Abstract
Iodonium ylides have recently attracted much attention on account of their synthetic applications. However, only a limited number of reports concerning the properties and reactivity of iodonium ylides exist, which is partly due to their instability. In this study, we synthesized several iodonium ylides that bear both an electron-withdrawing group and an aromatic ring with an ortho-t-BuSO2 group. Based on the crystal structures of the synthesized iodonium ylides in combination with natural-bond-orbital (NBO) calculations, we estimated the strength of the intra- and intermolecular halogen-bonding interactions. In addition, we investigated the reactivity of the iodonium ylides under photoirradiation.
1. Introduction
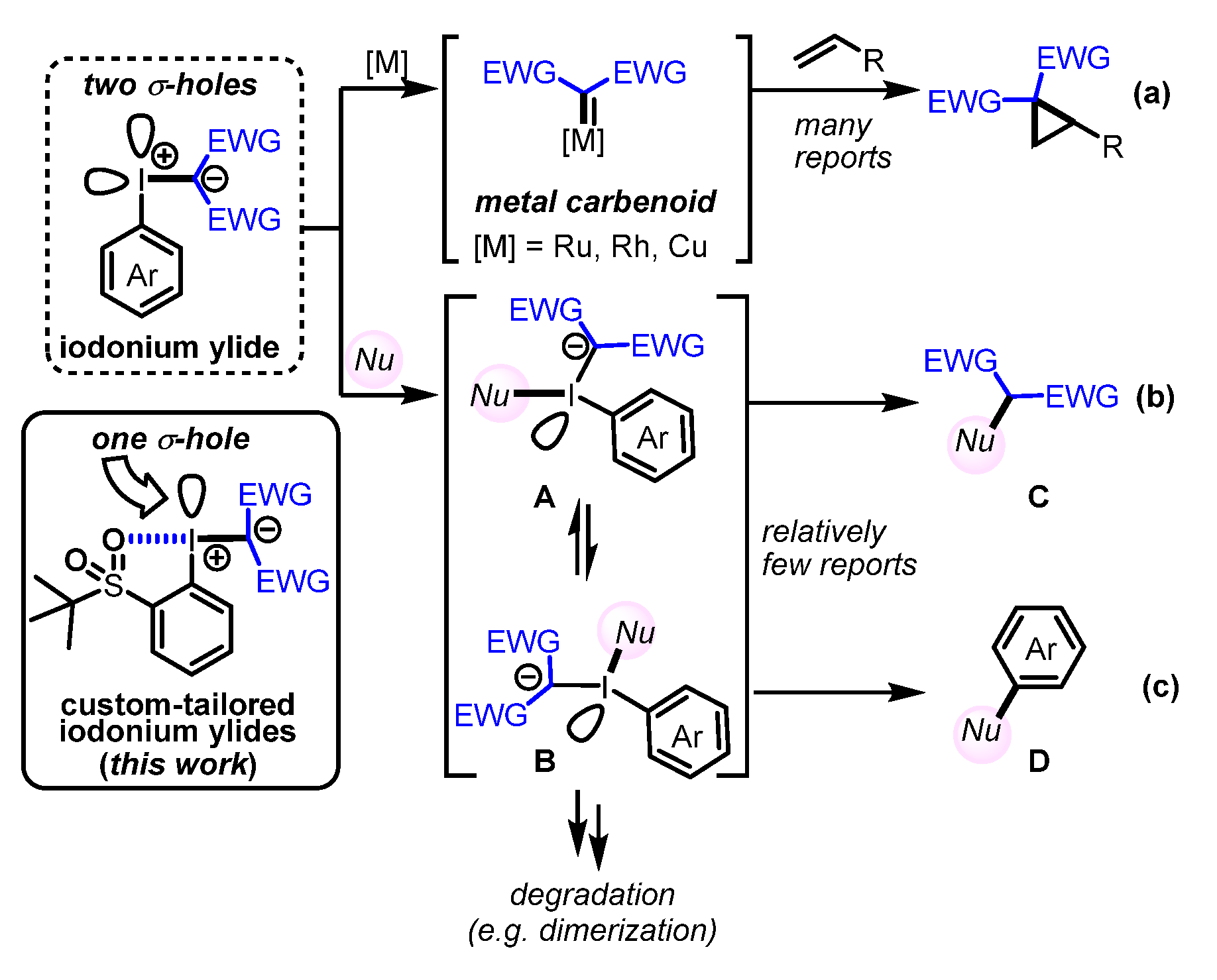
Iodonium ylides, a class of hypervalent iodine compounds [1], have attracted much attention due to their use as carbene precursors for C‒H-insertion and cyclopropanation reactions (Scheme 1a) [2,3]. Moreover, iodonium ylides have been used for various synthetic applications, including condensations with thioamides to produce thiazole rings in aqueous media (Scheme 1b; Nu = thioamide) [4,5], and for the introduction of 18F atoms into aromatic rings (Scheme 1c; Nu = F−) [6,7]. It has also been reported that depending on the iodonium ylide and nucleophile that is employed, the equilibrium between the T-shaped intermediates A and B can be influenced [6,7], and thus, the ratio of coupling products C and D can be controlled.
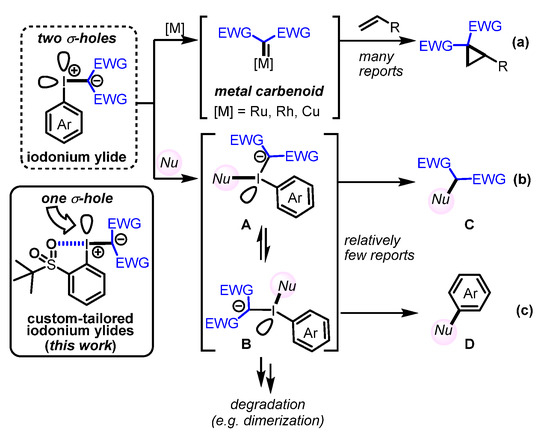
Scheme 1.
General synthetic utility of iodonium ylides. (a) Cyclopropanation of alkenes (b) Introduction of nucleophile into active methylene compound (c) Introduction of nucleophile into aromatic rings.
However, to date, only a limited number of reports concerning the properties and reactivity of iodonium ylides have been published, which is at least in part due to their instability (e.g., degradation via intermediates A and/or B) on account of the remaining σ-hole of intermediates A and B, which can react with nucleophiles such as water. We anticipate that the synthesis of a variety of iodonium ylides and the investigation of their properties would provide essential information for the advancement of synthetic applications such as chemoselective coupling reactions. Of particular interest to us are iodonium ylides that contain a coordinating ortho group on the aromatic ring [8], as such groups are known to stabilize iodonium ylides and other hypervalent iodine compounds [5,6,7,8,9], thus increasing their solubility via intramolecular halogen bonding (XB) [10]. In this study, we synthesized several iodonium ylides bearing both an electron-withdrawing group and an aromatic ring with an ortho-t-BuSO2 group [8,11]. Based on the crystal structures of the synthesized iodonium ylides in combination with natural-bond-order (NBO) analysis, we also estimated the strength of the intra- and intermolecular XBs [12,13,14,15,16,17,18,19,20,21,22,23]. In addition, we investigated the reactivity of these iodonium ylides under photoirradiation [24,25,26,27].
2. Results
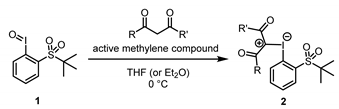
We began by synthesizing iodonium ylides 2 via the condensation of iodosobenzene 1 [11] with a variety of active methylene compounds (Table 1). The reaction of 1 and the active methylene compounds proceeded at 0 °C in etheric solvents such as THF or Et2O to furnish the corresponding iodonium ylides (2) as precipitates with H2O, as the sole byproduct. The desired products were collected by simple suction filtration and, following an n-hexane wash, produced the pure compounds in acceptable yield (for details, see the Supplementary Materials). The major advantage that this method [28] has over the conventional method that uses iodoarene diacetate and an active methylene compound under basic conditions is that it eliminates the need to remove the formed salt (e.g., KOAc or NaOAc) with an H2O wash [29]. We tested a wide range of active methylene compounds, which produced iodonium ylides 2a–h in 27–89% yield without significant decomposition during the purification. This is presumably due to the enhanced electrophilicity of the iodosobenzene compound and the increased stability of the product due to the presence of the coordinating ortho-sulfonyl group.

Table 1.
Synthesis of various iodonium ylides that bear an ortho-t-BuSO2 group on the aromatic ring (2) from iodosobenzene 1.
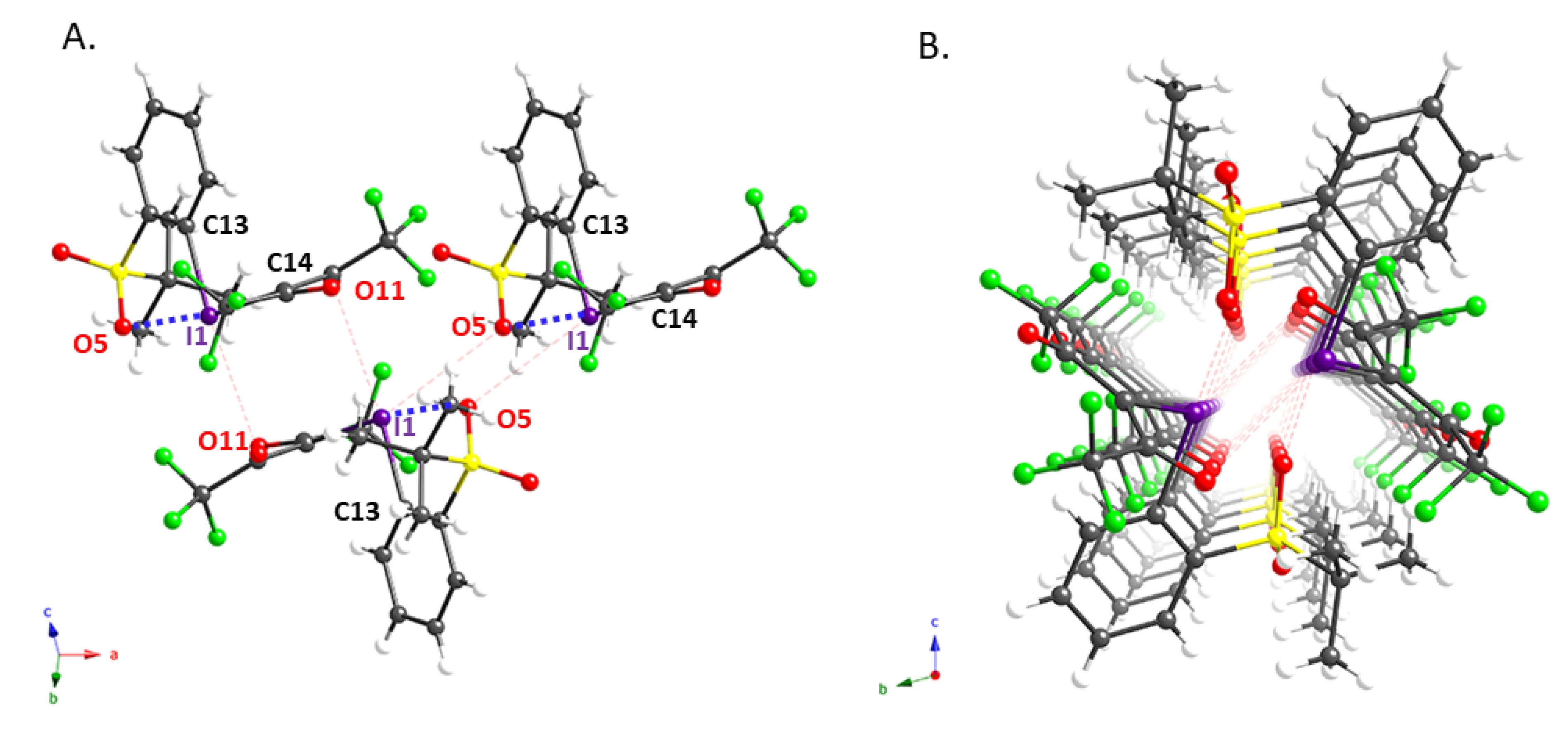
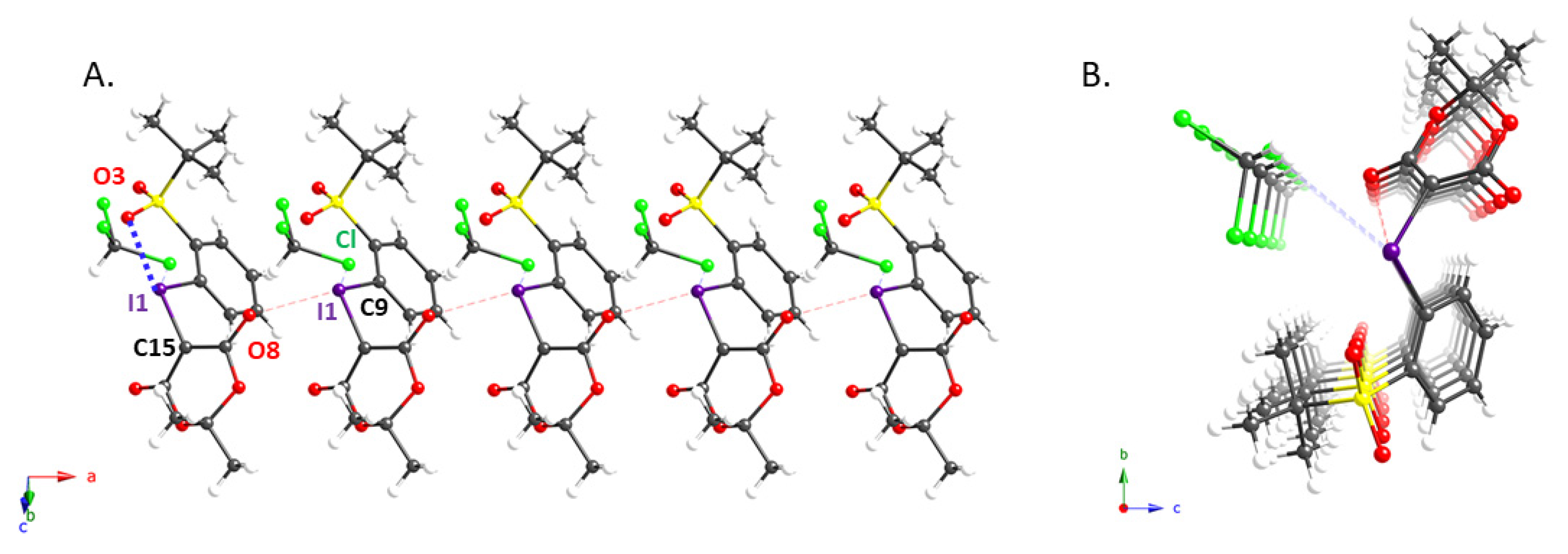
We also succeeded in obtaining single crystals of 2e and 2g that were suitable for an X-ray diffraction analysis; the molecular structures of these compounds are shown in Figure 1 and Figure 2, while pertinent crystallographic parameters are summarized in Table 2. As expected, the distance between the iodine atom (I1) and the oxygen atom (O5) of the ortho-sulfonyl group in 2e (2.737(3) Å) is within the sum of the van der Waals radii, implying the presence of an intramolecular XB interaction [10,19]. In addition, the analysis of the packing structure suggested the presence of an intermolecular XB in the solid state, in which the carbonyl oxygen (O11) and sulfonyl oxygen (O5) interact with the hypervalent iodine atom of 2e (Figure 1A). It is probably due to these interactions that 2e aligns to form the dimeric columnar structure shown in Figure 1B. The X-ray structure of 2g, which contains a residual molecule of chloroform, shows an intermolecular XB interaction in addition to the intramolecular XB with the ortho-t-BuSO2 group seen in 2e (Figure 2). This implies that this iodonium ylide may be able to recognize the substrates in coupling reactions via XB interactions.
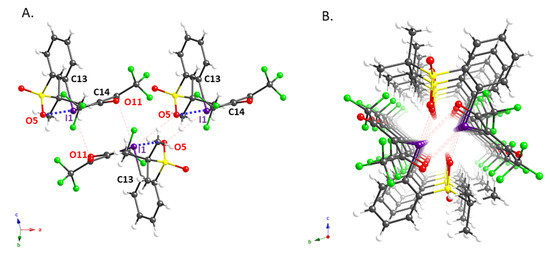
Figure 1.
Crystal structure of iodonium ylide 2e: (A) sideview of 2e; (B) dimeric columnar structure formed by two stacked columns of 2e.
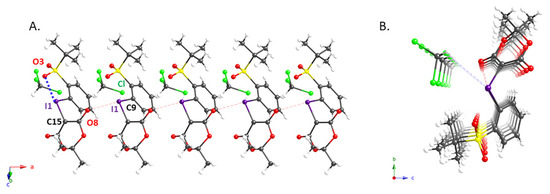
Figure 2.
Crystal structure of iodonium ylide 2g: (A) sideview of 2g; (B) columnar structure formed by one stacked column of 2g and residual chloroform molecules.

Table 2.
Crystallographic parameters of the intra- and intermolecular XB interactions in the crystals of iodonium ylides 2e and 2g.
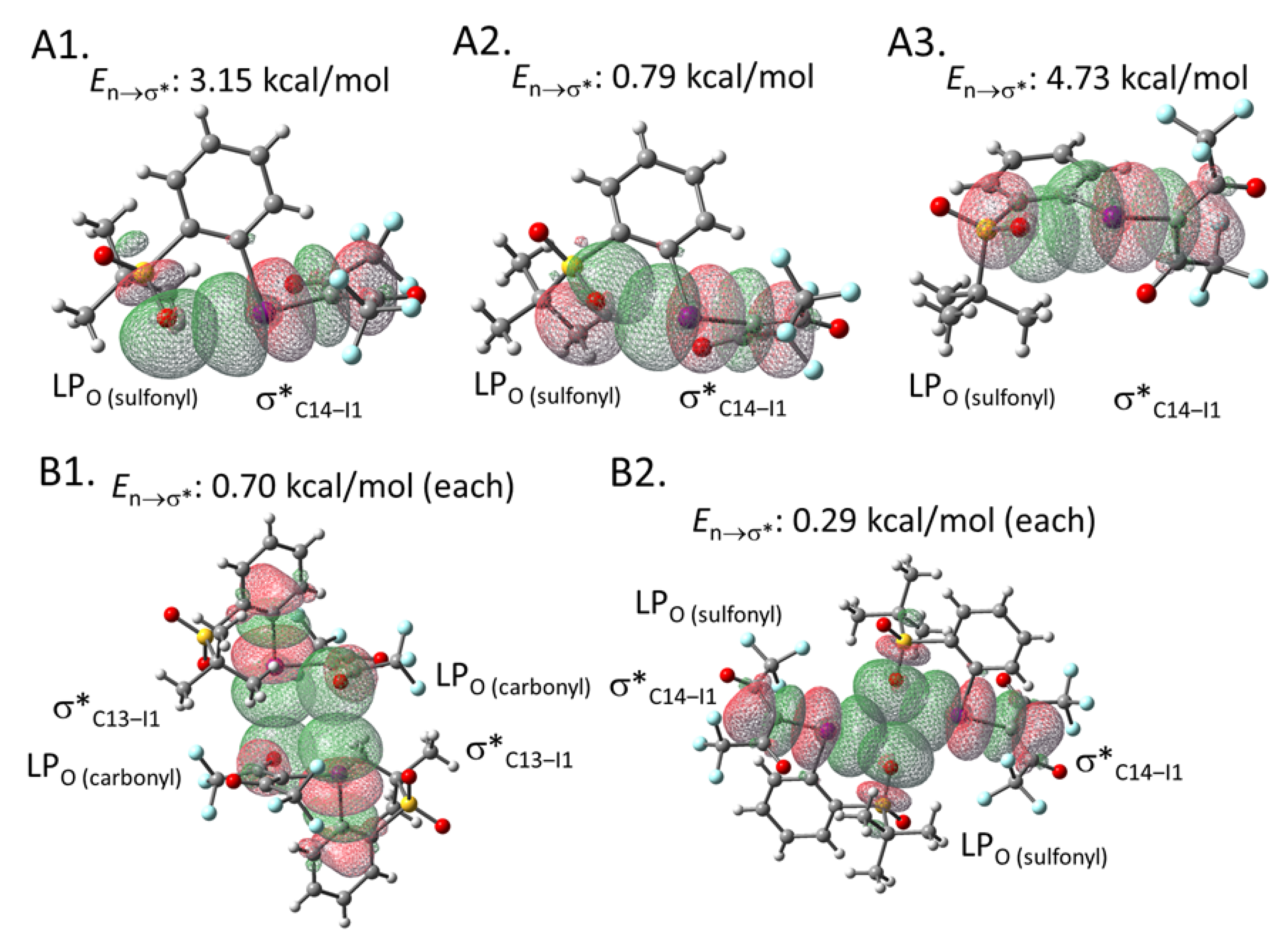
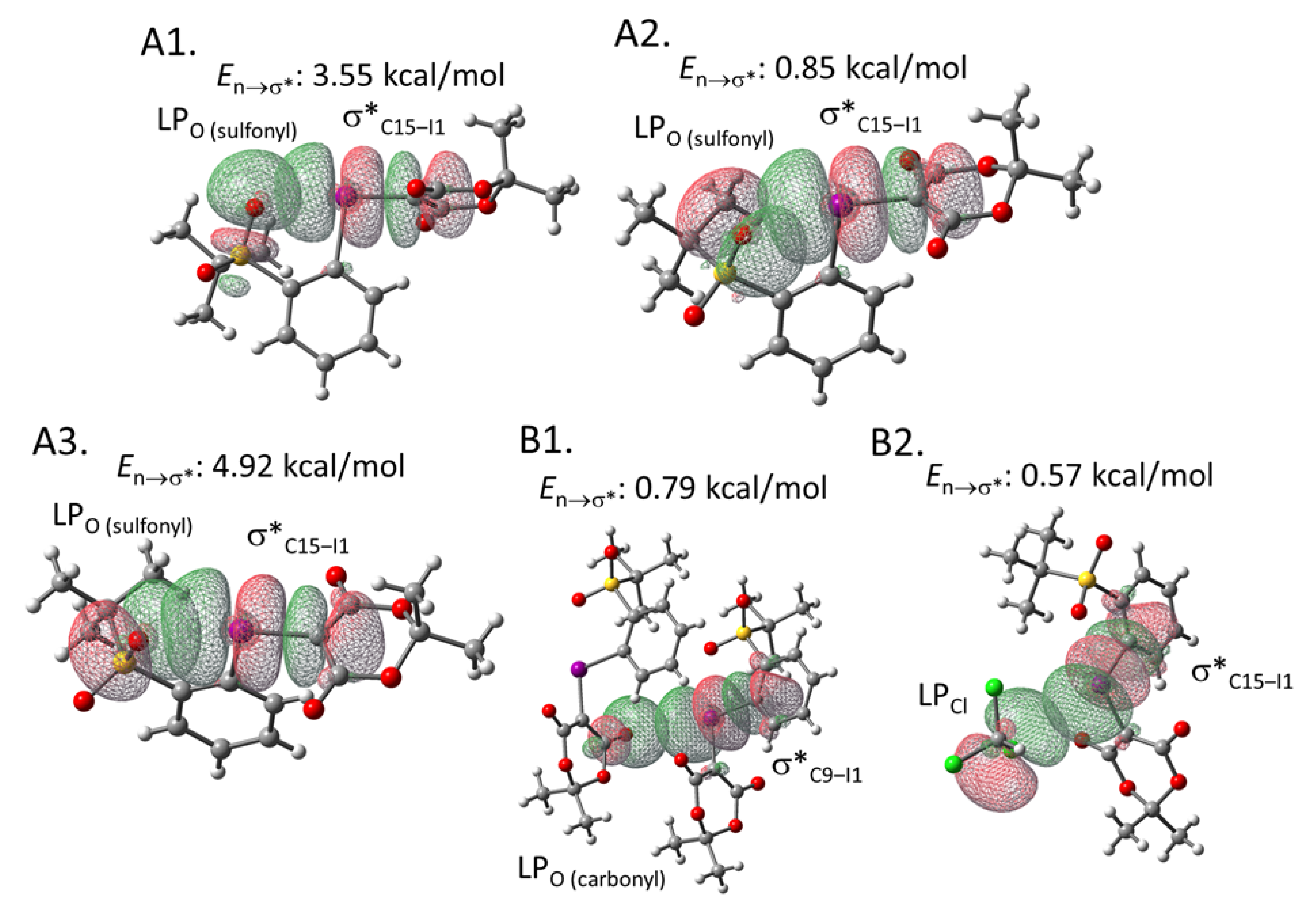
To estimate the interaction energies of the intra- and intermolecular XBs, we performed an NBO analysis based on the crystal structure of 2e, using the gaussian software package (Figure 3) [30,31]. The analysis demonstrated that the C14‒I1 σ* orbital of the hypervalent iodine moiety in 2e overlaps with the three types of lone pairs (LPs) of the intramolecular sulfonyl oxygen (O5) (See the Supplementary Materials for the details of orbitals). The energies of these interactions were calculated to be 3.15, 0.79, and 4.73 kcal/mol, respectively (Figure 3A). Moreover, the intermolecular XB energies were estimated to be 0.70 and 0.29 kcal/mol, respectively (Figure 3B). These interaction energies may contribute to the relative stability of this type of iodonium ylide. The corresponding results of the NBO analysis performed for iodonium ylide 2g are summarized in Figure 4. The interaction energies seem to be stronger when the interaction distance is shorter and the interaction angle is closer to 180º, which is an ideal situation for XB interactions (Table 2).
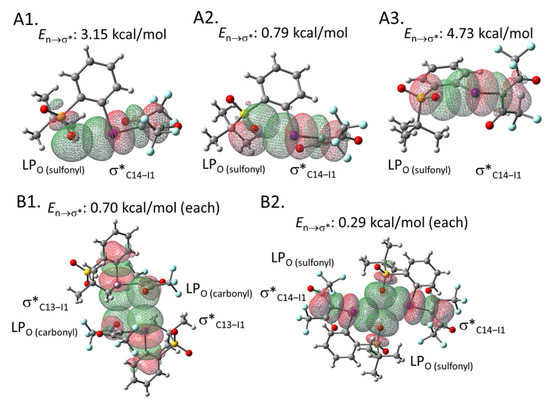
Figure 3.
NBO analysis of iodonium ylide 2e. (A1) Intramolecular XB between a lone pair of the sulfonyl group (Orbital#71) and the C14-I1 σ* orbital (Orbital#136) (type 1). (A2) Intramolecular XB between a lone pair of the sulfonyl group (Orbital#120) and the C14-I1 σ* orbital (Orbital#136) (type 2). (A3) Intramolecular XB between a lone pair of the sulfonyl group (Orbital#119) and the C14-I1 σ* orbital (type 3) (Orbital#136). (B1) Intermolecular XB between a lone pair of the carbonyl group and the C13-I1 σ* orbital. (B2) Intermolecular XB between a lone pair of sulfonyl group and the C13-I1 σ* orbital.
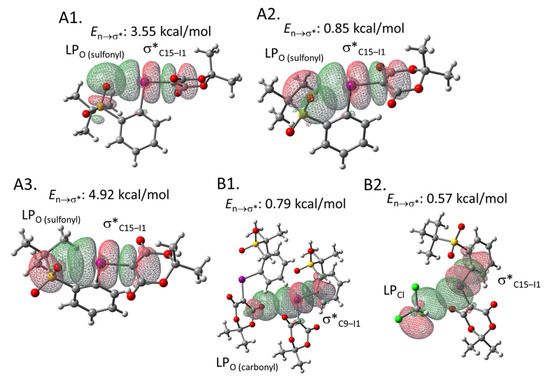
Figure 4.
NBO analysis of iodonium ylide 2g. (A1) Intramolecular XB between a lone pair of the sulfonyl group (Orbital#58) and the C15-I1 σ* orbital (Orbital#123) (type 1). (A2) Intramolecular XB between a lone pair of the sulfonyl group (Orbital#105) and the C15-I1 σ* orbital (Orbital#123) (type 2). (A3) Intramolecular XB between a lone pair of the sulfonyl group (Orbital#104) and the C15-I1 σ* orbital (Orbital#123) (type 3). (B1) Intermolecular XB between a lone pair of the carbonyl group and the C9-I1 σ* orbital. (B2) Intermolecular XB between a lone pair of the Cl group and the C9-I1 σ* orbital.
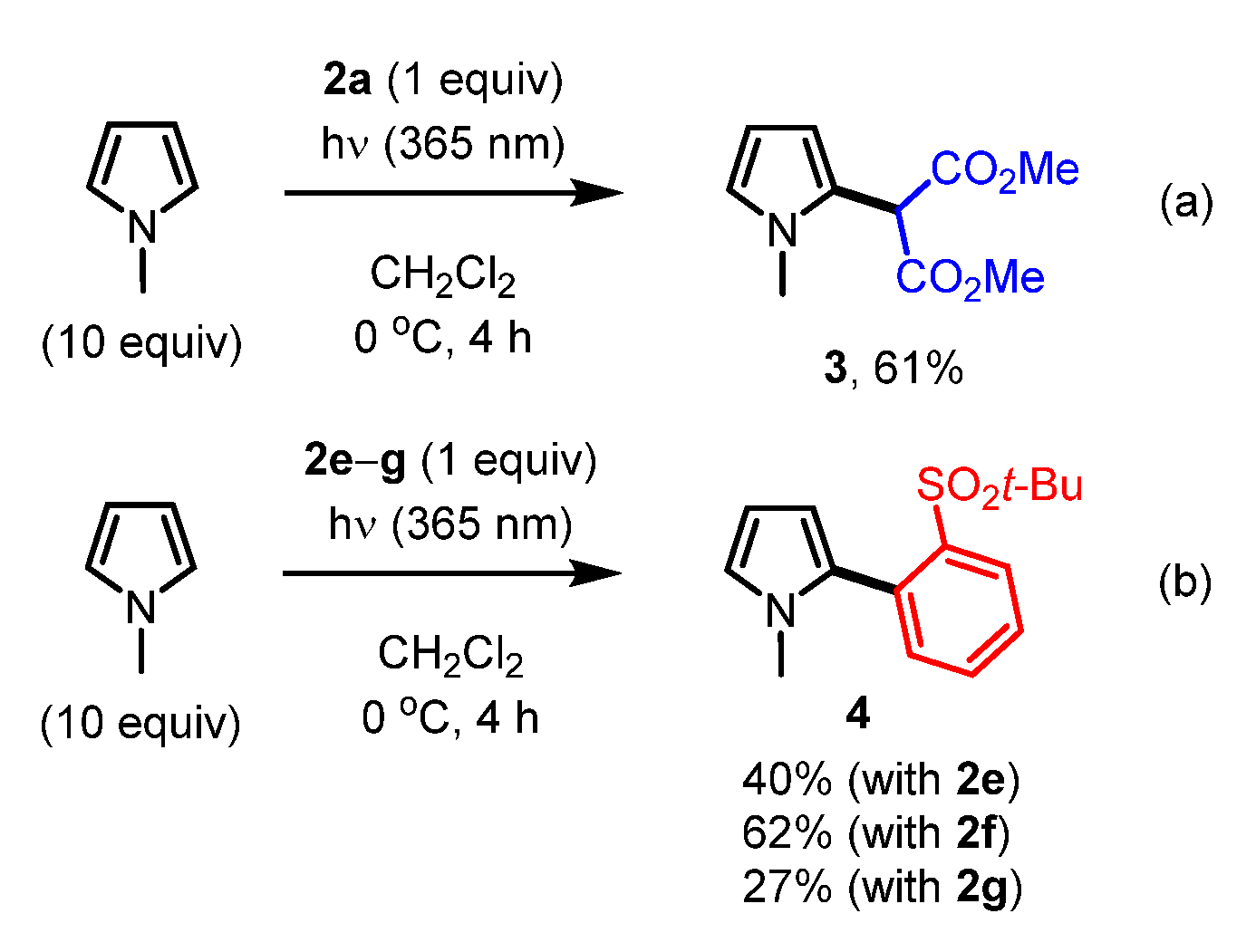
Finally, we investigated the reactivity of the synthesized iodonium ylides under photoirradiation conditions (Scheme 2) [9,24,25,26,27]. When ylide 2a and ten equivalents of N-methylpyrrole were irradiated at 365 nm, the active methylene group was introduced at the C2 position of N-methylpyrrole in 61% yield (Scheme 2a). Unexpectedly, when ylide 2e–g and ten equivalents of N-methylpyrrole were irradiated at 365 nm, the aryl group (2-t-BuSO2C6H4) of the ylide was exclusively introduced at the C2 position of N-methylpyrrole (Scheme 2b).
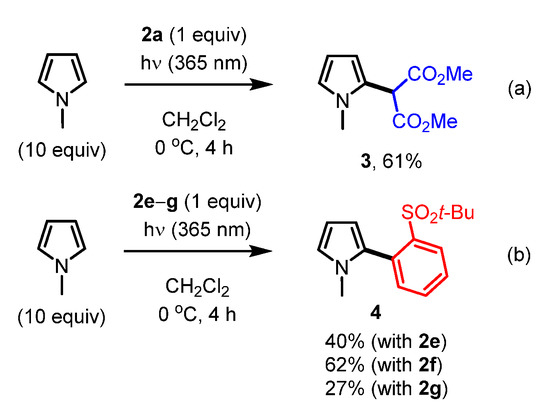
Scheme 2.
(a) Reactivity of iodonium ylide 2a under photoirradiation conditions. (b) Reactivity of iodonium ylide 2e–g under photoirradiation conditions.
Based on these observations and recent related reports by other groups [7], the reaction seems to proceed via a T-shaped tri-coordinated intermediate, which is different from a free carbene species [32]. The T-shaped tricoordinated intermediate could be formed via a biradical species generated via photoirradiation [33]. The unexpected selectivity of the reductive elimination might be explained by the steric hindrance of the dimedone and Meldrum’s acid moieties in 2f and 2g, although a detailed mechanism for this transformation, including the selectivity in the case of 2e, is not clear at this stage. Iodonium ylides 2c and 2h gave the coupling product 4 in 43% and 31%, respectively, while 2b and 2d did not afford any coupling products, presumably due to degradation under photoirradiation.
3. Conclusions
We have synthesized a series of novel iodonium ylides that bear a coordinating ortho-t-BuSO2 group on their aryl rings, and we have analyzed the crystal structures of two of these molecules. Intra- and intermolecular halogen bonding (XB) interactions were observed in the crystal structures of two ylides that were structurally characterized by X-ray diffraction analysis. The synthesized iodonium ylides were found to serve as active transfer reagents of methylene or aryl groups. Further synthetic applications and mechanistic studies are currently under investigation in our laboratory.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11091085/s1: Detailed compound characterization data and copies of NMR charts.
Author Contributions
Conceptualization, Y.K. and Y.T.; methodology, T.K.; software, S.H., T.F. and Y.K.; validation, S.H., T.F. and Y.K.; formal analysis, T.K.; investigation, T.K.; resources, T.K.; data curation, T.K.; writing—original draft preparation, Y.K.; writing—review and editing, S.H., T.F. and Y.T.; visualization, Y.K.; supervision, Y.K. and Y.T.; project administration, Y.K. and Y.T.; funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI grant 19K06974, as well as a research grant from the Hoansha Foundation.
Acknowledgments
We thank Kosuke Katagiri (Konan University) for assistance with preparing the graphics.
Conflicts of Interest
The funders neither had a role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, nor in the decision to publish the results.
References
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef]
- Mgller, P. Asymmetric Transfer of Carbenes with Phenyliodonium Ylides. Acc. Chem. Res. 2004, 37, 243–251. [Google Scholar] [CrossRef]
- Yusubov, M.S.; Yoshimura, A.; Zhdankin, V.V. Iodonium Ylides in Organic Synthesis. ARKIVOC Online J. Org. Chem. 2016, i, 342–374. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Kobayashi, Y.; Tsuzuki, S.; Takemoto, Y. Electrophilic Activation of Iodonium Ylides by Halogen-Bond-Donor Catalysis for Cross-Enolate Coupling. Angew. Chem. Int. Ed. 2017, 56, 7653–7657. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kobayashi, Y.; Takemoto, Y. Divergent and Chemoselective Transformations of Thioamides with Designed Carbene Equivalents. Chem.-Eur. J. 2019, 25, 10314–10318. [Google Scholar] [CrossRef]
- Yusubov, M.S.; Svitich, D.Y.; Larkina, M.S.; Zhdankin, V.V. Applications of Iodonium Salts and Iodonium Ylides as Precursors for Nucleophilic Fluorination in Positron Emission Tomography. ARKIVOC Online J. Org. Chem. 2013, 1, 364–395. [Google Scholar] [CrossRef] [Green Version]
- Rotstein, B.H.; Wang, L.; Liu, R.Y.; Patteson, J.; Kwan, E.E.; Vasdev, N.; Liang, S.H. Mechanistic Studies and Radiofluorination of Structurally Diverse Pharmaceuticals with Spirocyclic Iodonium(III) Ylides. Chem. Sci. 2016, 7, 4407–4417. [Google Scholar] [CrossRef] [Green Version]
- Zhdankin, V.V.; Protasiewicz, J.D. Development of New Hypervalent Iodine Reagents with Improved Properties and Reactivity by Redirecting Secondary Bonds at Iodine Center. Coord. Chem. Rev. 2014, 275, 54–62. [Google Scholar] [CrossRef]
- Zhu, C.; Yoshimura, A.; Ji, L.; Wei, Y.; Nemykin, V.N.; Zhdankin, V.V. Design, Preparation, X-ray Crystal Structure, and Reactivity of o-Alkoxyphenyliodonium Bis(methoxycarbonyl)methanide, a Highly Soluble Carbene Precursor. Org. Lett. 2012, 14, 3170–3173. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.L.; Huber, S.M. Catalysis of Organic Reactions through Halogen Bonding. ACS Catal. 2019, 9, 9622–9639. [Google Scholar] [CrossRef]
- Macikenas, D.; Skrzypczak-Jankun, E.; Protasiewicz, J.D. A New Class of Iodonium Ylides Engineered as Soluble Primary Oxo and Nitrene Sources. J. Am. Chem. Soc. 1999, 121, 7164–7165. [Google Scholar] [CrossRef]
- Novikov, A.S.; Ivanov, D.M.; Avdontceva, M.S.; Kukushkin, V.Y. Diiodomethane as a Halogen Bond Donor toward Metal-bound Halides. CrystEngComm 2017, 19, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Novikov, A.S.; Ivanov, D.M.; Bikbaeva, Z.M.; Bokach, N.A.; Kukushkin, V.Y. Noncovalent Interactions Involving Iodo fluorobenzenes: The Interplay of Halogen Bonding and Weak lp(O)···π-Holearene Interactions. Cryst. Growth Des. 2018, 18, 7641–7654. [Google Scholar] [CrossRef]
- Adonin, S.A.; Udalova, L.I.; Abramov, P.A.; Novikov, A.S.; Yushina, I.V.; Korolkov, I.V.; Semitut, E.Y.; Derzhavskaya, T.A.; Stevenson, K.J.; Troshin, P.A.; et al. A Novel Family of Polyiodo-Bromoantimonate(III) Complexes: Cation-Driven Self-Assembly of Photoconductive Metal-Polyhalide Frameworks. Chem. Eur. J. 2018, 24, 14707–14711. [Google Scholar] [CrossRef]
- Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S.M. Iodine(III) Derivatives as Halogen Bonding Organocatalysts. Angew. Chem. Int. Ed. 2018, 57, 3830–3833. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen Bonding in the Structures of Pentaiodobenzoic Acid and Its Salts. CrystEngComm 2019, 21, 6666–6670. [Google Scholar] [CrossRef]
- Gorokh, I.D.; Adonin, S.A.; Novikov, A.S.; Usoltsev, A.N.; Plyusnin, P.E.; Korolkov, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with 3-Iodopyridinium Cations: Halogen Bonding-assisted Crystal Packing. Polyhedron 2019, 166, 137–140. [Google Scholar] [CrossRef]
- Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guérin, V.; Legault, C.Y.; Kirsch, S.F.; Huber, S.M. Activation of a Metal-Halogen Bond by Halogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 16496–16500. [Google Scholar] [CrossRef]
- Heinen, F.; Engelage, E.; Cramer, C.J.; Huber, S.M. Hypervalent Iodine(III) Compounds as Biaxial Halogen Bond Donors. J. Am. Chem. Soc. 2020, 142, 8633–8640. [Google Scholar] [CrossRef]
- Adonin, S.A.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- and Two-Dimensional Iodine-Rich Iodobismuthate(III) Complexes: Structure, Optical Properties, and Features of Halogen Bonding in the Solid State. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Novikov, A.S.; Yusubov, M.S.; Postnikov, P.S.; Kukushkin, V.Y. Halogen Bonding Provides Heterooctameric Supramolecular Aggregation of Diaryliodonium Thiocyanate. Crystals 2020, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Aliyarova, I.S.; Ivanov, D.M.; Soldatova, N.S.; Novikov, A.S.; Postnikov, P.S.; Yusubov, M.S.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Diaryliodonium Cations as Iodine(III)-Based Double-σ-Hole Donors. Cryst. Growth Des. 2021, 21, 1136–1147. [Google Scholar] [CrossRef]
- Yunusova, S.N.; Novikov, A.S.; Soldatova, N.S.; Vovk, M.A.; Bolotin, D.S. Iodonium Salts as Efficient iodine(III)-based Noncovalent Organocatalysts for Knorr-type Reactions. RSC Adv. 2021, 11, 4574–4583. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Masakado, S.; Takemoto, Y. Photoactivated N-Acyliminoiodinanes Applied to Amination: An ortho-Methoxymethyl Group Stabilizes Reactive Precursors. Angew. Chem. Int. Ed. 2018, 57, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Masakado, S.; Kobayashi, Y.; Takemoto, Y. Photo-induced Aziridination of Alkenes with N-Sulfonyliminoiodinanes. Chem. Pharm. Bull. 2018, 66, 688–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masakado, S.; Kobayashi, Y.; Takemoto, Y. Photo-Irradiation-Promoted Aminoetherification of Glycals with N-Acyliminoiodinane and Alcohols. Heterocycles 2020, 101, 453–460. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Masakado, S.; Murai, T.; Hamada, S.; Furuta, T.; Takemoto, Y. A bench-stable N-trifluoroacetyl nitrene equivalent for a simple synthesis of 2-trifluoromethyl oxazoles. Org. Biomol. Chem. 2021, 19, 6628–6632. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.S.; Cui, J.; Hou, X.-S.; Zhang, C. A Mild and Efficient Direct α-Amination of β-Dicarbonyl Compounds Using Iodosobenzene and p-Toluenesulfonamide Catalyzed by Perchlorate Zinc Hexahydrate. Org. Lett. 2012, 14, 832–835. [Google Scholar] [CrossRef]
- Goudreau, S.R.; Marcoux, D.; Charette, A.B. General Method for the Synthesis of Phenyliodonium Ylides from Malonate Esters: Easy Access to 1,1-Cyclopropane Diesters. J. Org. Chem. 2009, 74, 470–473. [Google Scholar] [CrossRef]
- Gaussian, Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Camacho, M.B.; Clark, A.E.; Liebrecht, T.A.; DeLuca, J.P.A. Phenyliodonium Ylide as a Precursor for Dicarboethoxycarbene: Demonstration of a Strategy for Carbene Generation. J. Am. Chem. Soc. 2000, 122, 5210–5211. [Google Scholar] [CrossRef]
- Chidley, T.; Jameel, I.; Rizwan, S.; Peixoto, P.A.; Pouységu, L.; Quideau, S.; Hopkins, W.S.; Murphy, G.K. Blue LED Irradiation of Iodonium Ylides Gives Diradical Intermediates for Efficient Metal-free Cyclopropanation with Alkenes. Angew. Chem. Int. Ed. 2019, 58, 16959–16965. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).