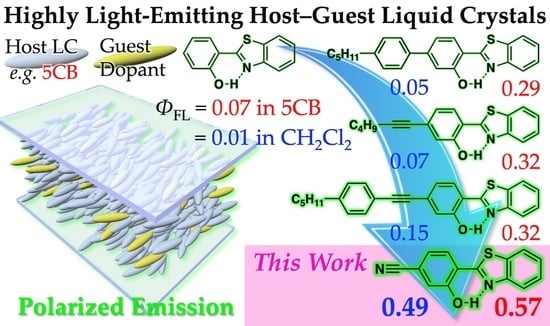
Remarkable Increase of Fluorescence Quantum Efficiency by Cyano Substitution on an ESIPT Molecule 2-(2-Hydroxyphenyl)benzothiazole: A Highly Photoluminescent Liquid Crystal Dopant
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Phase Characterizations
2.3. Absorption and Fluorescence Spectroscopy
2.4. Fluorescence Lifetime Measurements
2.5. Polarizing Fluorescence Microscopy
2.6. Synthesis
3. Results and Discussion
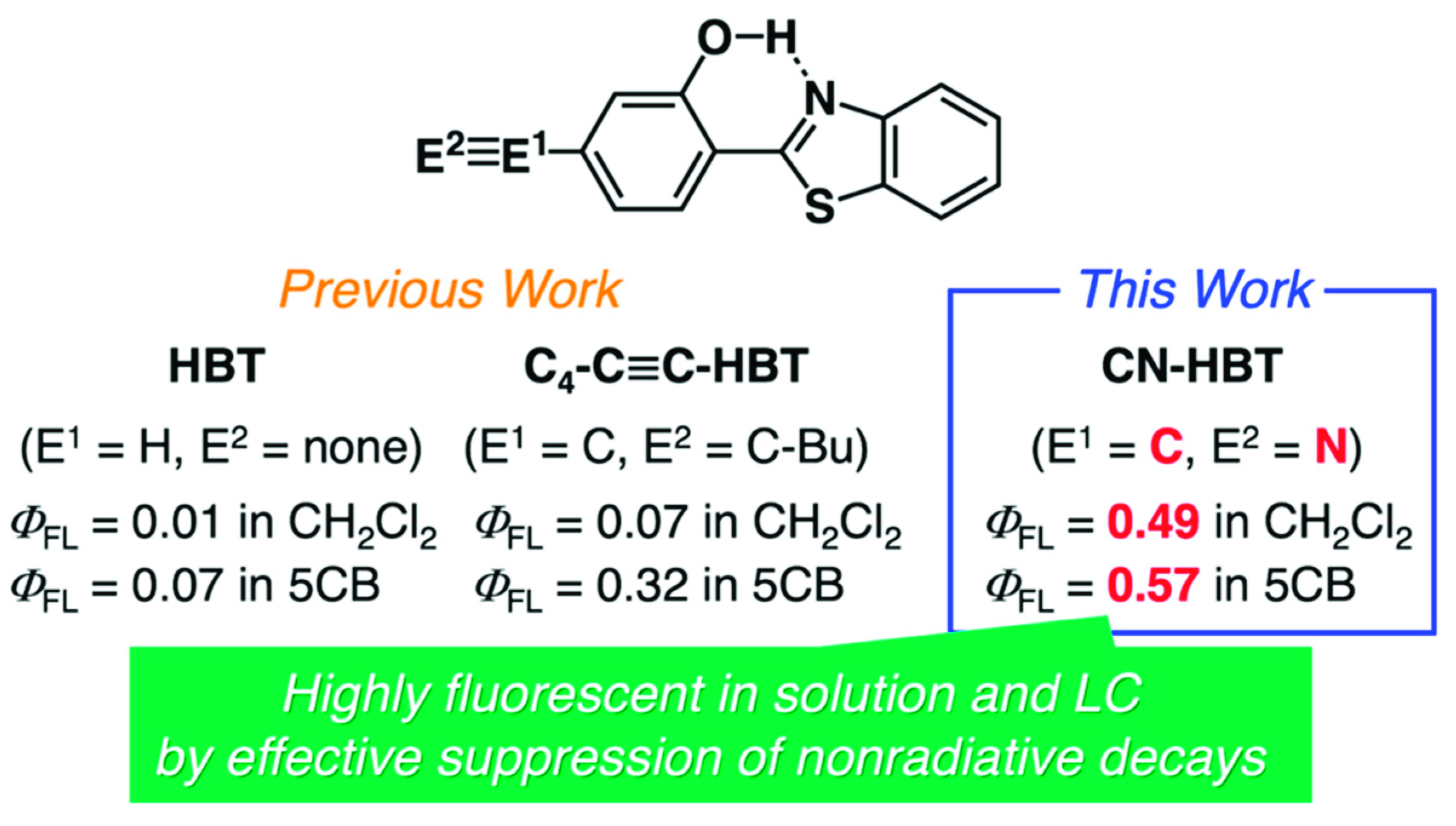
3.1. Synthesis and Characterization of CN-HBT
3.2. Preparation and Characterization of Host–Guest LCs Using CN-HBT as Guest Dopant
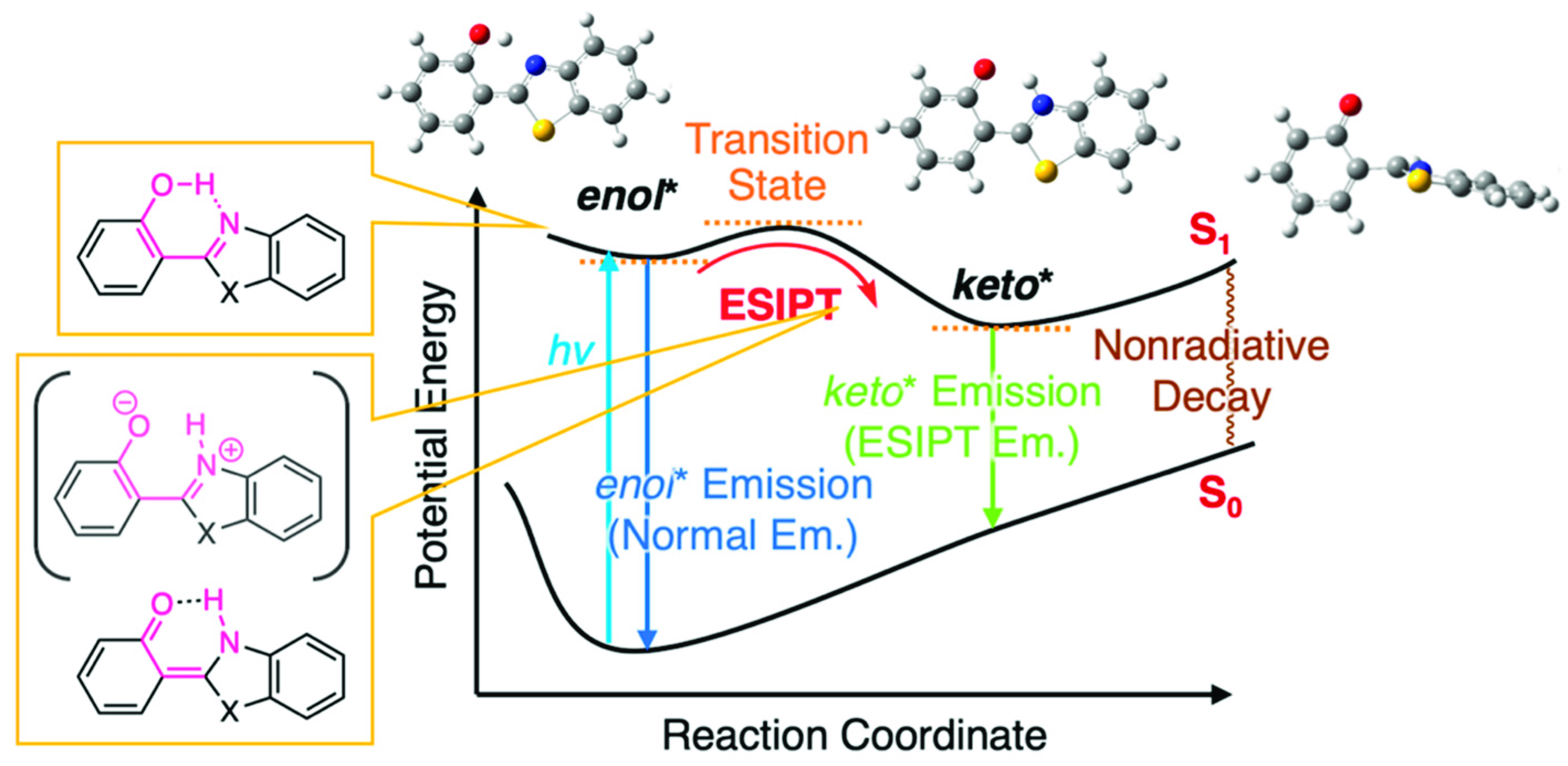
3.3. Rate Constant Analysis of Photoluminescence of CN-HBT in Solution and Nematic LCs
3.4. Polarizing Absorption and Fluorescence Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Raj, D. Dichroic display technology potentials and limitations. Mater. Chem. Phys. 1996, 43, 204–211. [Google Scholar] [CrossRef]
- Weder, C.; Sarwa, C.; Montali, A.; Bastiaansen, C.; Smith, P. Incorporation of photoluminescent polarizers into liquid crystal displays. Science 1998, 279, 835–837. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chen, J.; Zhu, W.; Baranoff, E. Recent progress in luminescent liquid crystal materials: Design, properties and application for linearly polarised emission. J. Mater. Chem. C 2015, 3, 7993–8005. [Google Scholar] [CrossRef]
- Zhao, D.; Bi, W.; Tang, B.Z. A light-emitting liquid crystal display device without polarizers and alignment layers. Adv. Opt. Mater. 2021, 9, 2100489. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Zanoni, L.A. Guest-host interactions in nematic liquid crystals. A new electro-optic effect. Appl. Phys. Lett. 1968, 13, 91–92. [Google Scholar] [CrossRef]
- White, D.L.; Taylor, G.N. New absorptive mode reflective liquid-crystal display device. J. Appl. Phys. 1974, 45, 4718–4723. [Google Scholar] [CrossRef]
- Sims, M.T. Dyes as guests in ordered systems: Current understanding and future directions. Liq. Cryst. 2016, 43, 2363–2374. [Google Scholar] [CrossRef] [Green Version]
- Lutfor, M.R.; Hegde, G.; Kumar, S.; Tschierske, C.; Chigrinov, V.G. Synthesis and characterization of bent-shaped azobenzene monomers: Guest-host effects in liquid crystals with Azo dyes for optical image storage devices. Opt. Mater. 2009, 32, 176–183. [Google Scholar] [CrossRef]
- Carrasco-Vela, C.; Quintana, X.; Oton, E.; Geday, M.; Otón, J. Security devices based on liquid crystals doped with a colour dye. Opto-Electron. Rev. 2011, 19, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Park, K.J.; Seok, S.; Ok, J.M.; Jung, H.-T.; Choe, J.; Oh, D.H.; Kim, D.H. Fabrication of microcapsules for dye-doped polymer-dispersed liquid crystal-based smart windows. ACS Appl. Mater. Interfaces 2015, 7, 17904–17909. [Google Scholar] [CrossRef]
- Zhang, W.; Sakurai, T.; Aotani, M.; Watanabe, G.; Yoshida, H.; Padalkar, V.S.; Tsutsui, Y.; Sakamaki, D.; Ozaki, M.; Seki, S. Highly fluorescent liquid crystals from excited-state intramolecular proton transfer molecules. Adv. Opt. Mater. 2019, 7, 1801349. [Google Scholar] [CrossRef]
- Zhang, W.; Suzuki, S.; Cho, S.; Watanabe, G.; Yoshida, H.; Sakurai, T.; Aotani, M.; Tsutsui, Y.; Ozaki, M.; Seki, S. Highly miscible hybrid liquid-crystal systems containing fluorescent excited-state intramolecular proton transfer molecules. Langmuir 2019, 35, 14031–14041. [Google Scholar] [CrossRef]
- Tsutsui, Y.; Zhang, W.; Ghosh, S.; Sakurai, T.; Yoshida, H.; Ozaki, M.; Akutagawa, T.; Seki, S. Electrically switchable amplified spontaneous emission from liquid crystalline phase of an AIEE-active ESIPT molecule. Adv. Opt. Mater. 2020, 8, 1902158. [Google Scholar] [CrossRef]
- Zhang, W.; Suzuki, S.; Sakurai, T.; Yoshida, H.; Tsutsui, Y.; Ozaki, M.; Seki, S. Extended conjugation of ESIPT-type dopants in nematic liquid crystalline phase for enhancing fluorescence efficiency and anisotropy. Phys. Chem. Chem. Phys. 2020, 22, 28393–28400. [Google Scholar] [CrossRef] [PubMed]
- Barbara, P.F.; Brus, L.E.; Rentzepis, P.M. Intramolecular proton transfer and excited-state relaxation in 2-(2-hydroxyphenyl)benzothiazole. J. Am. Chem. Soc. 1980, 102, 5631–5635. [Google Scholar] [CrossRef]
- Potter, C.A.S.; Brown, R.G.; Vollmer, F.; Rettig, W. Role of twisted intramolecular charge-transfer states in the decay of 2-(2′-hydroxyphenyl)benzothiazole following excited-state intramolecular proton transfer. J. Chem. Soc. Faraday Trans. 1994, 90, 59–67. [Google Scholar] [CrossRef]
- Barbatti, M.; Aquino, A.J.A.; Lischka, H.; Schriever, C.; Lochbrunner, S.; Riedle, E. Ultrafast internal conversion pathway and mechanism in 2-(2′-hydroxyphenyl)benzothiazole: A case study for excited-state intramolecular proton transfer systems. Phys. Chem. Chem. Phys. 2009, 11, 1406–1415. [Google Scholar] [CrossRef]
- Pijeau, S.; Foster, D.; Hohenstein, E.G. Excited-state dynamics of 2-(2′-Hydroxyphenyl) benzothiazole: Ultrafast proton transfer and internal conversion. J. Phys. Chem. A 2017, 121, 4595–4605. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Crespo-Otero, R.; Li, Q.; Blancafort, L. Exploring potential energy surfaces for aggregation-induced emission—From solution to crystal. Chem. Asian J. 2018, 14, 700–714. [Google Scholar] [CrossRef] [Green Version]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyšniauskas, A.; Kuimova, M.K. A twisted tale: Measuring viscosity and temperature of microenvironments using molecular rotors. Int. Rev. Phys. Chem. 2018, 37, 259–285. [Google Scholar] [CrossRef]
- Massue, J.; Ulrich, G.; Ziessel, R. Effect of 3,5-disubstitution on the optical properties of luminescent 2-(2-Hydroxyphenyl) benzoxazoles and their borate complexes. Eur. J. Org. Chem. 2013, 2013, 5701–5709. [Google Scholar] [CrossRef]
- Houari, Y.; Charaf-Eddin, A.; Laurent, A.D.; Massue, J.; Ziessel, R.; Ulrich, G.; Jacquemin, D. Modeling optical signatures and excited-state reactivities of substituted hydroxyphenylbenzoxazole (HBO) ESIPT dyes. Phys. Chem. Chem. Phys. 2014, 16, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Massue, J.; Jacquemin, D.; Ulrich, G. Molecular engineering of excited-state intramolecular proton transfer (ESIPT) dual and triple emitters. Chem. Lett. 2018, 47, 1083–1089. [Google Scholar] [CrossRef]
- Munch, M.; Curtil, M.; Vérité, P.M.; Jacquemin, D.; Massue, J.; Ulrich, G. Ethynyl-tolyl extended 2-(2′-Hydroxyphenyl) benzoxazole dyes: Solution and solid-state excited-state intramolecular proton transfer (ESIPT) emitters. Eur. J. Org. Chem. 2019, 2019, 1134–1144. [Google Scholar] [CrossRef]
- Pariat, T.; Munch, M.; Durko-Maciag, M.; Mysliwiec, J.; Retailleau, P.; Vérité, P.M.; Jacquemin, D.; Massue, J.; Ulrich, G. Impact of heteroatom substitution on dual-state emissive rigidified 2-(2′-hydroxyphenyl) benzazole dyes: Towards ultra-bright ESIPT fluorophores. Chem. Eur. J. 2021, 27, 3483–3495. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Niu, Y.; Wang, Y.; Yuan, M.-S.; Zhang, Y. Excited state intramolecular proton transfer in ethynyl-extended regioisomers of 2-(2′-Hydroxyphenyl) benzothiazole: Effects of the position and electronic nature of substituent groups. Chem. Asian J. 2016, 11, 3454–3464. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Q.; Zhang, Y. Electronic effect on the photophysical properties of 2-(2-hydroxyphenyl)benzothiazole-based excited state intramolecular proton transfer fluorophores synthesized by sonogashira-coupling reaction. Dye. Pigment. 2017, 136, 732–741. [Google Scholar] [CrossRef]
- Shah, S.S.; Paul, A.; Bera, M.; Venkatesh, Y.; Singh, N.D.P. Metallaphotoredox-Mediated Csp2–H Hydroxylation of Arenes under Aerobic Conditions. Org. Lett. 2018, 20, 5533–5536. [Google Scholar] [CrossRef]
- Shah, S.S.; Shee, M.; Singh, A.K.; Paul, A.; Singh, N.D.P. Direct oxygenation of CH bonds through photoredox and palladium catalysis. J. Org. Chem. 2020, 85, 3426–3439. [Google Scholar] [CrossRef]
- Bross, A.D.; Pla-Dalmau, A.; Spangler, C.W. Radiation damage to 2-(2′-Hydroxyphenyl) benzothiazoles. Radiat. Phys. Chem. 1993, 41, 379–387. [Google Scholar] [CrossRef]
- Iqbal, Z.; Lyubimtsev, A.; Hanack, M. Synthesis of phthalonitriles using a palladium catalyst. Synlett 2008, 19, 2287–2290. [Google Scholar]
- Suzuki, S.; Sasaki, S.; Sairi, A.S.; Iwai, R.; Tang, B.Z.; Konishi, G.-I. Principles of aggregation-induced emission: Design of deactivation pathways for advanced AIEgens and applications. Angew. Chem. Int. Ed. 2020, 59, 9856–9867. [Google Scholar] [CrossRef] [Green Version]
- Gim, M.-J.; Turlapati, S.; Debnath, S.; Rao, N.V.S.; Yoon, D.K. Highly polarized fluorescent illumination using liquid crystal phase. ACS Appl. Mater. Interfaces 2016, 8, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Lu, H.; Zhou, M.; Xia, G.; Fang, Y.; Zhang, G.; Qiu, L.; Yang, J.; Ding, Y. Highly polarized luminescence from an AIEE-active luminescent liquid crystalline film. Org. Electron. 2017, 50, 177–183. [Google Scholar] [CrossRef]
- Lu, H.; Xu, C.; Li, Z.; Xia, G.; Jing, S.; Bai, X.; Yang, J.; Qiu, L.; Ding, Y. High-contrast electrically switchable light-emitting liquid crystal displays. Liq. Cryst. 2018, 45, 32–39. [Google Scholar] [CrossRef]
- Ranjkesh, A.; Choi, J.-C.; Joo, K.-I.; Park, H.-W.; Zakerhamidi, M.S.; Kim, H.-R. Linear Dichroism and order parameters of nematics doped with azo dyes. Mol. Cryst. Liq. Cryst. 2017, 647, 107–118. [Google Scholar] [CrossRef]





| ΦFL/− | τFL/ns | kr/108 s–1 | knr/108 s–1 | |||||
|---|---|---|---|---|---|---|---|---|
| in CH2Cl2 | in 5CB | in CH2Cl2 | in 5CB | in CH2Cl2 | in 5CB | in CH2Cl2 | in 5CB | |
| HBT | 0.01 | 0.07 | 0.10 | 0.98 | 1.0 | 0.72 | 102 | 9.5 |
| C4-C≡C-HBT | 0.07 1 | 0.32 1 | 0.85 | 2.95 | 0.8 | 1.1 | 11 | 2.3 |
| CN-HBT | 0.49 | 0.57 | 4.81 | 4.94 | 1.0 | 1.1 | 1.1 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, T.; Kobayashi, M.; Yoshida, H.; Shimizu, M. Remarkable Increase of Fluorescence Quantum Efficiency by Cyano Substitution on an ESIPT Molecule 2-(2-Hydroxyphenyl)benzothiazole: A Highly Photoluminescent Liquid Crystal Dopant. Crystals 2021, 11, 1105. https://doi.org/10.3390/cryst11091105
Sakurai T, Kobayashi M, Yoshida H, Shimizu M. Remarkable Increase of Fluorescence Quantum Efficiency by Cyano Substitution on an ESIPT Molecule 2-(2-Hydroxyphenyl)benzothiazole: A Highly Photoluminescent Liquid Crystal Dopant. Crystals. 2021; 11(9):1105. https://doi.org/10.3390/cryst11091105
Chicago/Turabian StyleSakurai, Tsuneaki, Masaya Kobayashi, Hiroyuki Yoshida, and Masaki Shimizu. 2021. "Remarkable Increase of Fluorescence Quantum Efficiency by Cyano Substitution on an ESIPT Molecule 2-(2-Hydroxyphenyl)benzothiazole: A Highly Photoluminescent Liquid Crystal Dopant" Crystals 11, no. 9: 1105. https://doi.org/10.3390/cryst11091105
APA StyleSakurai, T., Kobayashi, M., Yoshida, H., & Shimizu, M. (2021). Remarkable Increase of Fluorescence Quantum Efficiency by Cyano Substitution on an ESIPT Molecule 2-(2-Hydroxyphenyl)benzothiazole: A Highly Photoluminescent Liquid Crystal Dopant. Crystals, 11(9), 1105. https://doi.org/10.3390/cryst11091105