New Copper Bromide Organic-Inorganic Hybrid Molecular Compounds with Anionic Inorganic Core and Cationic Organic Ligands
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, X.; Li, J.; Fu, H.X. The first covalent organic-inorganic networks of hybrid chalcogenides: Structures that may lead to a new type of quantum wells. J. Am. Chem. Soc. 2000, 122, 8789–8790. [Google Scholar] [CrossRef]
- Huang, X.Y.; Li, J. From single to multiple atomic layers: A unique approach to the systematic tuning of structures and properties of inorganic-organic hybrid nanostructured semiconductors. J. Am. Chem. Soc. 2007, 129, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Saverina, E.A.; Kapaev, R.R.; Stishenko, P.V.; Galushko, A.S.; Balycheva, V.A.; Ananikov, V.P.; Egorov, M.P.; Jouikov, V.V.; Troshin, P.A.; Syroeshkin, M.A. 2-Carboxyethylgermanium Sesquioxide as A Promising Anode Material for Li-Ion Batteries. ChemSusChem 2020, 13, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B.; Wang, S.; Feild, C.A.; Chess, C.A.; Guloy, A.M. Conducting layered organic-inorganic halides containing-oriented perovskite sheets. Science 1995, 267, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen Contacts-Induced Unusual Coloring in BiIII Bromide Complex: Anion-to-Cation Charge Transfer via Br⋅⋅⋅Br Interactions. Chem. Eur. J. 2017, 23, 15612–15616. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Frolova, L.A.; Sokolov, M.N.; Shilov, G.V.; Korchagin, D.V.; Fedin, V.P.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Antimony (V) Complex Halides: Lead-Free Perovskite-like Materials for Hybrid Solar Cells. Adv. Energy Mater. 2018, 8, 1701140. [Google Scholar] [CrossRef]
- He, Z.; Gao, T.; Duan, D.; Soucek, M.D. Effect of mixed sol-gel precursors on inorganic-organic polyurethane hybrid thermosets: DOE study. Prog. Org. Coat. 2019, 133, 237–248. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Chitosan-doped-hybrid/TiO2 nanocomposite-based sol-gel coating for the corrosion resistance of aluminum metal in 3.5% NaCl medium. Int. J. Biol. Macromol. 2017, 104 Pt B, 1730–1739. [Google Scholar]
- Li, L.; He, J.; Lei, J.; Xu, W.; Jing, X.; Ou, X.; Wu, S.; Li, N.; Zhang, S. A sol-bath-gel approach to prepare hybrid coating for corrosion protection of aluminum alloy. Surf. Coat. Technol. 2015, 279, 72–78. [Google Scholar] [CrossRef]
- Dohner, E.R.; Jaffe, A.; Bradshaw, L.R.; Karunadasa, H.I. Intrinsic white-light emission from layered hybrid perovskites. J. Am. Chem. Soc. 2014, 136, 13154–13157. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Wei, G.Z.; Banerjee, D.; Hu, Z.; Li, J. Systematic approach in designing rare-earth-free hybrid semiconductor phosphors for general lighting applications. J. Am. Chem. Soc. 2014, 136, 14230–14236. [Google Scholar] [CrossRef]
- Liu, W.; Fang, Y.; Wei, G.Z.; Teat, S.J.; Xiong, K.; Hu, Z.; Lustig, W.P.; Li, J. A family of highly efficient cui-based lighting phosphors prepared by a systematic, bottom-up synthetic approach. J. Am. Chem. Soc. 2015, 137, 9400–9408. [Google Scholar] [CrossRef]
- Liu, Z.; Qayyum, M.F.; Wu, C.; Whited, M.T.; Djurovich, P.I.; Hodgson, K.O.; Hedman, B.; Solomon, E.I.; Thompson, M.E. A codeposition route to CuI-pyridine coordination complexes for organic light-emitting diodes. J. Am. Chem. Soc. 2011, 133, 3700–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Qiu, J.; Wei, F.; Wang, J.; Liu, X.; Helander, M.G.; Rodney, S.; Wang, Z.; Bian, Z.; Lu, Z.; et al. Simple and high efficiency phosphorescence organic light-emitting diodes with codeposited copper(i) emitter. Chem. Mater. 2014, 26, 2368–2373. [Google Scholar] [CrossRef]
- Liu, W.; Lustig, W.P.; Li, J. Luminescent inorganic-organic hybrid semiconductor materials for energy-saving lighting applications. EnergyChem 2019, 1, 100008. [Google Scholar] [CrossRef]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent copper(I) complexes with halogenido-bridged dimeric core. Coord. Chem. Rev. 2016, 306 Pt 2, 636–651. [Google Scholar] [CrossRef]
- Yam, V.W.W.; Au, V.K.M.; Leung, S.Y.L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Dohner, E.R.; Hoke, E.T.; Karunadasa, H.I. Self-Assembly of Broadband White-Light Emitters. J. Am. Chem. Soc. 2014, 136, 1718–1721. [Google Scholar] [CrossRef]
- Ki, W.; Li, J. A Semiconductor Bulk Material That Emits Direct White Light. J. Am. Chem. Soc. 2008, 130, 8114–8115. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, C.C.; Luo, W.; Shu, S.; Liu, Z.; Liu, Y.; Kong, J.; Ma, E.; Cao, Y.; Liu, R.S.; et al. Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 2014, 5, 4312. [Google Scholar] [CrossRef] [Green Version]
- Ki, W.; Li, J.; Eda, G.; Chhowalla, M. Direct white light emission from inorganic-organic hybrid semiconductor bulk materials. J. Mater. Chem. 2010, 20, 10676–10679. [Google Scholar] [CrossRef]
- Wang, M.S.; Guo, G.C. Inorganic–organic hybrid white light phosphors. Chem. Commun. 2016, 52, 13194–13204. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ying, L.; Huang, F.; Cao, Y. Recent advances in high performance solution processed WOLEDs for solid-state lighting. J. Mater. Chem. C 2016, 4, 10993–11006. [Google Scholar] [CrossRef]
- Abdelbaky, M.S.; Amghouz, Z.; Blanco, D.M.; García-Granda, S.; García, J.R. Crystal structure and characterization of a novel layered copper-lithium phosphonate with antiferromagnetic intrachain Cu(II)···Cu(II) interactions. J. Solid State Chem. 2017, 248, 61–67. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, W.; Teat, S.J.; Dey, G.; Shen, Z.; An, L.; Yu, D.; Wang, L.; O’Carroll, D.M.; Li, J. A Systematic Approach to Achieving High Performance Hybrid Lighting Phosphors with Excellent Thermal- and Photostability. Adv. Funct. Mater. 2017, 27, 1603444. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Huang, G.; Lustig, W.P.; Wang, F.; Wang, H.; Teat, S.J.; Banerjee, D.; Zhang, D.; Li, J. Achieving exceptionally high luminescence quantum efficiency by immobilizing an AIE molecular chromophore into a metal–organic framework. Chem. Commun. 2015, 51, 3045–3048. [Google Scholar] [CrossRef]
- Huang, X. Red phosphor converts white LEDs. Nat. Photon. 2014, 8, 748. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, K.; Teat, S.J.; Dey, G.; Shen, Z.; Wang, L.; O’Carroll, D.M.; Li, J. All-in-One: Achieving robust, strongly luminescent and highly dispersible hybrid materials by combining ionic and coordinate bonds in molecular crystals. J. Am. Chem. Soc. 2017, 139, 9281–9290. [Google Scholar] [CrossRef]
- Hei, X.; Liu, W.; Zhu, K.; Teat, S.J.; Jensen, S.; Li, M.; O’Carroll, D.M.; Wei, K.; Tan, K.; Cotlet, M.; et al. Blending ionic and coordinate bonds in hybrid semiconductor materials: A general approach toward robust and solution-processable covalent/coordinate network structures. J. Am. Chem. Soc. 2020, 142, 4242–4253. [Google Scholar] [CrossRef]
- Yue, C.-Y.; Sun, C.; Li, D.-Y.; Dong, Y.-H.; Wang, C.-L.; Zhao, H.-F.; Jiang, H.; Jing, Z.-H.; Lei, X.-W. Organic–Inorganic Hybrid Heterometallic Halides with Low-Dimensional Structures and Red Photoluminescence Emissions. Inorg. Chem. 2019, 58, 10304–10312. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Li, G.; Hua, J.; Liu, X.; Hu, Y.; Shi, Z.; Feng, S. A coordination polymer of copper(i) iodide with 654 topology constructed from Cu4I4(DABCO)4. CrystEngComm 2007, 9, 984–986. [Google Scholar] [CrossRef]
- Bi, M.; Li, G.; Zou, Y.; Shi, Z.; Feng, S. Zeolite-like Copper Iodide Framework with New 66 Topology. Inorg. Chem. 2007, 46, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-Q.; Zhou, Q.-Q.; Zhang, Y.; Jin, L. Synthesis, structures and dielectric properties of two five-coordinate copper (II) complexes based on N-chloromethyl-1,4-diazabicyclo[2.2.2]octane. Inorg. Chem. Commun. 2013, 33, 161–164. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polyhalide-bonded metal complexes: Structural diversity in an eclectic class of compounds. Coord. Chem. Rev. 2018, 367, 1–17. [Google Scholar] [CrossRef]
- Bondarenko, M.A.; Novikov, A.S.; Sakhapov, I.F.; Sokolov, M.N.; Adonin, S.A. Heteroleptic Cu(I) halide complexes with perchlorinated 1,10-phenanthroline. J. Mol. Struct. 2021, 1234, 130199. [Google Scholar] [CrossRef]
- Liu, W.; Banerjee, D.; Lin, F.; Li, J. Strongly luminescent inorganic–organic hybrid semiconductors with tunable white light emissions by doping. J. Mater. Chem. C 2019, 7, 1484–1490. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Lv, L.; Lin, F.; Lin, F.; Zhang, Z.; Luo, C.; Luo, D.; Liu, W. Synthesis, characterization, luminescence properties of copper(I) bromide based coordination compounds. Inorg. Chim. Acta 2020, 512, 119893. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3648. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kamil, M.P.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mater. Sci. 2020, 112, 100663. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Zhang, H.; Yu, B.; Cong, H.; Shen, Y. Antibacterial material surfaces/interfaces for biomedical applications. Appl. Mater. Today 2021, 25, 101192. [Google Scholar] [CrossRef]







| Compound | 1 | 2 |
|---|---|---|
| Formula | C16H32Br4Cl2Cu2N4 | C16H32Br6Cu2N4 |
| Fw | 798.07 | 886.99 |
| Space Group | P21 | P21 |
| a (Å) | 8.5917(14) | 8.7020(5) |
| b (Å) | 12.765(2) | 12.8893(8) |
| c (Å) | 11.7691(18) | 11.6700(6) |
| α (°) | 90 | 90 |
| β (°) | 99.485(2) | 99.235(4) |
| γ (°) | 90 | 90 |
| V (Å3) | 1273.1(4) | 1291.97(13) |
| Z | 2 | 2 |
| T (K) | 296(2) | 296(2) |
| λ (Å) | 0.71073 | 0.71073 |
| CCDC # | 2033422 | 2033420 |
| Compound | 1 | 2 |
|---|---|---|
| Band Gap (eV) | 3.0 | 2.9 |
| λex (nm) | 320 | 320 |
| λem (nm) | 525 | 525 |
| Emission color | Green | Green |
| IQY (320 nm) | 11.5 % | 10.6 % |
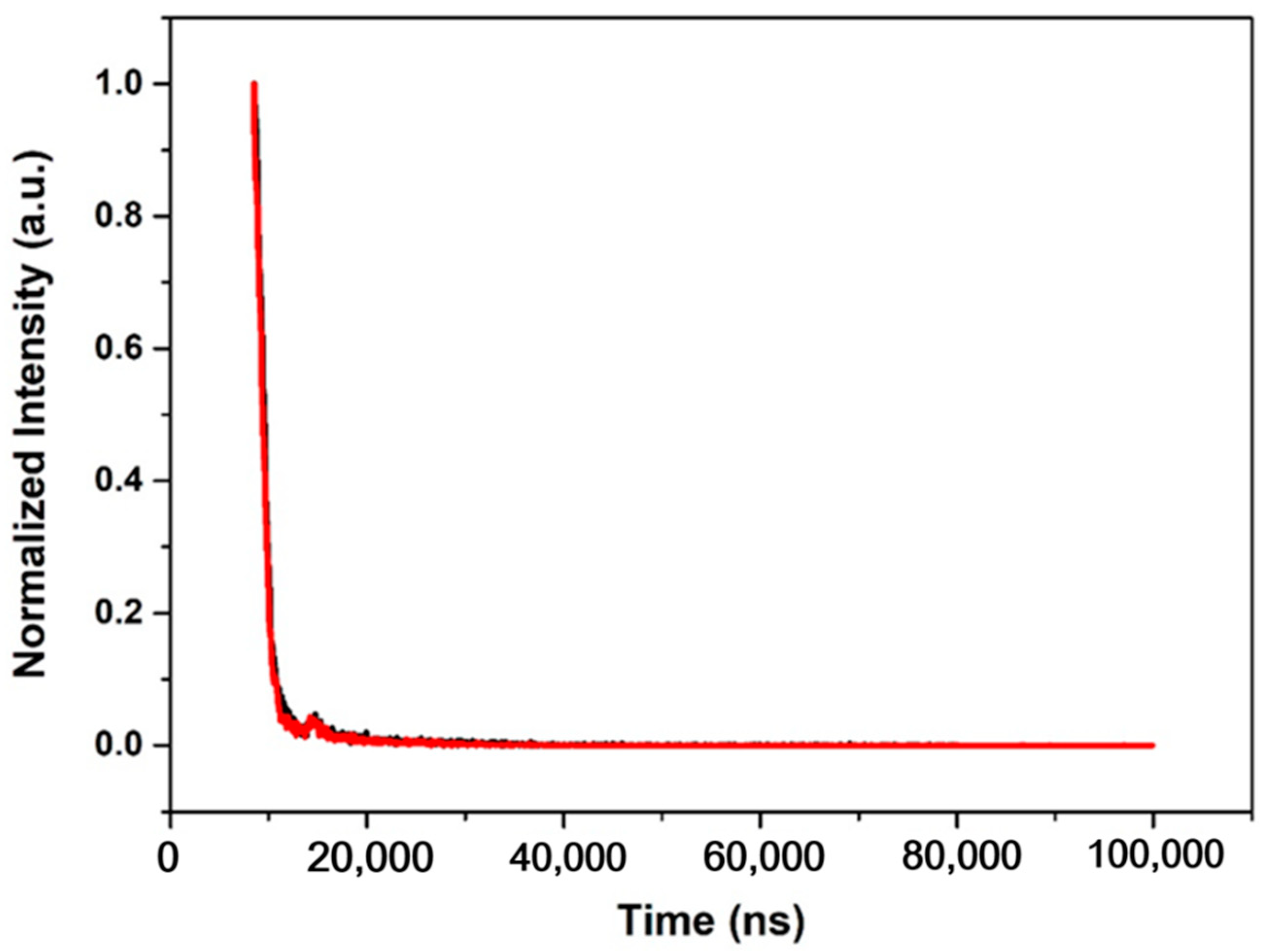
| τ (μs) | 10.66 | 10.34 |
| CIE | 0.28, 0.48 | 0.28, 0.48 |
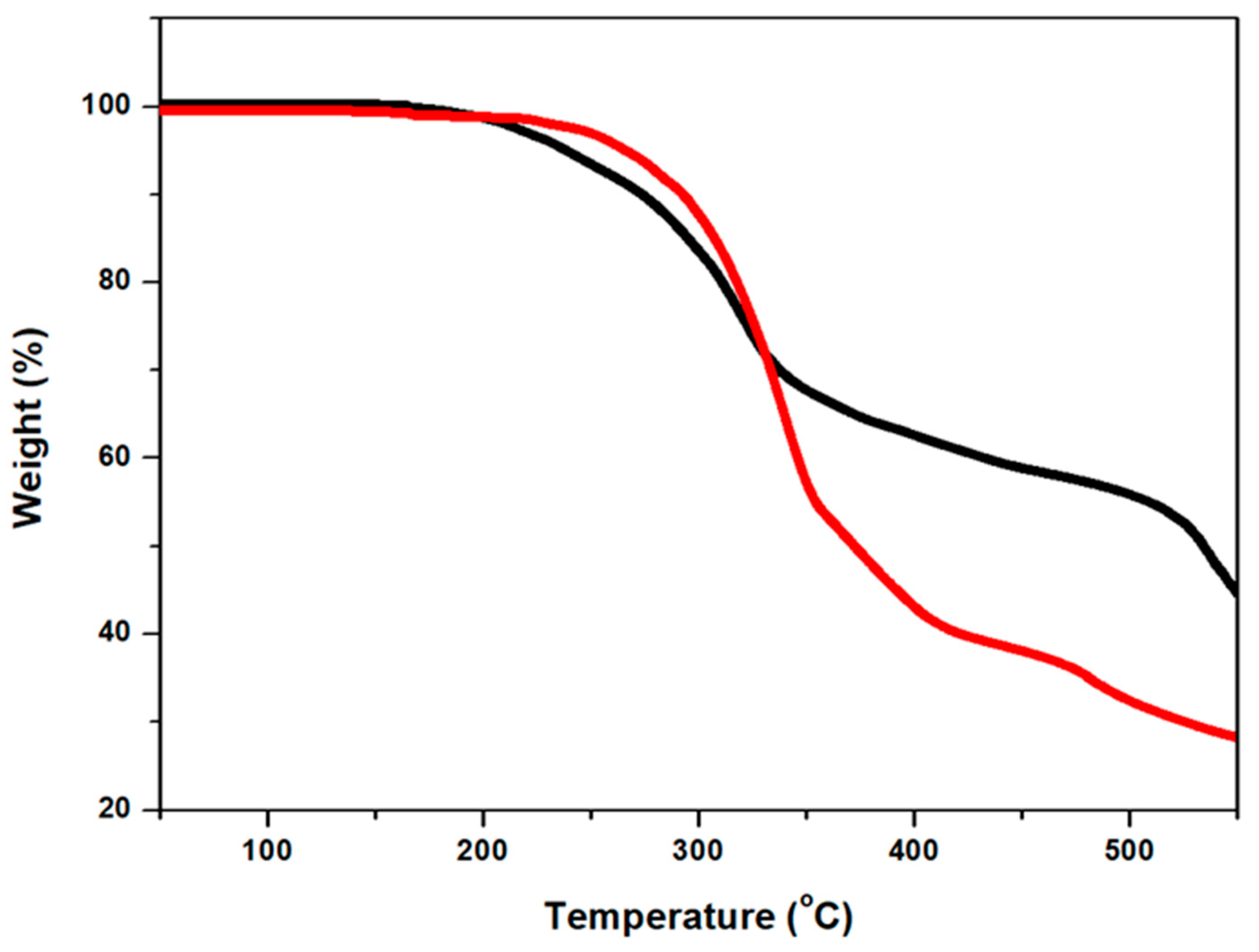
| TD (℃) | 190 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Zhang, J.; Liang, Y.; Ni, J.; Wang, F.; Liu, W. New Copper Bromide Organic-Inorganic Hybrid Molecular Compounds with Anionic Inorganic Core and Cationic Organic Ligands. Crystals 2022, 12, 19. https://doi.org/10.3390/cryst12010019
Luo D, Zhang J, Liang Y, Ni J, Wang F, Liu W. New Copper Bromide Organic-Inorganic Hybrid Molecular Compounds with Anionic Inorganic Core and Cationic Organic Ligands. Crystals. 2022; 12(1):19. https://doi.org/10.3390/cryst12010019
Chicago/Turabian StyleLuo, Dawei, Jian Zhang, Yu Liang, Jianling Ni, Fangming Wang, and Wei Liu. 2022. "New Copper Bromide Organic-Inorganic Hybrid Molecular Compounds with Anionic Inorganic Core and Cationic Organic Ligands" Crystals 12, no. 1: 19. https://doi.org/10.3390/cryst12010019
APA StyleLuo, D., Zhang, J., Liang, Y., Ni, J., Wang, F., & Liu, W. (2022). New Copper Bromide Organic-Inorganic Hybrid Molecular Compounds with Anionic Inorganic Core and Cationic Organic Ligands. Crystals, 12(1), 19. https://doi.org/10.3390/cryst12010019







