Corrosion Behaviour of Cu/Carbon Steel Gradient Material
Abstract
:1. Introduction
2. Materials and Experiment Methods
2.1. Meterials
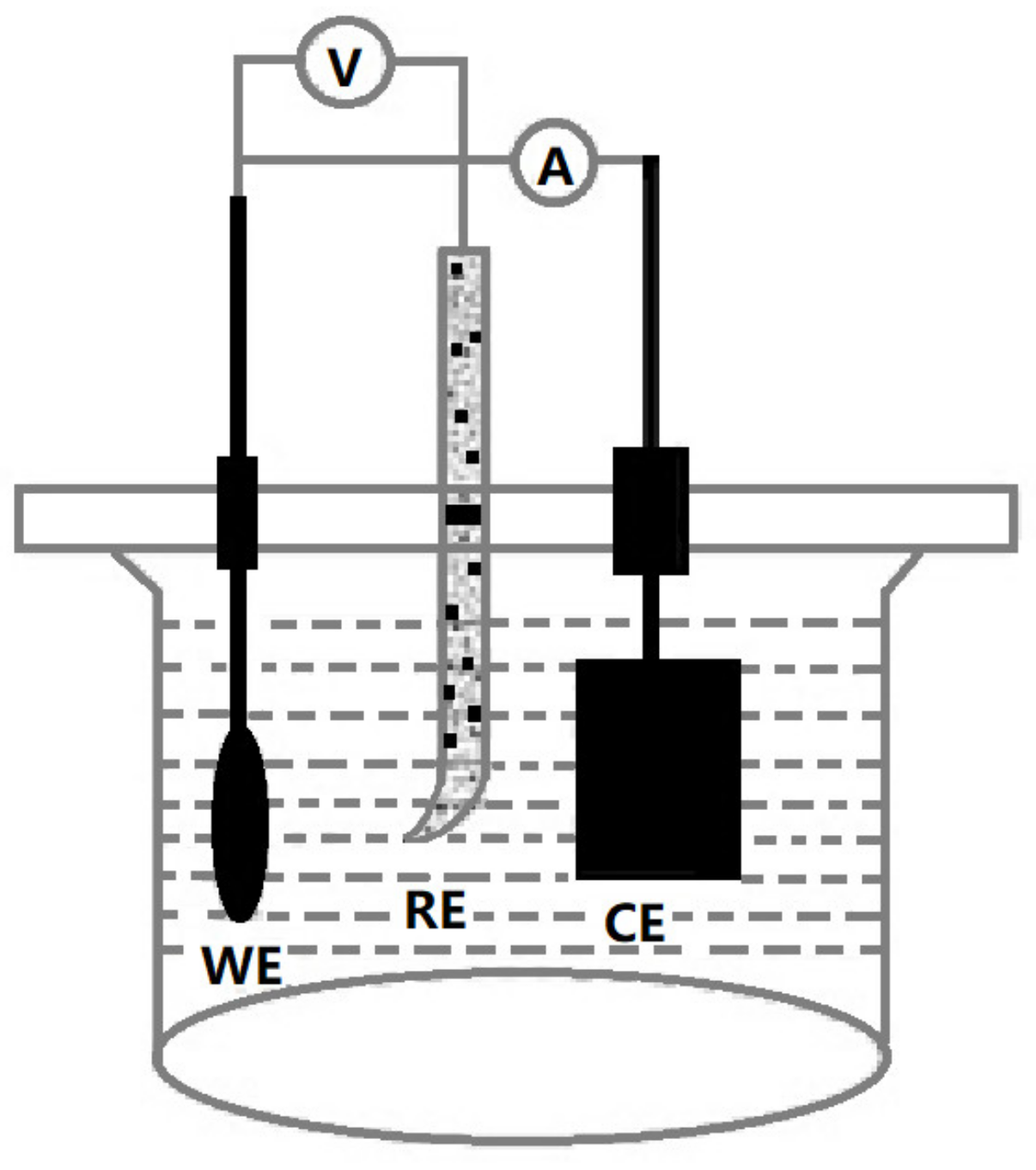
2.2. Experiment Procedure
3. Results
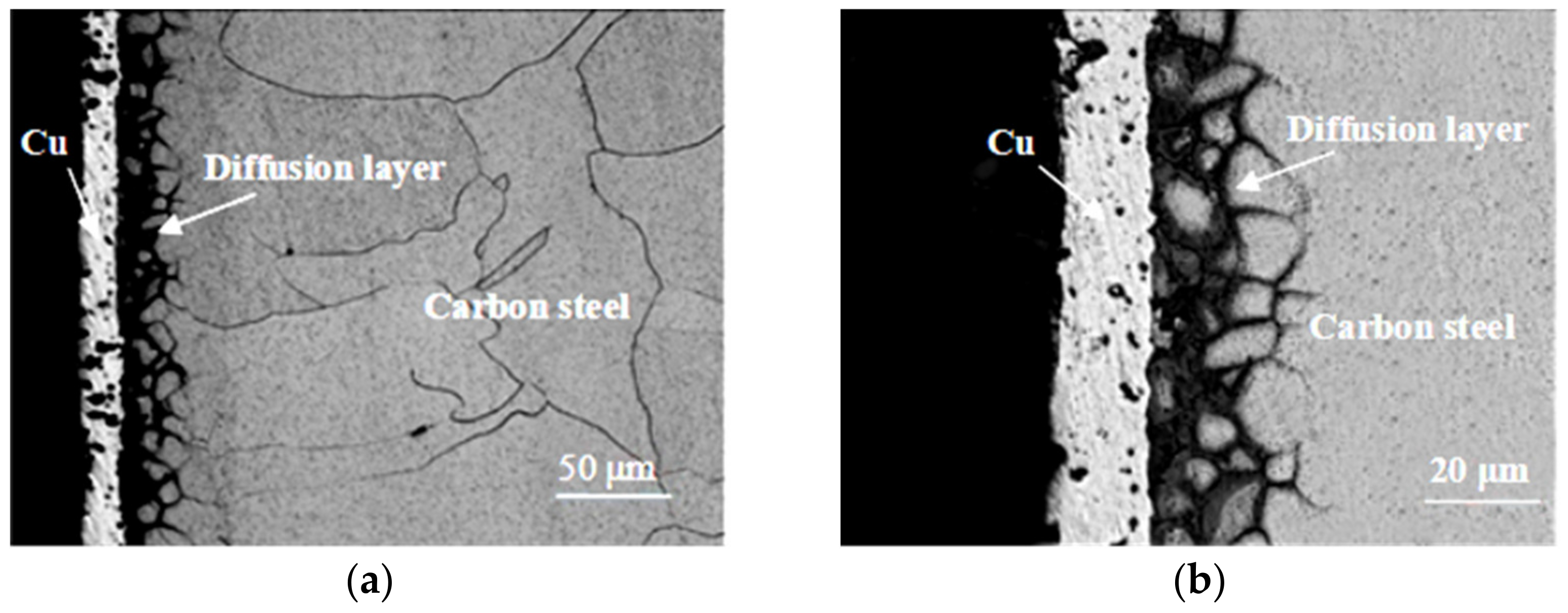
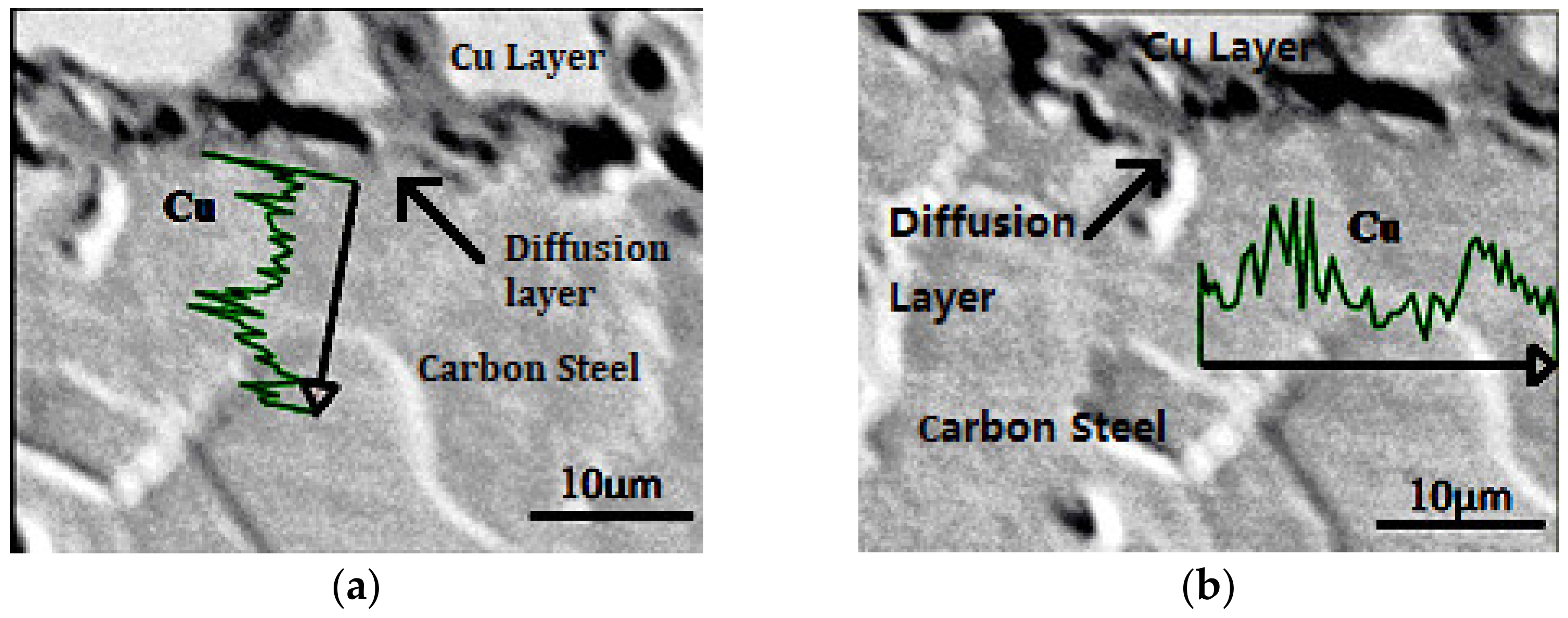
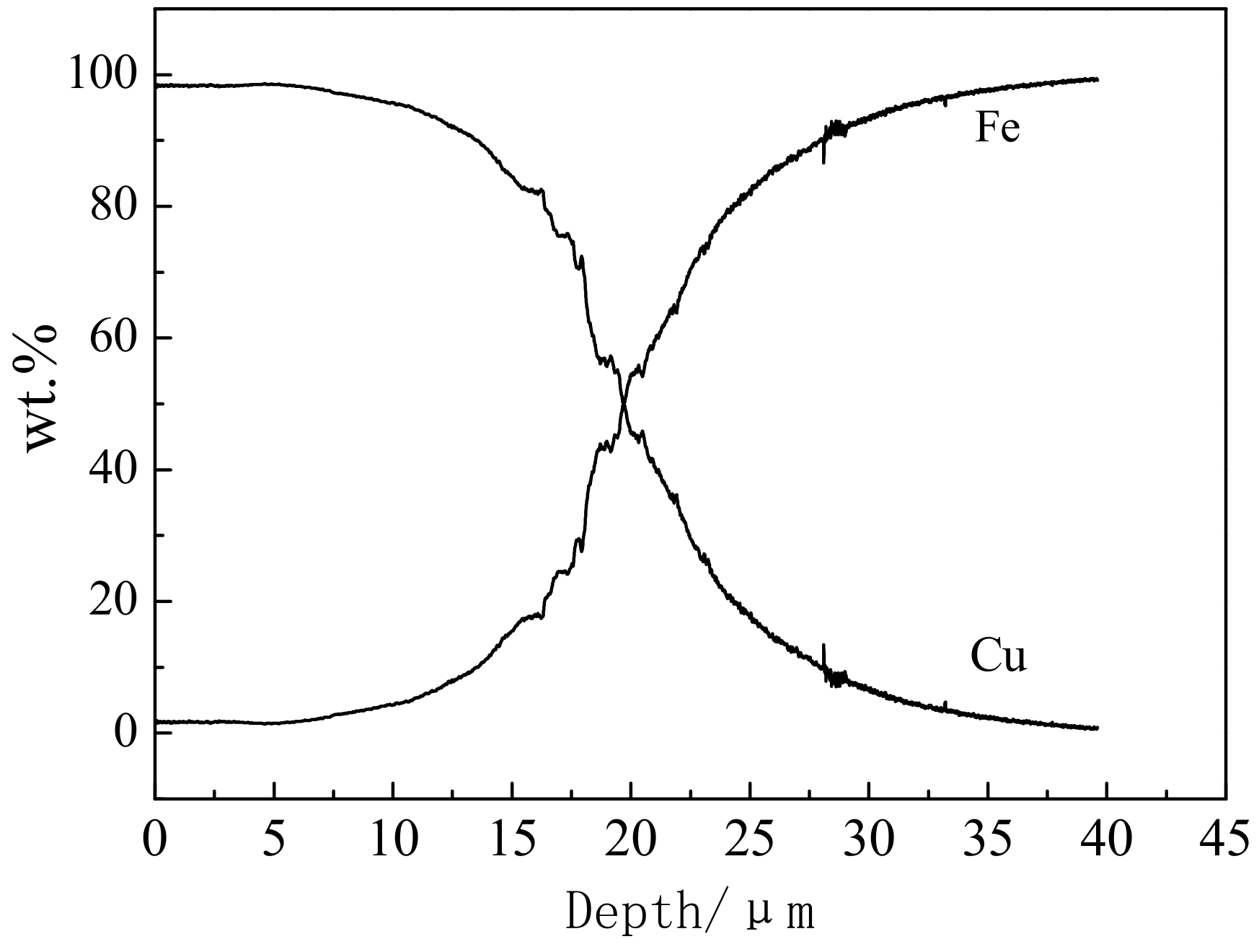
3.1. Effect of Copper Diffusion on Microstructure of Carbon Steel
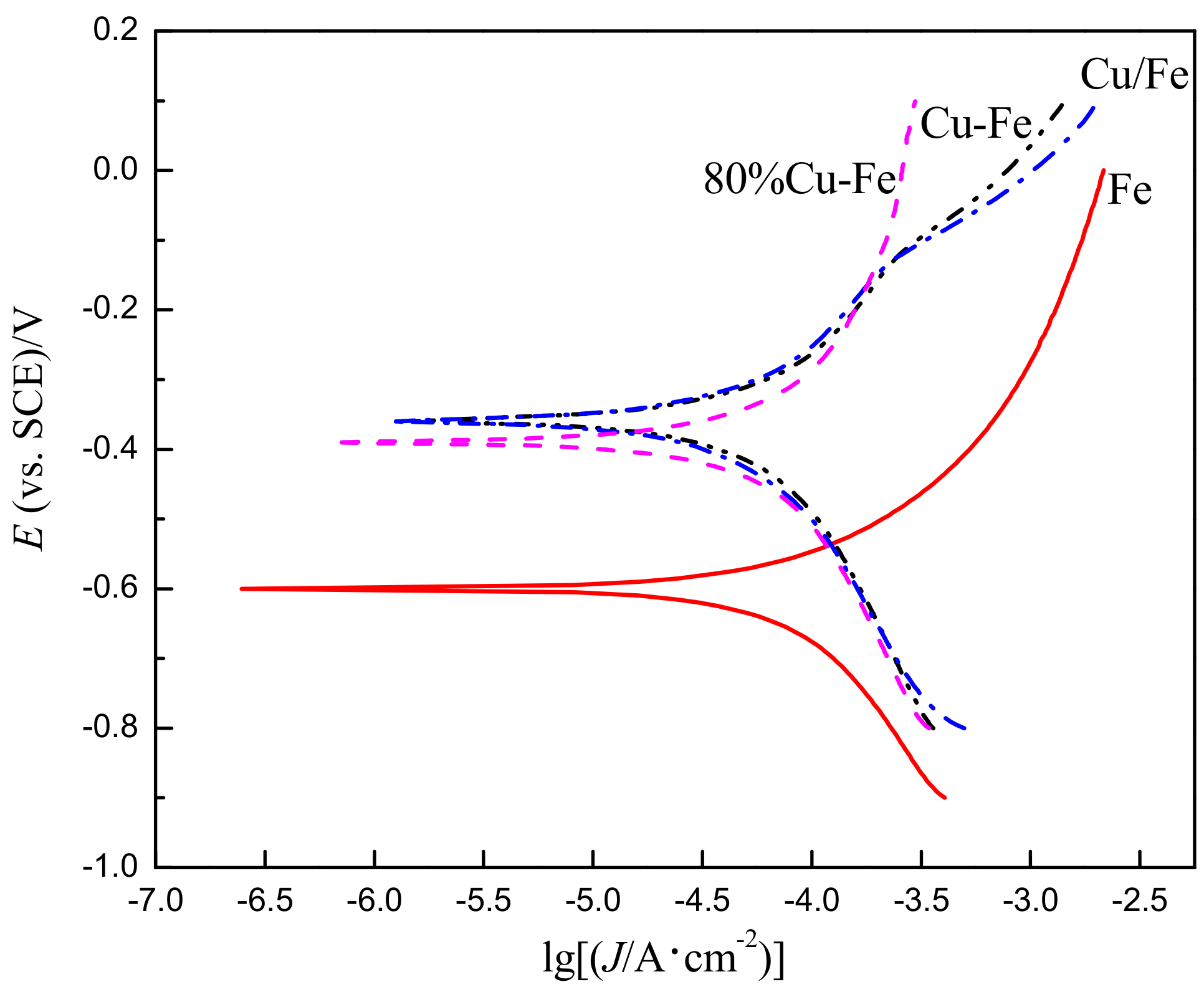
3.2. Polarization Curve
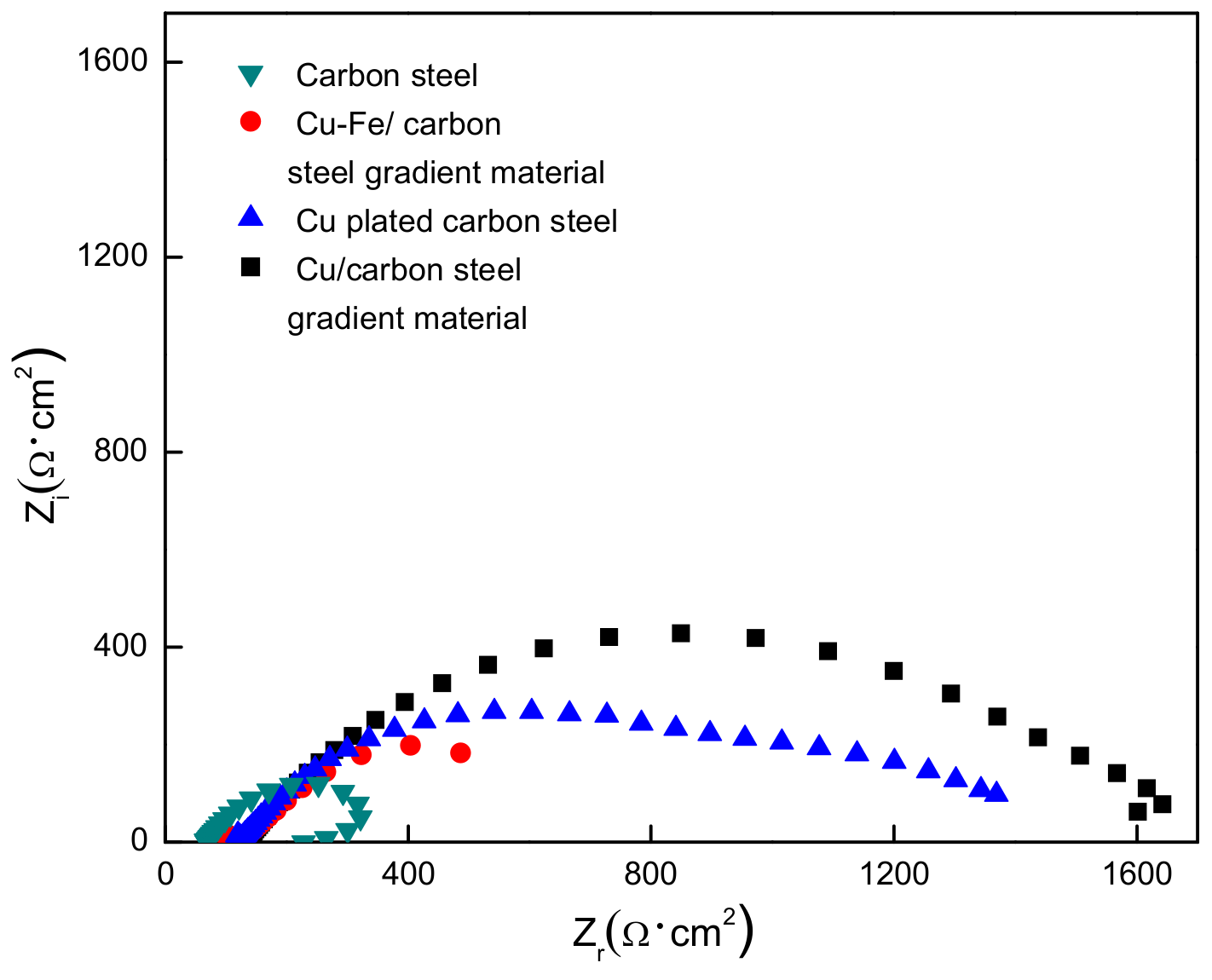
3.3. EIS
3.4. Surface Morphology of Materials after Polarization
4. Discussion
5. Conclusions
- As for the Cu-Fe, during the electrodeposition-diffusion process, the copper coating on the surface of carbon steel will diffuse mainly along the grain boundary to the inside of carbon steel, and the diffusion of copper will restrain the grain growth of carbon steel during high-temperature heat treatment.
- The corrosion current density (30.6 μA/cm2) of Cu-Fe prepared by the electrodeposition-diffusion process is smaller than that of carbon steel (53.0 μA/cm2), meaning that the corrosion rate of Cu-Fe is lower. The polarization resistance (1742 Ω·cm2) of the Cu-Fe is obviously higher than that of the carbon steel (266 Ω·cm2), indicating that the corrosion resistance of the Cu-Fe is stronger than that of the carbon steel material. The corrosion resistance of the Cu-Fe is mainly obtained through a pure copper layer on the surface of the Cu-Fe.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafari, E. Improving the Corrosion Resistance of Carbon Steel by Ni–P Nano-Structured Coating. Russ. J. Electrochem. 2021, 57, 663–670. Available online: https://link.springer.com/article/10.1134/S1023193520120083 (accessed on 12 July 2021). [CrossRef]
- Karim, M.; Daniel, S.; Haq, E.U.; Ahmad, A.A.; Khan, K.; Raza, S. Improving the Corrosion Resistance of API X56 and API X70 Pipeline Steels by Hot Dip Aluminization. Key Eng. Mater. 2021, 875, 315–321. Available online: https://www.scientific.net/KEM.875.315 (accessed on 1 February 2021). [CrossRef]
- Burduhos-Nergis, D.P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Phosphate Surface Treatment for Improving the Corrosion Resistance of the C45 Carbon Steel Used in Carabiners Manufacturing. Materials 2020, 13, 3410. Available online: https://www.researchgate.net/publication/343397480_Phosphate_Surface_Treatment_for_Improving_the_Corrosion_Resistance_of_the_C45_Carbon_Steel_Used_in_Carabiners_Manufacturing (accessed on 1 August 2020). [CrossRef] [PubMed]
- Liu, R.; Chen, X.P.; Wang, X.D.; Shi, Q.N. Effect of alloy elements on corrosion resistance of weathering steels in marine atmosphere environment. Hot Work. Technol. 2014, 43, 19–22. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-SJGY201420005.htm (accessed on 21 October 2014).
- Yue, L.J.; Wang, L.M.; Han, J.S. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. Available online: https://www.sciencedirect.com/science/article/pii/S1002072109602192 (accessed on 1 December 2010). [CrossRef]
- Ren, F. Effect of niobium content on microstructure and properties of rolling weathering steel for construction. Hot Work. Technol. 2016, 45, 156–161. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-SJGY201608020.htm (accessed on 8 July 2016).
- Morcillo, M.; Diza, I.; Chico, B.; Cano, H.; de la Fuente, D. Weathering steels: From empirical development to scientific design. A review. Corros. Sci. 2014, 83, 6–31. Available online: https://www.sciencedirect.com/science/article/pii/S0010938X14001103 (accessed on 1 June 2014). [CrossRef] [Green Version]
- Chen, X.H.; Dong, J.H.; Han, E.H.; Wei, K.E. Effect of Cu, Mn on the corrosion performance of carbon steels in wet/dry environments. Mater. Prot. 2007, 40, 19–22. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-CLBH200710008.htm (accessed on 15 October 2007).
- Cen, S.B.; Chen, H.; Liu, Y.; Ma, Y.; Wu, Y. Effect of CeO2 on corrosion behavior of WC-12Co coatings by high velocity oxygen fuel. Acta Metall. Sin. 2016, 52, 1441–1448. Available online: https://www.researchgate.net/publication/311640009_Effect_of_CeO2_on_corrosion_behavior_of_WC-12Co_coatings_by_high_velocity_oxygen_fuel (accessed on 1 November 2016).
- Liu, S.; Yue, C.; Chen, X.; Zhu, Q.; Tu, Y. Pitting Corrosion Resistance on Annealing Treated Super Duplex Stainless Steel S32750. Crystals 2020, 10, 294. Available online: https://www.mdpi.com/2073-4352/10/4/294 (accessed on 24 March 2020). [CrossRef]
- Sherif, E.-S.M.; El Rayes, M.M.; Abdo, H.S. Cr3C2-NiCr Coating for the Protection of API Steel Corrosion in Concentrated Sodium Chloride Solution. Crystals 2020, 10, 249. Available online: https://www.mdpi.com/2073-4352/10/4/249 (accessed on 24 February 2020). [CrossRef] [Green Version]
- Chen, C.Y.; Xiang, S.; Hu, Y.N.; Liang, Y.; Shi, W. Electrochemical behavior of SAF2507 in high temperature concentrated phosphoric acid. Mater. Rev. 2016, 30, 62–66. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-CLDB201620015.htm (accessed on 25 October 2016).
- Li, L.; Yang, M.; Zhang, J. Effects of magnetron sputtering Ti films on wear resistance and corrosion resistance of AZ91 magnesium alloy. Surf. Technol. 2018, 47, 172–175. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-BMJS201803029.htm (accessed on 20 March 2018).
- Zhang, X.; Wen, J.X.; Liu, Y.X.; Yang, X.M. Corrosion inhibition effect of galium aparine linn extracts on Q235 steel in hydrochloric acid solution. Surf. Technol. 2018, 47, 231–236. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-BMJS201803038.htm (accessed on 20 March 2018).
- Song, G.L.; Cao, C.N.; Lin, H.C. General circuit for EIS of an irreversible electrode under electrochemical step control and parameters analysis of the circuit. J. Chin. Soc. Corros. Prot. 1994, 8, 113–122. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-ZGFF402.002.htm (accessed on 30 June 1994).
- Xie, F.; Wang, D.; Wu, M.; Li, R. Effect of sulfate reducing bacteria in seawater on corrosion behavior of Q235 steel. Mater. Rev. 2017, 31, 51–55. Available online: https://www.researchgate.net/publication/323682629_Effect_of_Sulfate_Reducing_Bacteria_in_Seawater_on_Corrosion_Behavior_of_Q235_Steel (accessed on 1 April 2017).
- Li, X.; Yan, B.; Dong, P. Crystallization and electrochemical corrosion behaviors of amorphous and nanocrystal line Fe-based alloys. Electrochemistry 2009, 15, 269–274. Available online: https://www.researchgate.net/publication/339276812_Crystallization_and_electrochemical_corrosion_behaviors_of_nanometer_amorphous_Ni-P_alloys (accessed on 1 February 2020).
- Liu, Z.Y.; Li, X.G.; Cheng, Y. Understand the occurrence of pitting corrosion of pipeline carbon steel under cathodic polarization. Electrochim. Acta 2012, 60, 259–263. Available online: https://www.sciencedirect.com/science/article/pii/S0013468611017245 (accessed on 15 January 2012). [CrossRef]
- Wan, Y.; Zhang, D.; Liu, H.Q.; Li, Y.J.; Hou, B.R. Influence of sulphate-reducing bacteria on environmental parameters and marine corrosion behavior of Q235 steel in aerobic conditions. Electrochim. Acta 2010, 55, 1528–1534. Available online: https://www.sciencedirect.com/science/article/pii/S0013468609012821 (accessed on 1 February 2010). [CrossRef]
- Zhang, J.; Liu, F.L.; Li, W.H.; Duan, J.Z.; Hou, B.R. Effects of srb on corrosion of Zn-Al-Cd anode in marine sediment. Acta Metall. Sin. 2010, 46, 1250–1257. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-JSXB201010015.htm (accessed on 11 October 2010). [CrossRef]
- Gao, N.; Wang, C.; Zhang, F.H.; Li, F.; Zhao, S.L.; Li, P. The influence of major ions on the corrosion of penetrating zinc steel in oil field water. J. Petrochem. Univ. 2011, 24, 31–34. Available online: http://www.en.cnki.com.cn/Article_en/CJFDTOTAL-SYHX201103008.htm (accessed on 15 June 2011).
- Ma, T.; Li, H.R.; Gao, J.X.; Li, Y.G. Diffusion behavior of Cu in carbon steel and its influence on corrosion resistance of carbon steel. Chin. J. Mater. Res. 2019, 33, 225–231. Available online: https://www.researchgate.net/publication/333563173_Diffusion_Behavior_of_Cu_in_Carbon_Steel_and_Its_Influence_on_Corrosion_Resistance_of_Carbon_Steel (accessed on 25 March 2019).


| Name of the Sample | Process | Surface Copper Content/% | Thickness of the Diffusion Layer/μm |
|---|---|---|---|
| Fe | - | 0 | 0 |
| Cu/Fe | plating | 100 | 0 |
| Cu-Fe | plating + diffusion annealing | 100 | 16 |
| 80%Cu-Fe | grinding and polishing the surface of the copper/carbon steel gradient material | 80 | 15 |
| Sample | Ecorr/mV(SCE) | Icorr/(μA/cm2) | βc/(mV/dec) | βa/(mV/dec) |
|---|---|---|---|---|
| Fe | −600 | 53.0 | 301 | 261 |
| Cu/Fe | −357 | 29.0 | 333 | 252 |
| Cu-Fe | −362 | 30.6 | 329 | 240 |
| 80%Cu-Fe | −389 | 49.0 | 455 | 500 |
| Sample | Rs/Ω·cm2 | Rt/Ω·cm2 | Ca/F·cm2 | Ra/Ω·cm2 | Rp/Ω·cm2 |
|---|---|---|---|---|---|
| Fe | 69 | 84 | 8.27 × 10−3 | 182 | 266 |
| Cu/Fe | 129 | 1539 | 9.48 × 10−3 | 203 | 1742 |
| Cu-Fe | 121 | 974 | 4.21 × 10−3 | 258 | 1232 |
| 80%Cu-Fe | 103 | 163 | 2.23 × 10−3 | 186 | 349 |
| Sample | Mass Fraction/% | ||
|---|---|---|---|
| O/% | Cu/% | Fe/% | |
| Fe | 11.27 | 0 | 88.73 |
| Cu/Fe | 2.94 | 97.06 | 0 |
| Cu-Fe | 3.06 | 96.94 | 0 |
| 80%Cu-Fe | 6.97 | 75.87 | 17.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Li, H.; Gao, J.; Li, Y. Corrosion Behaviour of Cu/Carbon Steel Gradient Material. Crystals 2021, 11, 1091. https://doi.org/10.3390/cryst11091091
Ma T, Li H, Gao J, Li Y. Corrosion Behaviour of Cu/Carbon Steel Gradient Material. Crystals. 2021; 11(9):1091. https://doi.org/10.3390/cryst11091091
Chicago/Turabian StyleMa, Tao, Huirong Li, Jianxin Gao, and Yungang Li. 2021. "Corrosion Behaviour of Cu/Carbon Steel Gradient Material" Crystals 11, no. 9: 1091. https://doi.org/10.3390/cryst11091091
APA StyleMa, T., Li, H., Gao, J., & Li, Y. (2021). Corrosion Behaviour of Cu/Carbon Steel Gradient Material. Crystals, 11(9), 1091. https://doi.org/10.3390/cryst11091091





