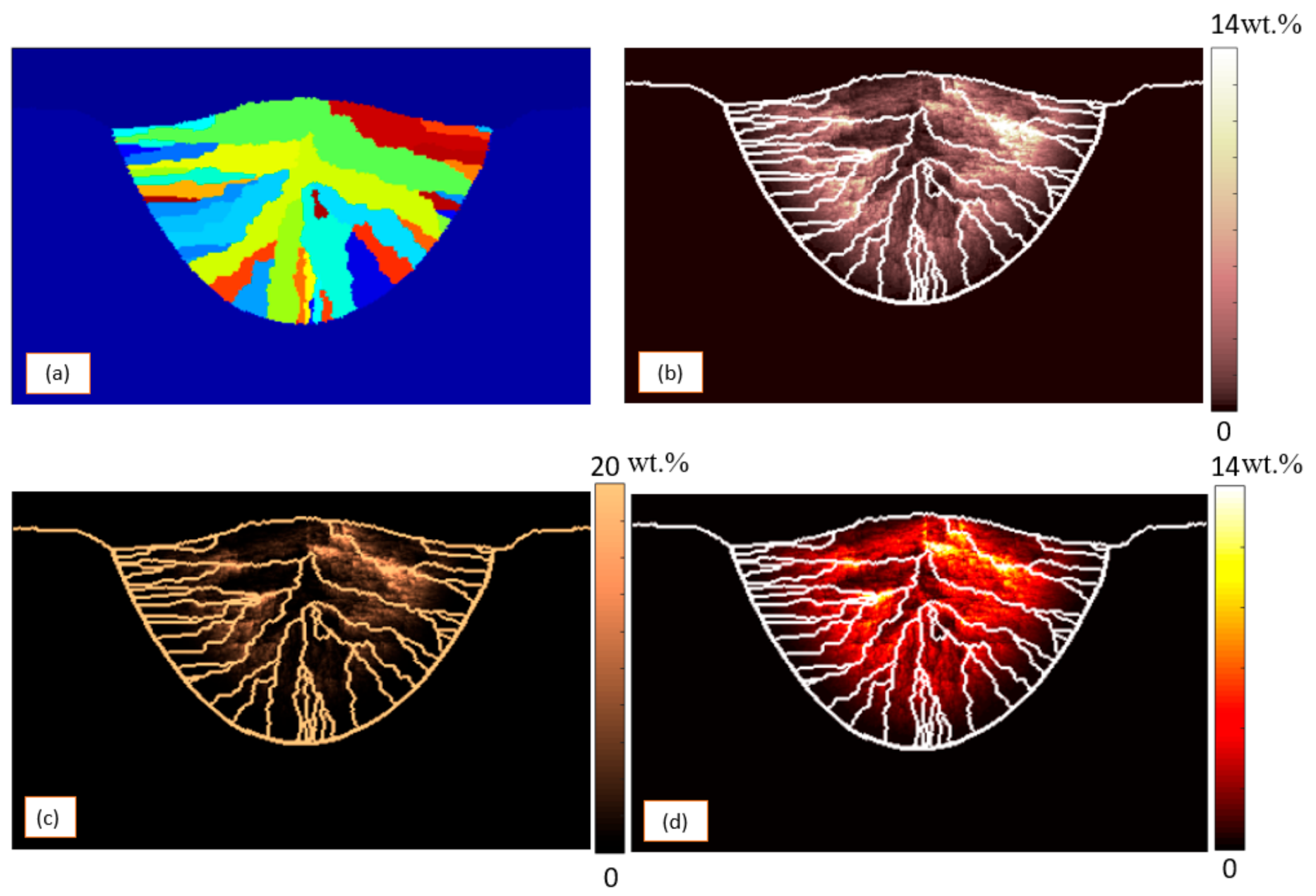
3. Results and Discussion
4. Conclusions
- (1)
- Solidification speed can be controlled by heat input. Faster cooling rate and solidification speed are obtained by decreasing the laser heat input.
- (2)
- The simulation results show that the micro segregation phenomenon is a diffusion-controlled process, in which the elements with a lower partition coefficient and higher diffusivity experience a higher rate of segregation.
- (3)
- It is shown that element segregation is enhanced under a slower solidification rate since the elements spend a longer time in their respective precipitation windows and thus have sufficient time to move from the solid phase to the liquid phase.
- (4)
- As the laser heat input reduces, the solidification speed increases and the element partitioning coefficients approach unity. Consequently, a more uniform solid phase is formed with only minimal segregation at the grain boundaries.
- (5)
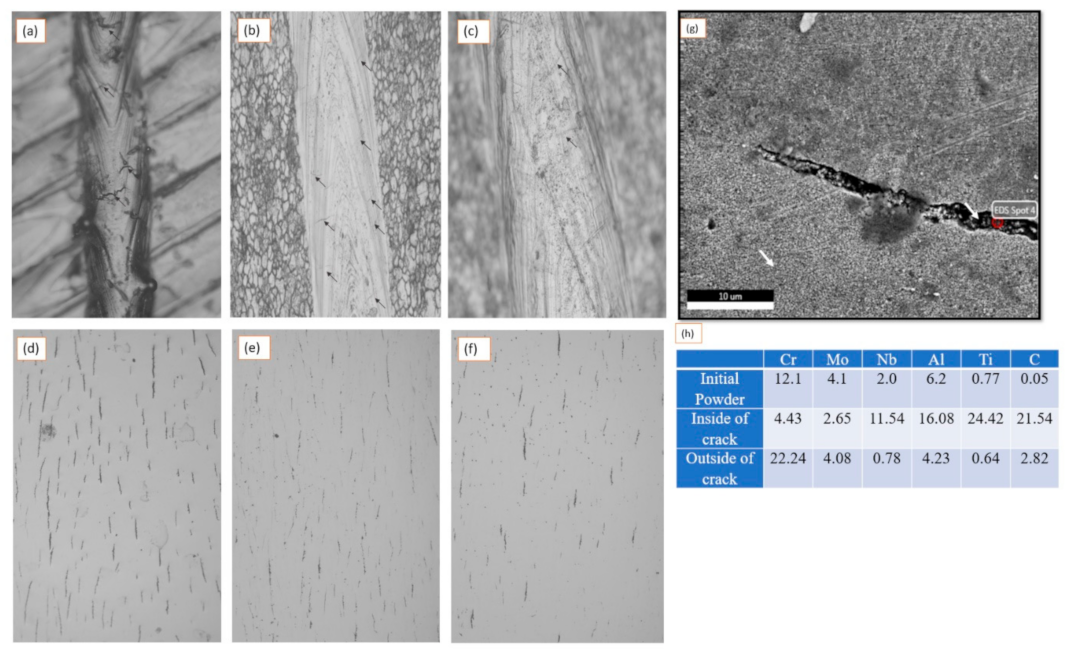
- It has been shown that the crack length and crack density decrease with a reducing laser heat input.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cantó, J.S.; Winwood, S.; Rhodes, K.; Birosca, S. A study of low cycle fatigue life and its correlation with microstructural parameters in IN713C nickel based superalloy. Mater. Sci. Eng. A 2018, 718, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Kunz, L.; Lukáš, P.; Konečná, R.; Fintová, S. Casting defects and high temperature fatigue life of IN 713LC superalloy. Int. J. Fatigue 2012, 41, 47–51. [Google Scholar] [CrossRef]
- Matysiak, H.; Zagorska, M.; Balkowiec, A.; Adamczyk-Cieslak, B.; Cygan, R.; Cwajna, J.; Nawrocki, J.; Kurzydłowski, K.J. The Microstructure Degradation of the IN 713C Nickel-Based Superalloy after the Stress Rupture Tests. J. Mater. Eng. Perform. 2014, 23, 3305–3313. [Google Scholar] [CrossRef] [Green Version]
- Amirjan, M.; Bozorg, M.; Sakiani, H. Investigation of microstructure and corrosion behavior of IN718 superalloy fabricated by selective laser melting. Mater. Chem. Phys. 2021, 263, 124368. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, K.; Gao, M.; Li, X.; Chen, X.; Zeng, X. The microstructure and mechanical properties of deposited-IN718 by selective laser melting. J. Alloy. Compd. 2012, 513, 518–523. [Google Scholar] [CrossRef]
- Li, S.; Wei, Q.; Shi, Y.; Zhu, Z.; Zhang, D. Microstructure Characteristics of Inconel 625 Superalloy Manufactured by Selective Laser Melting. J. Mater. Sci. Technol. 2015, 31, 946–952. [Google Scholar] [CrossRef]
- Tian, Y.; Tomus, D.; Rometsch, P.; Wu, X. Influences of processing parameters on surface roughness of Hastelloy X produced by selective laser melting. Addit. Manuf. 2017, 13, 103–112. [Google Scholar] [CrossRef]
- Shepeleva, L.; Medres, B.; Kaplan, W.; Bamberger, M.; Weisheit, A. Laser cladding of turbine blades. Surf. Coat. Technol. 2000, 125, 45–48. [Google Scholar] [CrossRef]
- Łyczkowska, K.; Adamiec, J.; Jachym, R.; Kwieciński, K. Properties of the Inconel 713 Alloy Within the High Temperature Brittleness Range. Arch. Foundry Eng. 2017, 17, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Dezfoli, A.R.A.; Hwang, W.-S.; Huang, W.-C.; Tsai, T.-W. Determination and controlling of grain structure of metals after laser incidence: Theoretical approach. Sci. Rep. 2017, 7, srep41527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Liu, D.; Yang, Y.; Liu, C.; Zhang, Z.; Wang, H.; Wang, J. Investigation on the meta-dynamic recrystallization behavior of Inconel 718 superalloy in the presence of δ phase through a modified cellular automaton model. J. Alloy. Compd. 2020, 817, 152773. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Yang, H.; Li, F.; Wang, Z.; Zeng, X. Prediction of microstructure in selective laser melted Ti6Al4V alloy by cellular automaton. J. Alloys Compd. 2018, 748, 281–290. [Google Scholar]
- Michael, A.; Zhou, Y.; Yavuz, M.; Khan, M. Modelling the alloy element composition change in NiTi achieved through laser induced vaporization. Mater. Chem. Phys. 2019, 231, 87–94. [Google Scholar] [CrossRef]
- Ao, X.; Xia, H.; Liu, J.; He, Q. Simulations of microstructure coupling with moving molten pool by selective laser melting using a cellular automaton. Mater. Des. 2020, 185, 108230. [Google Scholar] [CrossRef]
- Trivedi, R. Morphological stability of a solid particle growing from a binary alloy melt. J. Cryst. Growth 1980, 48, 93–99. [Google Scholar] [CrossRef]
- Zinoviev, A.; Zinovieva, O.; Ploshikhin, V.; Romanova, V.; Balokhonov, R. Evolution of grain structure during laser additive manufacturing. Simulation by a cellular automata method. Mater. Des. 2016, 106, 321–329. [Google Scholar] [CrossRef]
- Lian, Y.; Gan, Z.; Yu, C.; Kats, D.; Liu, W.K.; Wagner, G.J. A cellular automaton finite volume method for microstructure evolution during additive manufacturing. Mater. Des. 2019, 169, 107672. [Google Scholar] [CrossRef]
- Simonelli, M.; McCartney, D.G.; Barriobero-Vila, P.; Aboulkhair, N.T.; Tse, Y.Y.; Clare, A.; Hague, R. The Influence of Iron in Minimizing the Microstructural Anisotropy of Ti-6Al-4V Produced by Laser Powder-Bed Fusion. Metall. Mater. Trans. A 2020, 51, 2444–2459. [Google Scholar] [CrossRef] [Green Version]
- Chamanfar, A.; Jahazi, M.; Bonakdar, A.; Morin, E.; Firoozrai, A. Cracking in fusion zone and heat affected zone of electron beam welded Inconel-713LC gas turbine blades. Mater. Sci. Eng. A 2015, 642, 230–240. [Google Scholar] [CrossRef]
- Xu, J.; Lin, X.; Guo, P.; Hu, Y.; Wen, X.; Xue, L.; Liu, J.; Huang, W. The effect of preheating on microstructure and mechanical properties of laser solid forming IN-738LC alloy. Mater. Sci. Eng. A 2017, 691, 71–80. [Google Scholar] [CrossRef]
- Andreotta, R.; Ladani, L.; Brindley, W. Finite element simulation of laser additive melting and solidification of Inconel 718 with experimentally tested thermal properties. Finite Elem. Anal. Des. 2017, 135, 36–43. [Google Scholar] [CrossRef]
- Hirt, C.; Amsden, A.; Cook, J. An arbitrary Lagrangian-Eulerian computing method for all flow speeds. J. Comput. Phys. 1974, 14, 227–253. [Google Scholar] [CrossRef]
- De Baere, D.; Bayat, M.; Mohanty, S.; Hattel, J. Thermo-fluid-metallurgical modelling of the selective laser melting process chain. Procedia CIRP 2018, 74, 87–91. [Google Scholar] [CrossRef]
- Morville, S.; Carin, M.; Muller, M.; Gharbi, M.; Peyre, P.; Carron, D.; Masson, P.L.E.; Fabbro, R. 2D axial-symmetric model for fluid flow and heat transfer in the melting and resolidification of a vertical cylinder. In Proceedings of the COMSOL Conference, Paris, France, 17–19 November 2010. [Google Scholar]
- Li, S.; Xiao, H.; Liu, K.; Xiao, W.; Li, Y.; Han, X.; Mazumder, J.; Song, L. Melt-pool motion, temperature variation and dendritic morphology of Inconel 718 during pulsed- and continuous-wave laser additive manufacturing: A comparative study. Mater. Des. 2017, 119, 351–360. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Xiang, Z.; Yin, M.; Dong, G.; Mei, X.; Yin, G. Modeling of the thermal physical process and study on the reliability of linear energy density for selective laser melting. Results Phys. 2018, 9, 939–946. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, D.; Liu, Z.; Kang, Z.; Gao, Y.; Li, L. Microscopic simulation of the effect of process parameters by the Marangoni convection on the temperature field of Inconel 718 alloy using selective laser melting. In: Advanced Laser Processing and Manufacturing II. Int. Soc. Opt. Photonics 2018, 108130. [Google Scholar]
- Sih, S.S.; Barlow, J.W. The Prediction of the Emissivity and Thermal Conductivity of Powder Beds. Part. Sci. Technol. 2004, 22, 427–440. [Google Scholar] [CrossRef]
- Loh, L.-E.; Chua, C.-K.; Yeong, W.Y.; Song, J.; Mapar, M.; Sing, S.L.; Liu, Z.-H.; Zhang, D.-Q. Numerical investigation and an effective modelling on the Selective Laser Melting (SLM) process with aluminium alloy 6061. Int. J. Heat Mass Transf. 2015, 80, 288–300. [Google Scholar] [CrossRef]
- Trivedi, R.; Kurz, W. Dendritic growth. Int. Mat. Rev. 1994, 39, 49–74. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D. Dendrite growth at the limit of stability: Tip radius and spacing. Acta Metall. 1981, 29, 11–20. [Google Scholar] [CrossRef]
- Tsai, D.C.; Hwang, W.S. Numerical simulation of solidification morphologies of Cu-0.6 Cr casting alloy using modified cellular automaton model. Trans. Nonferrous Metals Soc. China 2010, 20, 1072–1077. [Google Scholar] [CrossRef]
- Zlá, S.; Smetana, B.; Žaludová, M.; Dobrovská, J.; Vodárek, V.; Konečná, K.; Matějka, V.; Francová, H. Determination of thermophysical properties of high temperature alloy IN713LC by thermal analysis. J. Therm. Anal. Calorim. 2012, 110, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Queva, A.; Guillemot, G.; Moriconi, C.; Metton, C.; Bellet, M. Numerical study of the impact of vaporisation on melt pool dynamics in Laser Powder Bed Fusion—Application to IN718 and Ti-6Al-4V. Addit. Manuf. 2020, 35, 101249. [Google Scholar] [CrossRef]
- Krajewski, W.K.; Piwowarski, G.; Buraś, J.; Rutkowski, P.; Szeliga, D. Thermo-Physical Properties of Selected Inconel. Arch. Metall. Mater. 2014, 59, 1055–1058. [Google Scholar] [CrossRef]
- Mills, K.C. Recommended Values of Thermophysical Properties for Selected Commercial Alloys; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Queva, A.; Mayi, Y.; Bellet, M.; Guillemot, G.; Peyre, P.; Dal, M.; Moriconi, C.; Metton, C. Thermo-Mechanical Simulation of Track Development in the Laser Beam Melting Process-Effect of Laser-Metal Interaction. IOP Conf. Ser. Mater. Sci. Eng. 2019, 529, 012005. [Google Scholar] [CrossRef]
- Kundin, J.; Mushongera, L.; Emmerich, H. Phase-field modeling of microstructure formation during rapid solidification in Inconel 718 superalloy. Acta Mater. 2015, 95, 343–356. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lo, Y.-L.; Tran, H.-C.; Raza, M.M.; Le, T.-N. Systematic Approach for Reducing Micro-Crack Formation in Inconel 713LC Components Fabricated by Laser Powder Bed Fusion. Rapid Prototype J. 2021, 27, 1548–1561. [Google Scholar] [CrossRef]
- Rashid, R.; Masood, S.; Ruan, D.; Palanisamy, S.; Elambasseril, J.; Brandt, M. Effect of energy per layer on the anisotropy of selective laser melted AlSi12 aluminium alloy. Addit. Manuf. 2018, 22, 426–439. [Google Scholar] [CrossRef]


| Parameter | Cr | Mo | Nb | Al | Ti | C |
|---|---|---|---|---|---|---|
| C0 × % [mass] | 12.1 | 4.10 | 2.0 | 6.2 | 0.77 | 0.05 |
| DL × 1010 [m2/s] [39] | 8.98 | 10.76 | 10.53 | 11.10 | 10.99 | 43.0 |
| k [16] | 0.96 | 0.82 | 0.46 | 0.83 | 0.55 | 0.12 |
| Property (Unit) | Value | Reference | |
|---|---|---|---|
| Liquidus temperature [°C] | Tl | 1349 | [34] |
| Solidus temperature [°C] | Ts | 1250 | [34] |
| Density of liquid [kg.m3] | 7300 | [35] | |
| Density of solid [kg.m3] | 8190 | [35] | |
| Thermal Conductivity [J/m.s.K] | −1.9 × 10−8T3 + 2.6×10−5T2 + 1.7 × 10−2T + 9.53 | [36] | |
| Specific heat [J/kg.K] | 2.9 × 10−10T3 − 4.1 × 10−7T2 + 5.5 × 10−4T + 4.210−1 | [36] | |
| Latent heat of fusion [KJ/kg] | 236 | [37] | |
| Viscosity [kg/ms] | µ | 7.8 × 10−3 | [38] |
| Surface tension [N/m] | 1.89 | [38] | |
| Marangoni coefficient [N/m.K] | −1.1 × 10−4 | [38] | |
| Absorption (liquid) [mm−1] | A | 25 | [35,38] |
| Reflection coefficient | R | 0.7 | [35] |
| CA cell length [µm] | 0.2 | - | |
| Laser radius [µm] | 50 | - |
| Heat Input [J/m] | Cr | Mo | Nb | Al | Ti | C |
|---|---|---|---|---|---|---|
| 360 | 0.97 | 0.87 | 0.60 | 0.84 | 0.71 | 0.15 |
| 280 | 0.98 | 0.89 | 0.63 | 0.85 | 0.73 | 0.17 |
| 210 | 0.98 | 0.90 | 0.71 | 0.86 | 0.76 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari Dezfoli, A.R.; Lo, Y.-L.; Raza, M.M. Microstructure and Elements Concentration of Inconel 713LC during Laser Powder Bed Fusion through a Modified Cellular Automaton Model. Crystals 2021, 11, 1065. https://doi.org/10.3390/cryst11091065
Ansari Dezfoli AR, Lo Y-L, Raza MM. Microstructure and Elements Concentration of Inconel 713LC during Laser Powder Bed Fusion through a Modified Cellular Automaton Model. Crystals. 2021; 11(9):1065. https://doi.org/10.3390/cryst11091065
Chicago/Turabian StyleAnsari Dezfoli, Amir Reza, Yu-Lung Lo, and M. Mohsin Raza. 2021. "Microstructure and Elements Concentration of Inconel 713LC during Laser Powder Bed Fusion through a Modified Cellular Automaton Model" Crystals 11, no. 9: 1065. https://doi.org/10.3390/cryst11091065
APA StyleAnsari Dezfoli, A. R., Lo, Y.-L., & Raza, M. M. (2021). Microstructure and Elements Concentration of Inconel 713LC during Laser Powder Bed Fusion through a Modified Cellular Automaton Model. Crystals, 11(9), 1065. https://doi.org/10.3390/cryst11091065









