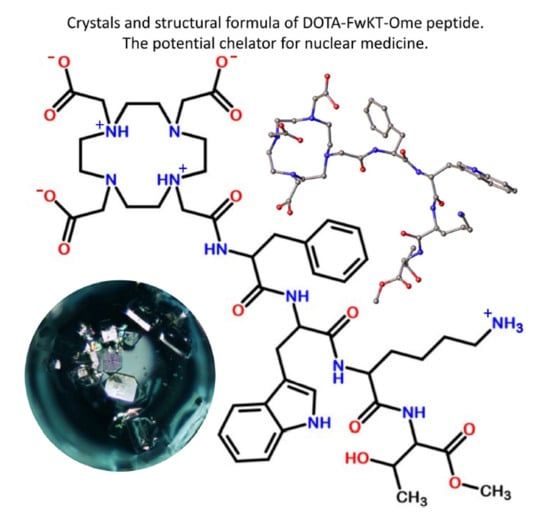
The Crystal Structure Elucidation of a Tetrapeptide Analog of Somatostatin DOTA-Phe-D-Trp-Lys-Thr-OMe
Abstract
:1. Introduction
2. Experimental
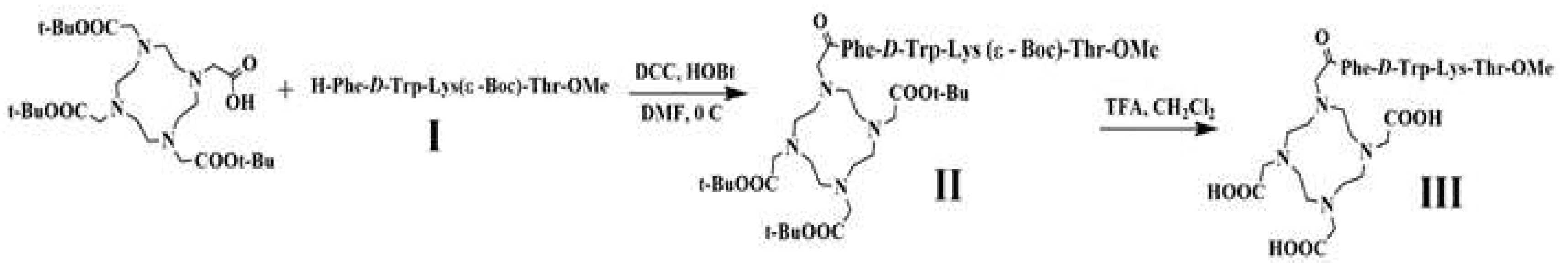
2.1. Synthesis
2.2. Crystallization
2.3. X-ray Diffraction Data Acquisition and Analysis
3. Results and Discussion
3.1. Structure Solution and Refinement
3.2. Structure Description and Crystal Structure Analysis
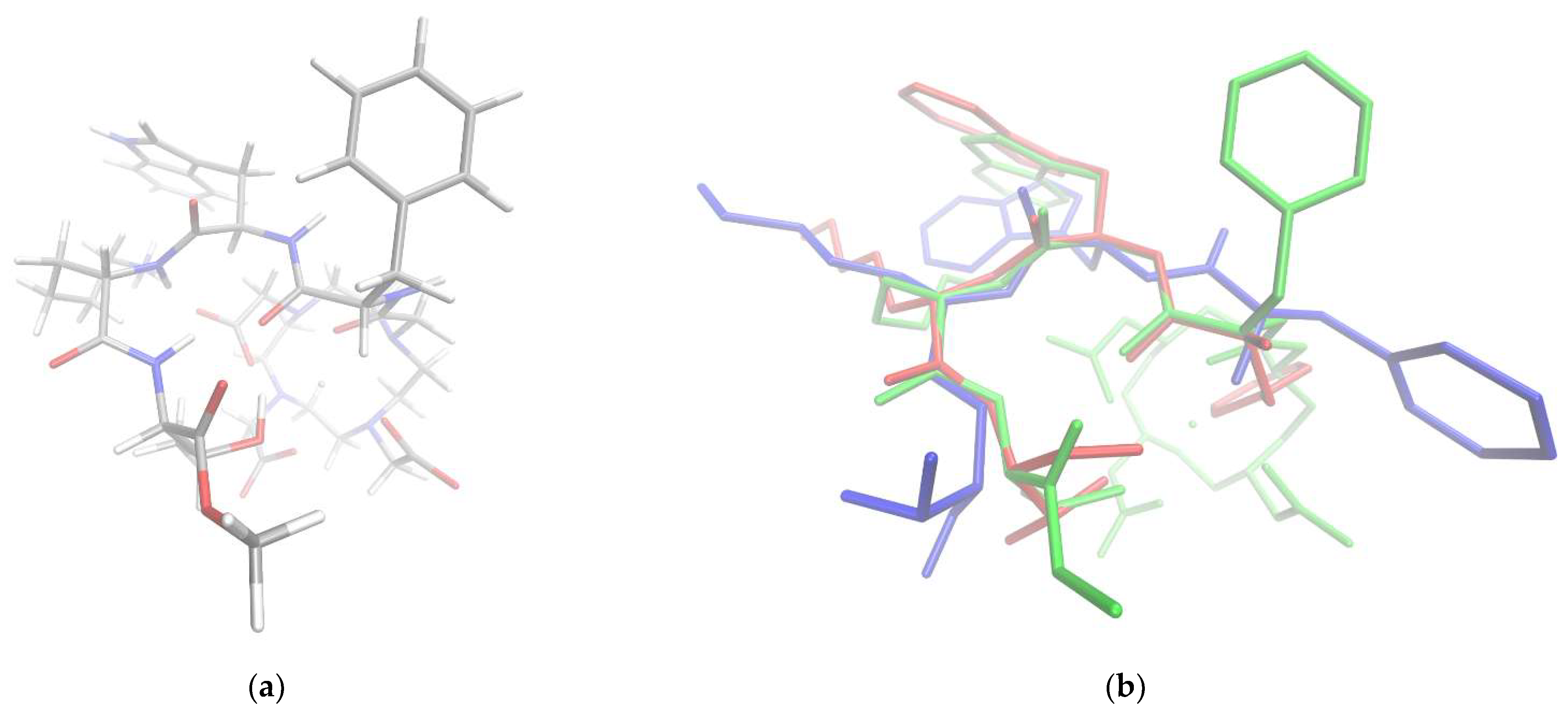
3.3. Computational Analysis of the Peptide Backbone Conformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidorova, M.V.; Molokoedov, A.S.; Az’muko, A.A.; Kudryavtseva, E.V.; Krause, E.; Ovchinnikov, M.V.; Bespalova, Z.D. The Use of Hydrogen Peroxide for Closing Disulfide Bridges in Peptides. Russ. J. Bioorganic Chem. 2004, 30, 101–110. [Google Scholar] [CrossRef]
- Balaev, A.N.; Osipov, V.N.; Okhmanovich, K.A.; Ruchko, E.A.; Baryshnikova, M.A.; Khachatryan, D.S. Pentapeptide Analogs of Somatostatin Containing a Thiazolidine Fragment: Synthesis and Cytotoxic Activity. Russ. Chem. Bull. 2016, 65, 2948–2951. [Google Scholar] [CrossRef]
- Balaev, A.N.; Osipov, V.N.; Okhmanovich, K.A.; Ruchko, E.A.; Kolotaev, A.V.; Khachatryan, D.S. Synthesis and Cytotoxic Activity of Boc-Protected Pentapeptide Amide Analogs of Somatostatin. Russ. Chem. Bull. 2016, 65, 2766–2769. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef] [Green Version]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef]
- Avdeev, D.V.; Sidorova, M.V.; Ovchinnikov, M.V.; Moiseeva, N.I.; Osipov, V.N.; Balaev, A.N.; Khachatryan, D.S. Synthesis and Antitumor Activity of Conjugates Based on the Phe-D-Trp-Lys-Thr Peptide Fragment of Somatostatin. Russ. J. Bioorganic Chem. 2019, 45, 248–252. [Google Scholar] [CrossRef]
- Yakusheva, A.; Titchenko, N.; Egorova, B.; Matazova, E.; Podkhalyuzina, N.; Osipov, V.; Khachatryan, D.; Avdeev, D.; Posypanova, G.; Kalmykov, S. From Octreotide to Shorter Analogues: Synthesis, Radiolabeling, Stability. J. Label. Compd. Radiopharm. 2019, 62, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Fili, S.; Valmas, A.; Spiliopoulou, M.; Kontou, P.; Fitch, A.; Beckers, D.; Degen, T.; Barlos, K.; Barlos, K.K.; Karavassili, F.; et al. Revisiting the Structure of a Synthetic Somatostatin Analogue for Peptide Drug Design. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.; Heine, A.; Sheldrick, G.M.; Dauter, Z.; Wilson, K.S.; Kallen, J.; Huber, W.; Pfaffli, P.J. Structure of Octreotide, a Somatostatin Analogue. Acta Crystallogr. Sect. D Biol. Crystallogr. 1995, 51, 48–59. [Google Scholar] [CrossRef]
- Balaev, A.N.; Osipov, V.N.; Okhmanovich, K.A.; Fedorov, V.E.; Reshetnikov, E.V. Obtaining H-Phe-D-Trp-Lys (ε-BOC) -Thr-OMe-a Tetrapeptide Fragment of the Synthesis of Somatostatin Analogs. Russ. Biother. J. 2011, 4, 43–45. [Google Scholar]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-Throughput Small-Molecule Crystallography at the ‘Belok’ Beamline of the Kurchatov Synchrotron Radiation Source: Transition Metal Complexes with Azomethine Ligands as a Case Study. Crystals 2017, 7, 325. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A: Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXL-2016/6: Program for Crystal Structure Determination; University of Göttingen: Göttingen, Germany, 2016. [Google Scholar] [CrossRef]
- Dolomanov, O.v.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refine-ment and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Van der Sluis, P.; Spek, A.L. BYPASS: An Effective Method for the Refinement of Crystal Structures Containing Disordered Solvent Regions. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B: Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melacini, G.; Zhu, Q.; Goodman, M. Multiconformational NMR Analysis of Sandostatin (Octreotide): Equilibrium between β-Sheet and Partially Helical Structures. Biochemistry 1997, 36, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Anoop, A.; Ranganathan, S.; Dhaked, B.D.; Jha, N.N.; Pratihar, S.; Ghosh, S.; Sahay, S.; Kumar, S.; Das, S.; Kombrabail, M.; et al. Elucidating the Role of Disulfide Bond on Amyloid Formation and Fibril Reversibility of Somatostatin-14: Relevance to Its Storage and Secretion. J. Biol. Chem. 2014, 289, 16884–16903. [Google Scholar] [CrossRef] [Green Version]
- Spiliopoulou, M.; Karavassili, F.; Triandafillidis, D.P.; Valmas, A.; Fili, S.; Kosinas, C.; Barlos, K.; Barlos, K.K.; Morin, M.; Reinle-Schmitt, M.L.; et al. New Perspectives in Macromolecular Powder Diffraction Using Single-Photon-Counting Strip Detectors: High-Resolution Structure of the Pharmaceutical Peptide Octreotide. Acta Crystallogr. Sect. A Found. Adv. 2021, 77. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—an Integrated Software System for Modeling Organic and Bioorganic Molecules Using Molecular Mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Parish, C.; Lombardi, R.; Sinclair, K.; Smith, E.; Goldberg, A.; Rappleye, M.; Dure, M. A Comparison of the Low Mode and Monte Carlo Conformational Search Methods. J. Mol. Graph. Model. 2002, 21, 129–150. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision d. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Gulde, R.; Pollak, P.; Weigend, F. Error-Balanced Segmented Contracted Basis Sets of Double-ζ to Quadruple-ζ Valence Quality for the Lanthanides. J. Chem. Theory Comput. 2012, 8, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Mennucci, B. Polarizable Continuum Model; Wiley: Hoboken, NJ, USA, 2012; Volume 2. [Google Scholar]



| 41/43 | 42 | n- | -b- | -c- | -n- | -21- | -c | |
|---|---|---|---|---|---|---|---|---|
| N | 93 | 59 | 1754 | 5254 | 5162 | 5192 | 87 | 3158 |
| N·I > 3 s | 28 | 7 | 1005 | 2469 | 2347 | 2276 | 25 | 1604 |
| <I> | 81.9 | 30.0 | 247.2 | 224.6 | 216.7 | 181.4 | 70.8 | 218.4 |
| <I/σ> | 2.5 | 1.3 | 4.7 | 3.9 | 3.9 | 3.6 | 2.4 | 4.1 |
| CCDC Ref. Code | RMS (Main Chain), Å |
| GOGZOU [9] | 1.50 |
| YICMUS [10] | 1.46 |
| PDB Ref. Code | RMS (Main Chain), Å |
| 1SOC [23] | 1.21 |
| 2SOC [23] | 0.896 |
| 2MI1 [24] | 0.946 |
| 6VC1 [25] | 1.23 |
| D–H...A | D–H | H–A | D–A | DHA |
|---|---|---|---|---|
| O3–H3...O9Aiv | 0.84 | 1.90 | 2.738 (8) | 179.8 |
| O3–H3...O9Biv | 0.84 | 2.12 | 2.87 (2) | 147.3 |
| N1–H1...O7v | 0.88 | 1.95 | 2.812 (7) | 165.0 |
| N3–H3A...O15 | 0.88 | 2.04 | 2.922 (8) | 175.6 |
| N4–H4...O10Bvi | 0.88 | 1.95 | 2.791 (15) | 159.5 |
| N5–H5...O5v | 0.88 | 2.16 | 3.017 (7) | 163.7 |
| N6–H6...O14 | 0.88 | 2.09 | 2.934 (6) | 159.3 |
| N8–H8...O11B | 1.00 | 2.26 | 3.003 (14) | 129.8 |
| N9–H9...O11A | 1.00 | 2.08 | 2.739 (15) | 121.4 |
| N10–H10...O11A | 1.00 | 2.14 | 3.036 (18) | 148.2 |
| N2A–H2AC...O10Avi | 0.91 | 2.14 | 2.728 (17) | 121.9 |
| N2B–H2BA...O15iii | 0.91 | 1.98 | 2.72 (3) | 136.7 |
| N2B–H2BC...O11Bii | 0.91 | 2.13 | 3.01 (4) | 161.0 |
| O14–H14C...O8A | 0.85 | 2.02 | 2.667 (9) | 132.1 |
| O14–H14D...O4i | 0.85 | 1.91 | 2.731 (7) | 161.8 |
| O15–H15A...O14iii | 0.87 | 2.02 | 2.731 (8) | 138.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diusenova, S.; Arkhipov, S.; Avdeev, D.; Dorovatovskii, P.; Khachatryan, D.; Lazarenko, V.; Medvedev, M.; Nikolaeva, A.; Ovchinnikov, M.; Sidorova, M.; et al. The Crystal Structure Elucidation of a Tetrapeptide Analog of Somatostatin DOTA-Phe-D-Trp-Lys-Thr-OMe. Crystals 2022, 12, 12. https://doi.org/10.3390/cryst12010012
Diusenova S, Arkhipov S, Avdeev D, Dorovatovskii P, Khachatryan D, Lazarenko V, Medvedev M, Nikolaeva A, Ovchinnikov M, Sidorova M, et al. The Crystal Structure Elucidation of a Tetrapeptide Analog of Somatostatin DOTA-Phe-D-Trp-Lys-Thr-OMe. Crystals. 2022; 12(1):12. https://doi.org/10.3390/cryst12010012
Chicago/Turabian StyleDiusenova, Sabina, Sergey Arkhipov, Dmitry Avdeev, Pavel Dorovatovskii, Derenik Khachatryan, Vladimir Lazarenko, Michael Medvedev, Alena Nikolaeva, Mikhail Ovchinnikov, Maria Sidorova, and et al. 2022. "The Crystal Structure Elucidation of a Tetrapeptide Analog of Somatostatin DOTA-Phe-D-Trp-Lys-Thr-OMe" Crystals 12, no. 1: 12. https://doi.org/10.3390/cryst12010012
APA StyleDiusenova, S., Arkhipov, S., Avdeev, D., Dorovatovskii, P., Khachatryan, D., Lazarenko, V., Medvedev, M., Nikolaeva, A., Ovchinnikov, M., Sidorova, M., & Zubavichus, Y. (2022). The Crystal Structure Elucidation of a Tetrapeptide Analog of Somatostatin DOTA-Phe-D-Trp-Lys-Thr-OMe. Crystals, 12(1), 12. https://doi.org/10.3390/cryst12010012