Annealing Induced Saturation in Electron Concentration for V-Doped CdO
Abstract
:1. Introduction
2. Experimental
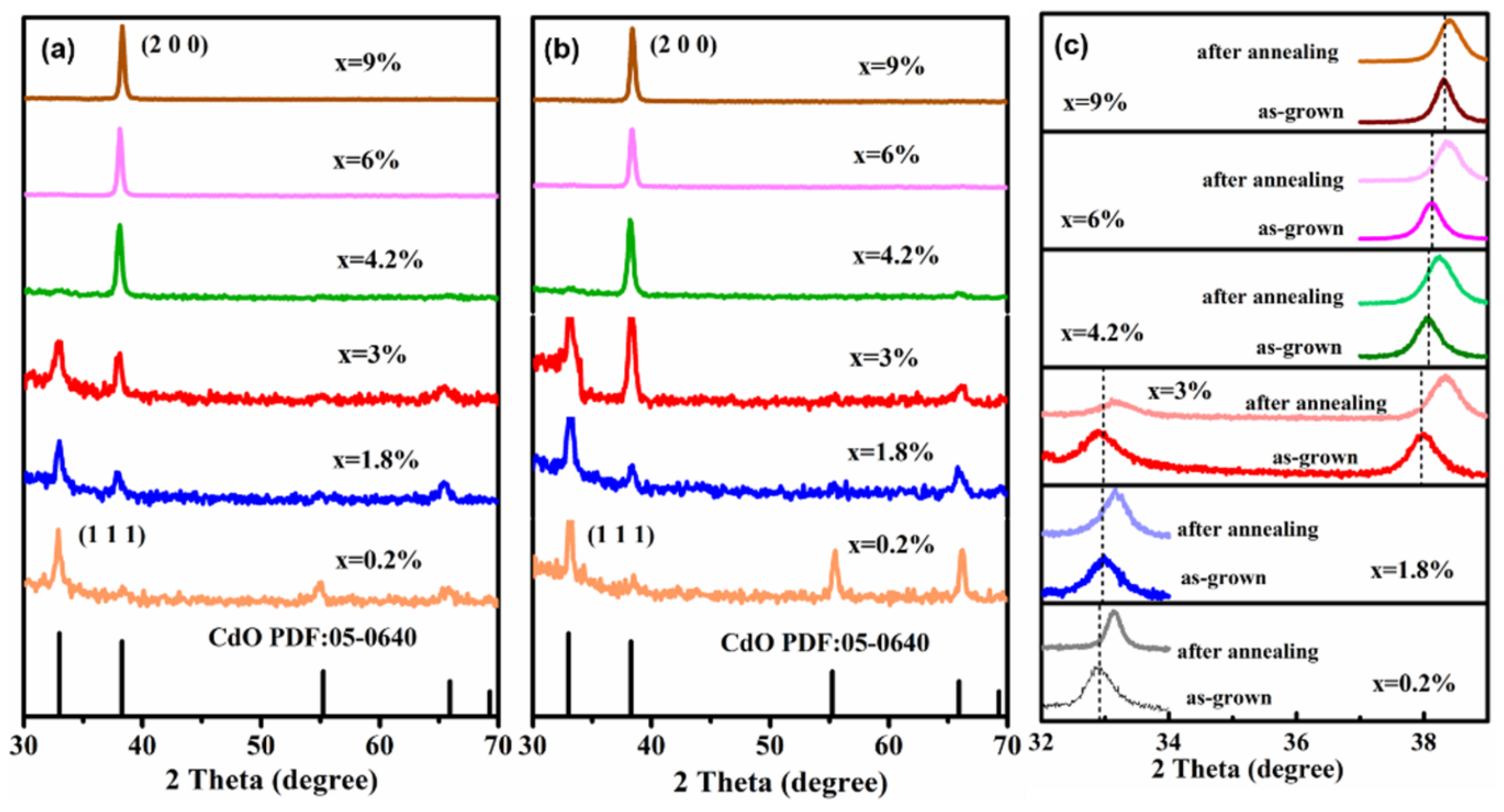
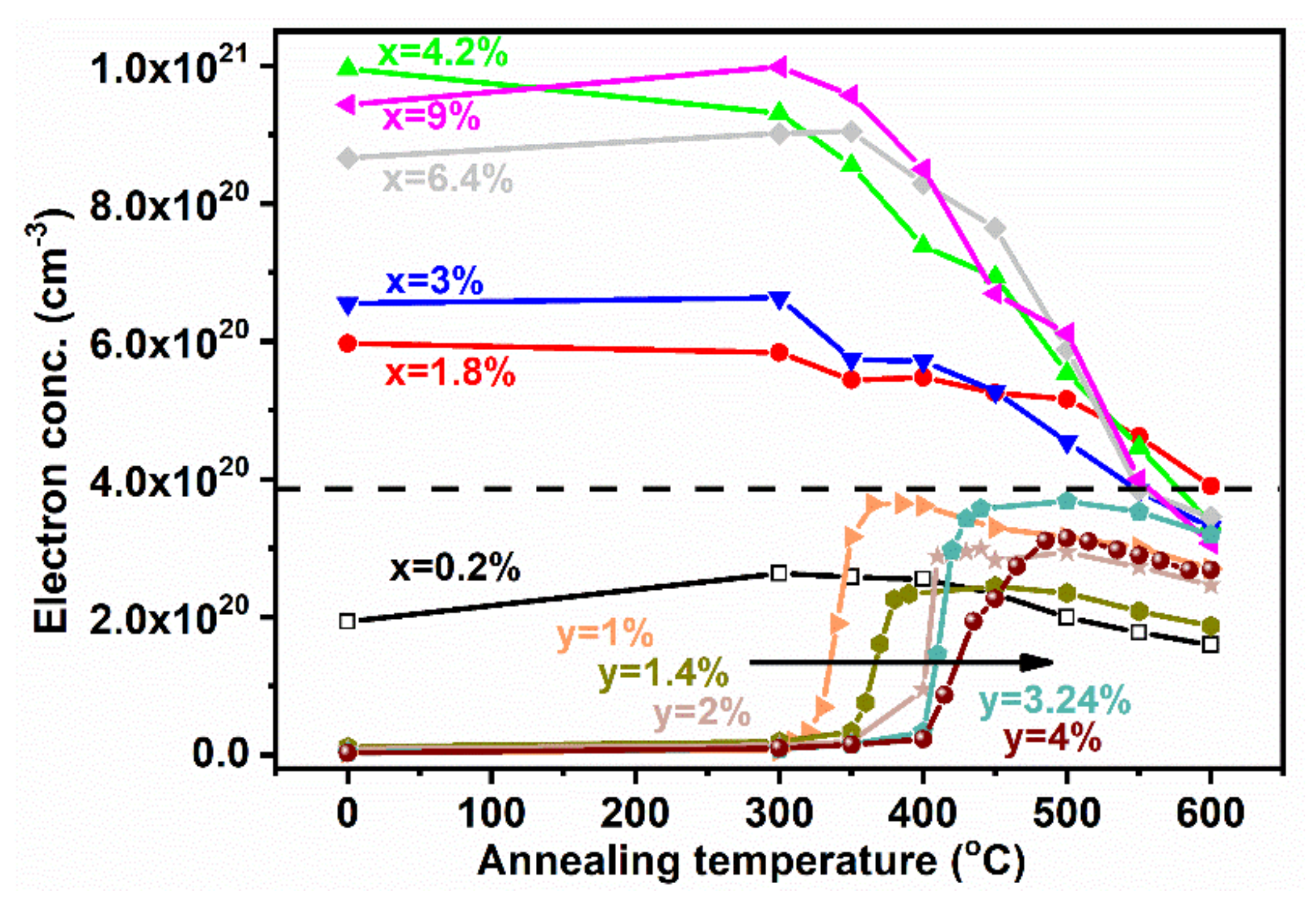
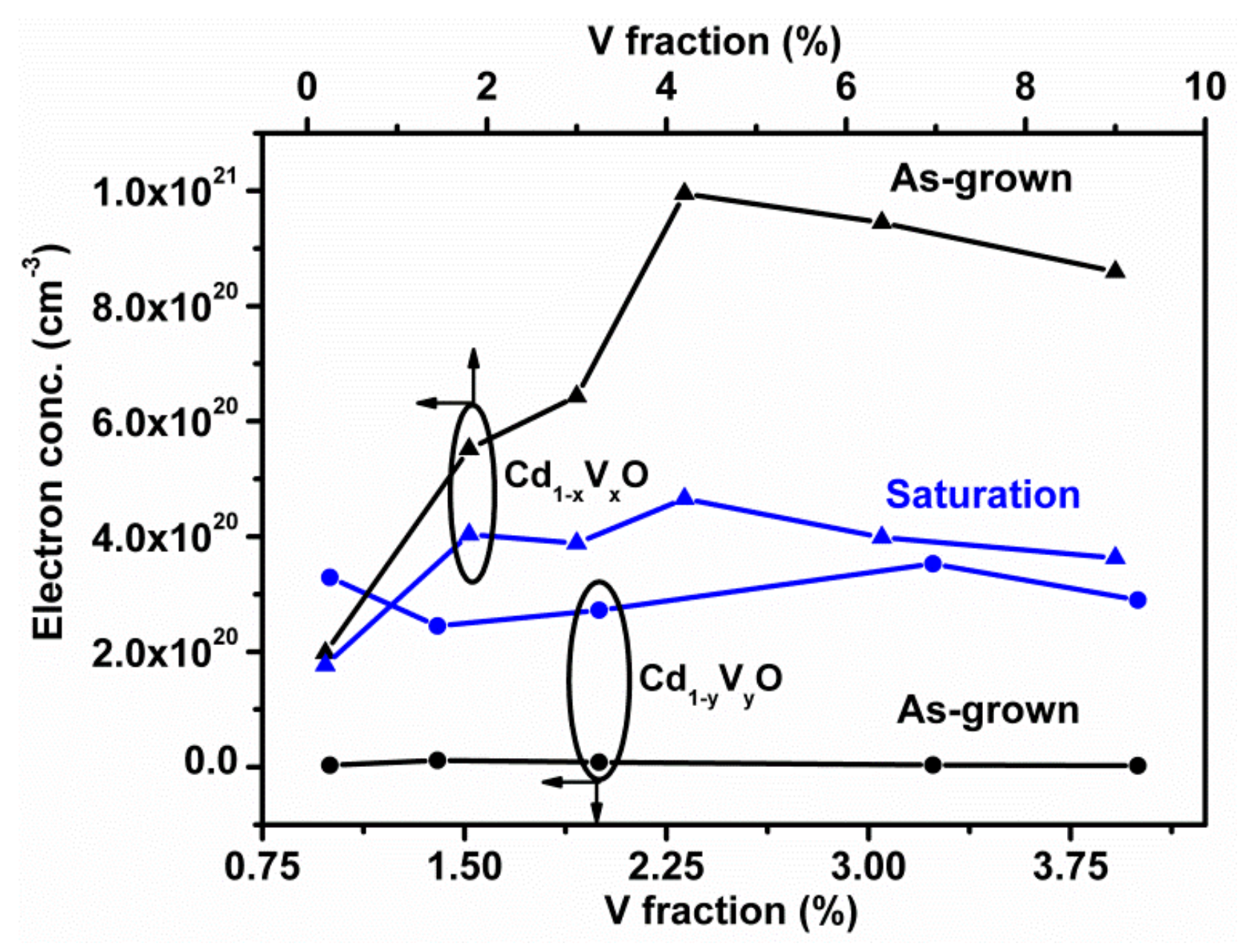
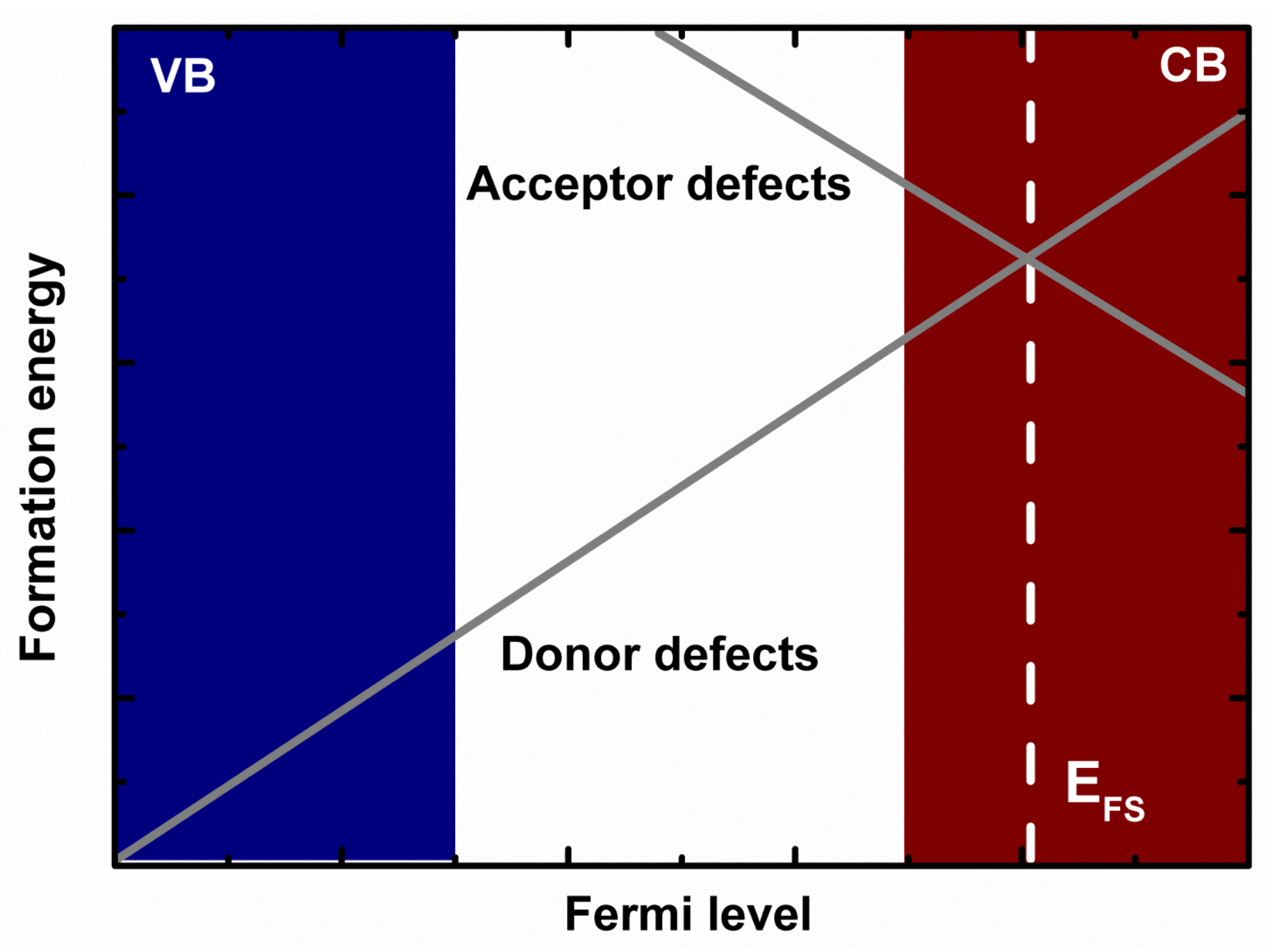
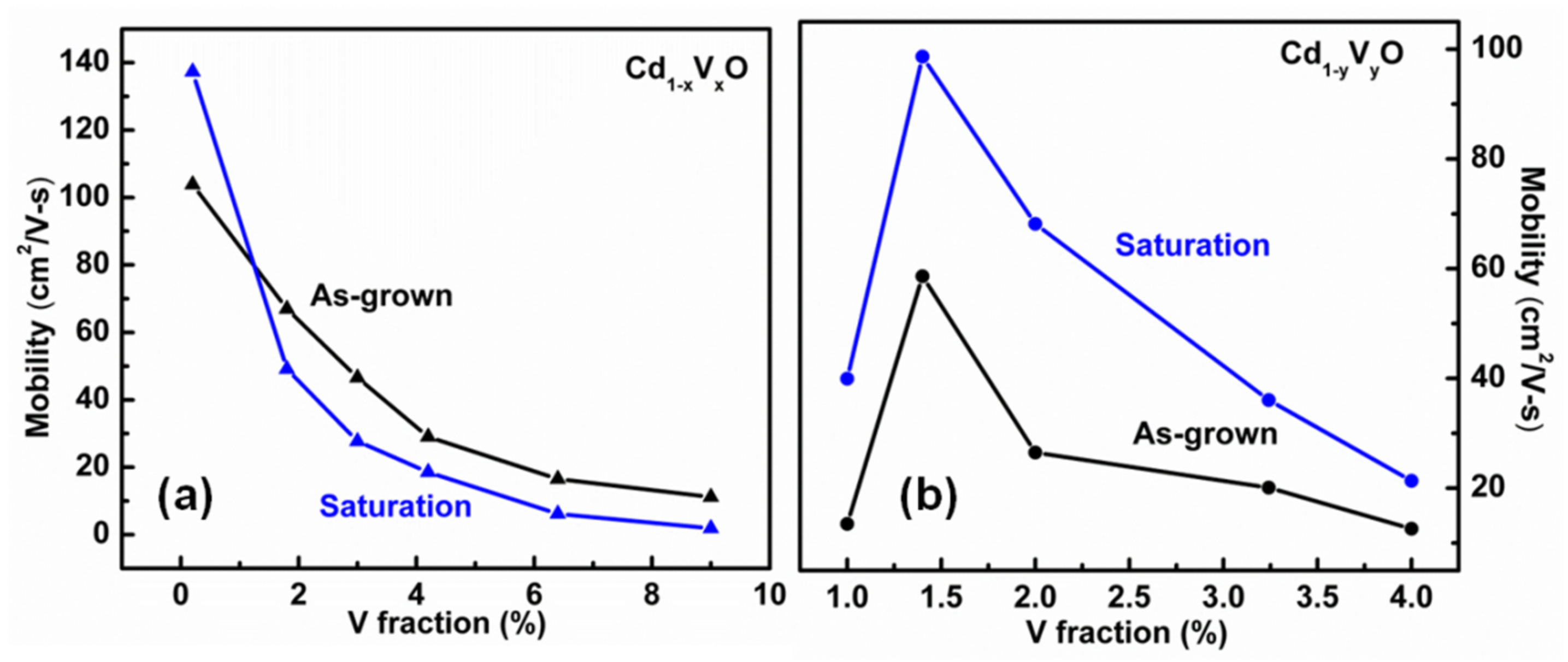
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, R.A. Improved characte ristics of sprayed CdO films by rapid thermal annealing. J. Mater. Sci. Mater. Electron. 2019, 20, 1219–1224. [Google Scholar] [CrossRef]
- Franco-Linton, B.; Castanedo-Pérez, R.; Torres-Delgado, G.; Márquez-Marín, J.; Zelaya-Ángel, O. Influence of vacuum and Ar/CdS atmospheres-rapid thermal annealing (RTA) on the properties of Cd2SnO4 thin films obtained by sol-gel technique. Mater. Sci. Semicond. Process. 2016, 56, 302. [Google Scholar] [CrossRef]
- Xie, M.T.; Zhu, W.; Yu, K.M.; Zhu, Z.S.; Wang, G.Z. Fe-embedded Au (111) monolayer as an electrocatalyst for N2 reduction reaction: A first-principles investigation. J. Alloys Compd. 2019, 776, 259. [Google Scholar] [CrossRef]
- Morales, G.E.M.; Garcia, M.E.A.; Cruz, S.C.; Plata, B.R.; Moreno, O.P.; Pérez, R.G. CdCO3 nanocrystalline thin film grown by chemical bath and its transition to porous CdO by thermal annealing treatment. Optik 2018, 171, 347–355. [Google Scholar] [CrossRef]
- Yu, K.M.; Detert, D.M.; Chen, G.B.; Zhu, W.; Liu, C.P.; Grankowska, S.; Hsu, L.; Dubon, O.D.; Walukiewicz, W. Defects and properties of cadmium oxide based transparent conductors. J. Appl. Phys. 2016, 119, 18150. [Google Scholar] [CrossRef]
- Yu, K.M.; Mayer, M.A.; Speaks, D.T.; He, H.; Zhao, R.; Hsu, L.; Mao, S.S.; Haller, E.E.; Walukiewicz, W. Ideal transparent conductors for full spectrum photovoltaics. J. Appl. Phys. 2012, 111, 123505. [Google Scholar] [CrossRef]
- Segura, A.; Sanchez-Royo, J.F.; Garia-Domene, B.; Almonacid, G. Current underestimation of the optical gap and Bursterin-Moss shift in CdO thin films: A consequence of extended misuse of α2-versus-hν plots. Appl. Phys. Lett. 2011, 99, 151907. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, K.M.; Chen, G.B.; Liu, C.P.; Walukiewicz, W. Conduction band modifications by d states in vanadium doped CdO. J. Alloys Compd. 2020, 822, 153567. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, J.H.; Park, D.S.; Jeong, T.S.; Youn, C.J.; Hong, K.J. Effect of rapid thermal annealing on Zn1−xCdxO layers grown by radio-frequency magnetron co-sputtering. Cryst. Res. Technol. 2010, 45, 1050–1056. [Google Scholar] [CrossRef]
- Sakthivel, P.; Murugan, R.; Asaithambi, S.; Karuppaiah, M.; Rajendran, S.; Ravi, G. Radio frequency magnetron sputtered CdO thin films for optoelectronic applications. J. Phys. Chem. Solids 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Saha, B.; Thapa, R.; Chattopadhyay, K.K. Wide range tuning of electrical conductivity of RF sputtered CdO thin films through oxygen partial pressure variation. Sol. Energy Mater. Sol. Cells 2008, 92, 1077–1080. [Google Scholar] [CrossRef]
- Sakthivela, P.; Murugana, R.; Asaithambia, S.; Karuppaiaha, M.; Rajendrana, S.; Ravi, G. Influence of radiofrequency power on structural, morphological, optical and electrical properties of magnetron sputtered CdO: Sm thin films as alternative TCO for optoelectronic applications. J. Alloys Compd. 2018, 765, 146. [Google Scholar] [CrossRef]
- Devika, M.; Koteeswara, N.; Reddy, S.; Venkatramana Reddy, K.; Ramesh, K.; Gunasekhar, R. Influence of rapid thermal annealing (RTA) on the structural and electrical properties of SnS films. J. Mater. Sci. Mater. Electron. 2009, 20, 1129. [Google Scholar] [CrossRef]
- Huang, T.Z.; Yuan, X.X.; Yu, J.M.; Wu, Z.; Han, J.T.; Sun, G.X.; Xu, N.X.; Zhang, Y.H. Effects of annealing treatment and partial substitution of Cu for Co on phase composition and hydrogen storage performance of La0.7Mg0.3Ni3.2Co0.35 alloy. Int. J. Hydrogen Energy 2012, 37, 1074. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; Gryse, R.D. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Kwon, H.; Thompson, L.T.; Eng, J., Jr.; Chenz, J.G. n-Butane dehydrogenation over vanadium carbides: Correlating catalytic and electronic properties. J. Catal. 2000, 190, 60–68. [Google Scholar] [CrossRef]
- Walukiewicz, W. Amphoteric native defects in semiconductors. Appl. Phys. Lett. 1989, 54, 2094–2096. [Google Scholar] [CrossRef] [Green Version]
- King, P.D.C.; Veal, T.D.; Jefferson, P.H.; Zúñiga-Pérez, J.; Muñoz-Sanjosé, V.; McConville, C.F. Unification of the electrical behavior of defects, impurities, and surface states in semiconductors: Virtual gap states in CdO. Phys. Rev. B 2009, 79, 035203. [Google Scholar] [CrossRef] [Green Version]
- Langer, J.M.; Delerue, C.; Lannoo, M. Transition-metal impurities in semiconductors and heterojunction band lineups. Phys. Rev. B 1998, 38, 7723. [Google Scholar] [CrossRef] [PubMed]
- Burbano, M.; Scanlon, D.O.; Watson, G.W. Sources of conductivity and doping limits in CdO from hybrid density functional theory. J. Am. Chem. Soc. 2011, 133, 15065–15072. [Google Scholar] [CrossRef]
- Speaks, D.T.; Mayer, M.A.; Yu, K.M.; Mao, S.S.; Haller, E.E.; Walukiewicz, W. Fermi level stabilization energy in cadmium oxide. J. Appl. Phys. 2010, 107, 113706. [Google Scholar] [CrossRef] [Green Version]
- Seidel, T.E.; Lischner, D.J.; Pai, C.S.; Lau, S.S. Temperature transients in heavily doped and undoped silicon using rapid thermal annealing. J. Appl. Phys. 1985, 57, 1317–1321. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, G.; Yu, K.; Walukiewicz, W.; Gong, W. Annealing Induced Saturation in Electron Concentration for V-Doped CdO. Crystals 2021, 11, 1079. https://doi.org/10.3390/cryst11091079
Li Y, Chen G, Yu K, Walukiewicz W, Gong W. Annealing Induced Saturation in Electron Concentration for V-Doped CdO. Crystals. 2021; 11(9):1079. https://doi.org/10.3390/cryst11091079
Chicago/Turabian StyleLi, Yajie, Guibin Chen, Kinman Yu, Wladyslaw Walukiewicz, and Weiping Gong. 2021. "Annealing Induced Saturation in Electron Concentration for V-Doped CdO" Crystals 11, no. 9: 1079. https://doi.org/10.3390/cryst11091079
APA StyleLi, Y., Chen, G., Yu, K., Walukiewicz, W., & Gong, W. (2021). Annealing Induced Saturation in Electron Concentration for V-Doped CdO. Crystals, 11(9), 1079. https://doi.org/10.3390/cryst11091079





