Structural Diversities of a Series of Cd(II) Coordination Complexes Based on a Flexible Tripodal N-donor Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Single-Crystal X-ray Diffraction
2.3. Preparation of Compounds 1–3
2.3.1. Synthesis of {[Cd(tttmb)(Hbtc)]·5H2O}n (1)
2.3.2. Synthesis of {[Cd(tttmb)(m-phda)(H2O)]·2H2O}n (2)
2.3.3. Synthesis of {[Cd(tttmb)(o-cpla)]·(CH3CN)·(H2O)}n (3)
3. Results and Discussion
3.1. Structural Description
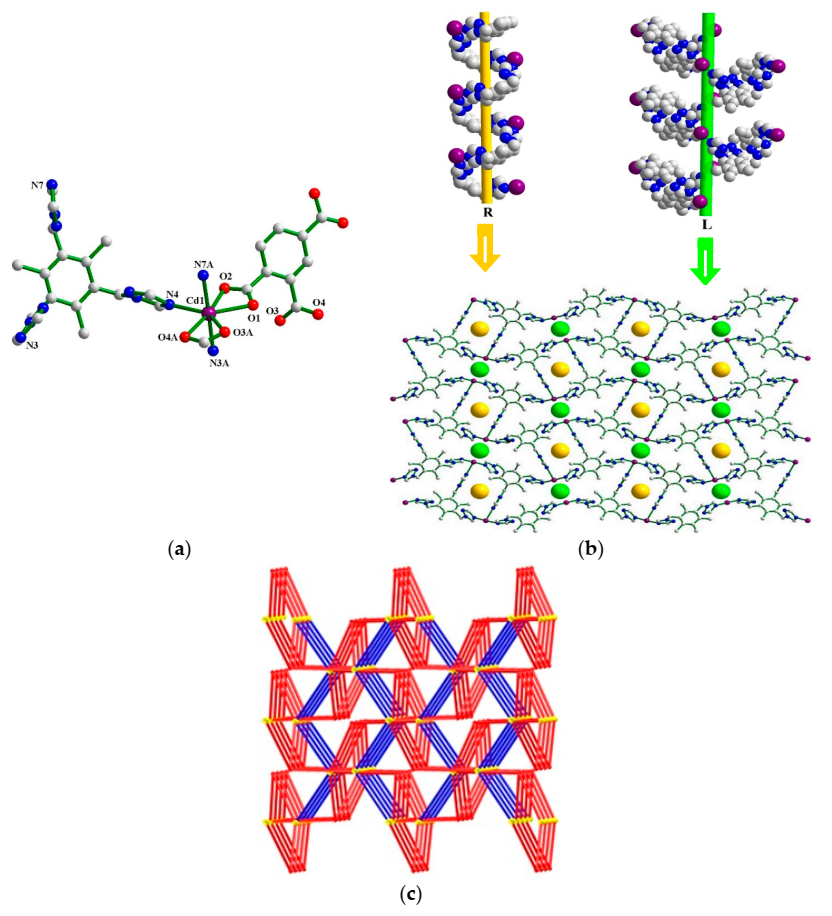
3.1.1. Crystal Structure of 1
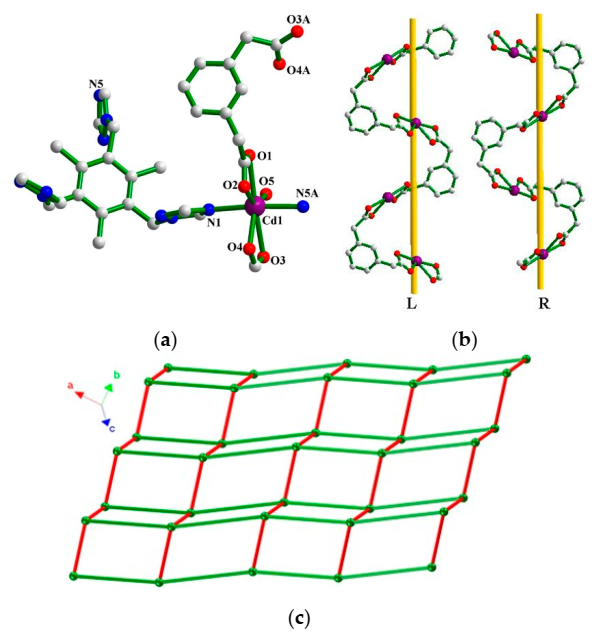
3.1.2. Crystal Structure of 2
3.1.3. Crystal Structure of 3
3.1.4. Coordination Modes of tttmb Ligand in 1–3
3.2. Powder X-ray Diffraction (PXRD) and Thermal Analyses
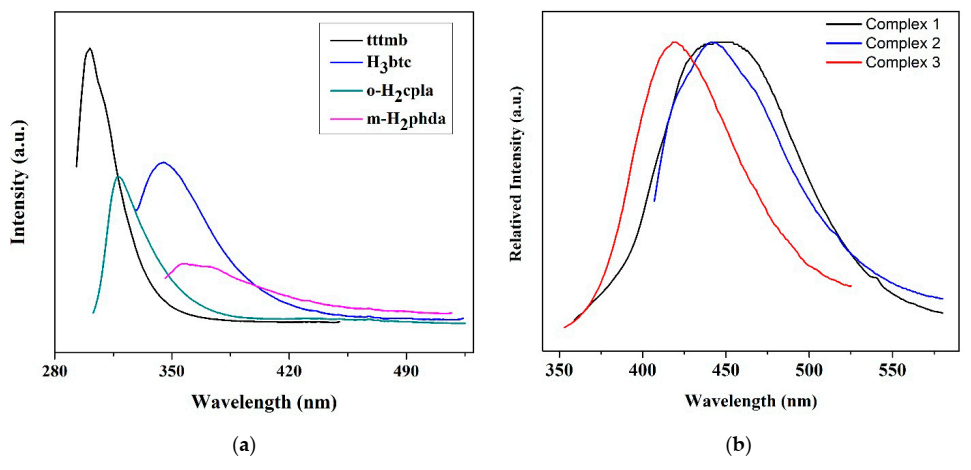
3.3. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Z.-J.; Lü, J.; Hong, M.; Cao, R. Metal-organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.-J.; Han, S.-D.; Mu, Y.; Pan, J.; Li, J.-H.; Wang, G.-M. Two Cobalt-Diphosphonates Templated by Long-Chain Flexible Amines: Synthesis, Structures, Proton Conductivity, and Magnetic Properties. Cryst. Growth Des. 2018, 18, 3477–3483. [Google Scholar] [CrossRef]
- Hawes, C.S.; Hamilton, S.E.; Hicks, J.; Knowles, G.P.; Chaffee, A.L.; Turner, D.R.; Batten, S.R. Coordination Chemistry and Structural Dynamics of a Long and Flexible Piperazine-Derived Ligand. Inorg. Chem. 2016, 55, 6692–6702. [Google Scholar] [CrossRef] [PubMed]
- Hawes, C.S.; Byrne, K.; Schmitt, W.; Gunnlaugsson, T. Flexible porous coordination polymers from divergent photoluminescent 4-oxo-1,8-naphthalimide ligands. Inorg. Chem. 2016, 55, 11570–11582. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-H.; Qin, W.-J.; Xiao, Z.; Yang, J.-K.; Wang, H.-R.; Yang, X.-G.; Li, D.-S.; Ma, L.-F. Efficient energy-transfer-induced high photoelectric conversion in a dye-encapsulated ionic pyrene-based metal–organic framework. Inorg. Chem. 2021, 60, 18593–18597. [Google Scholar] [CrossRef]
- Kang, Y.S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.Y. Metal-organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Zhai, Q.G.; Bu, X.; Mao, C.; Zhao, X.; Feng, P. Systematic and dramatic tuning on gas sorption performance in heterometallic metal-organic frameworks. J. Am. Chem. Soc. 2016, 138, 2524–2527. [Google Scholar] [CrossRef]
- Qin, J.-H.; Huang, Y.-D.; Zhao, Y.; Yang, X.-G.; Li, F.-F.; Wang, C.; Ma, L.-F. Highly Dense Packing of Chromophoric Linkers Achievable in a Pyrene-Based Metal–Organic Framework for Photoelectric Response. Inorg. Chem. 2019, 58, 15013–15016. [Google Scholar] [CrossRef]
- Yang, X.-G.; Ma, L.-F.; Yan, D.-P. Facile synthesis of 1D organic--inorganic perovskite micro-belts with high water stability for sensing and photonic applications. Chem. Sci. 2019, 10, 4567–4572. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.-H.; Xu, P.; Huang, Y.-D.; Xiao, L.-Y.; Lu, W.; Yang, X.-G.; Ma, L.; Zang, S.-Q. High loading of Mn(II)-metalated porphyrin in MOF for photocatalytic CO2 reduction in gas-solid condition. Chem. Commun. 2021, 57, 8468–8471. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Young, D.J.; Hor, T.S.A. Nitrogen-rich azoles as ligand spacers in coordination polymers. Chem.-Asian J. 2011, 6, 292–304. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, J.; Chen, W. Organometallic chemistry of bis(N-heterocyclic carbene) ligands containing a heteroarene spacer. J. Organomet. Chem. 2017, 848, 249–280. [Google Scholar] [CrossRef]
- Liu, X.; Du, L.; Li, R.; Ma, N.; You, M.; Feng, X. Different effects in the selective detection of aniline and Fe3+ by lanthanide-based coordination polymers containing multiple reactive sites. CrystEngComm 2020, 22, 2837–2844. [Google Scholar] [CrossRef]
- Qin, J.-H.; Zhang, H.; Sun, P.; Huang, Y.-D.; Shen, Q.; Yang, X.-G.; Ma, L.-F. Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy. Dalton Trans. 2020, 49, 17772–17778. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Li, R.; Du, L.; Feng, X.; Ding, Y. A highly Sensitive and Selective “Turn off-on” Fluorescent Sensor Based on Sm-MOF for the Detection of Tertiary Butylhydroquinone. Dyes Pigm. 2020, 178, 108347. [Google Scholar] [CrossRef]
- Xue, X.-F.; Liu, Y.-Q.; Liu, Q.; Wang, X.-Y.; Li, W. Four Novel Coordination Polymers Based on Flexible 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene Ligand: Synthesis, Structure, Luminescence and Magnetic Properties. J. Clust. Sci. 2019, 30, 777–787. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. The coordination chemistry of Zn(II), Cd(II) and Hg(II) complexes with 1,2,4-triazole derivatives. Dalton Trans. 2011, 40, 8475–8490. [Google Scholar] [CrossRef]
- Yang, P.; Wu, X.X.; Huo, J.Z.; Ding, B.; Wang, Y.; Wang, X.G. Hydrothermal synthesis and characterization of a series of luminescent Zn(II) and Cd(II) coordination polymers with the new versatile multidentate ligand 1,3-di-(1,2,4-triazol-4yl)benzene. CrystEngComm 2013, 15, 8097–8109. [Google Scholar] [CrossRef]
- Gautier, R.; Clérac, R. Tuning the Crystal Structure Dimensionality of Cobalt(II)/1,2,4-Triazole Complexes. Cryst. Growth Des. 2017, 17, 864–869. [Google Scholar] [CrossRef]
- Zhang, M.N.; Fan, T.T.; Wang, Q.S.; Han, H.L.; Li, X. Zn/Cd/Cu-frameworks constructed by 3,3′-diphenyldicarboxylate and 1,4-bis(1,2,4-triazol-1-yl)butane: Syntheses, structure, luminescence and luminescence sensing for metal ion in aqueous medium. J. Solid State Chem. 2018, 258, 744–752. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Xu, C.; Guo, Q.; Hou, H.; Fan, Y. Series of Cd(II) Metal–Organic Frameworks Based on a Flexible Tripodal Ligand and Polycarboxylate Acids: Syntheses, Structures, and Photoluminescent Properties. Cryst. Growth Des. 2012, 12, 2435–2444. [Google Scholar] [CrossRef]
- Wu, Q.; Han, Y.B.; Shao, Z.C.; Li, J.X.; Hou, H.W. Stable Fe(II)-based coordination polymers: Synthesis, structural diversity and catalytic applications in homo-coupling reactions. Dalton Trans. 2018, 47, 8063–8069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Zhang, G.-D.; Sheng, X.-X.; Ding, Q.-W.; Bai, Y.-Z.; Su, Y.; Liu, H.-K.; Su, Z. Efficient MO dye degradation catalyst of Cu(I)-based coordination complex from dissolution-recrystallization structural transformation. Cryst. Growth Des. 2021, 21, 333–343. [Google Scholar] [CrossRef]
- Chang, X.-H.; Zhao, Y.; Han, M.-L.; Ma, L.-F.; Wang, L.-Y. Five Cd(II) coordination polymers based on 2,3′,5,5′-biphenyltetracarboxylic acid and N-donor coligands: Syntheses, structures and fluorescent properties. CrystEngComm 2014, 16, 6417–6424. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, L.; Zhang, W.Y.; Yan, Y.T.; Wu, Y.L.; Yang, R.F.; Cao, F.; Wang, Y.Y. Two metal-organic frameworks based on a flexible benzimidazole carboxylic acid ligand: Selective gas sorption and luminescence. Dalton Trans. 2017, 46, 15118–15123. [Google Scholar] [CrossRef]
- Miao, S.-B.; Li, Z.-H.; Xu, C.-Y.; Ji, B.-M. A New 3-Fold Interpenetrating 3D Zn(II) Metal-organic Framework: Synthesis, Structure and Luminescent Property. Chin. J. Struct. Chem. 2016, 35, 1960–1966. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 342–346. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Meng, W.; Liu, Y.; Zhao, Y.; Hou, H.; Fan, Y. Coordination polymers based on a flexible tripodal ligand and aromatic dicarboxylate anions: Syntheses, structures and fluorescent properties. Polyhedron 2013, 52, 1219–1226. [Google Scholar] [CrossRef]
- Wang, K.; Tang, H.; Zhang, D.; Ma, Y.; Wang, Y. Selective and recyclable sensing of aqueous phase 2,4,6-Trinitrophenol (TNP) based on Cd(II) coordination polymer with Zwitterionic ligand. Crystals 2018, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Miao, S.-B.; Sun, X.-J.; Wang, K.-X.; Xu, C.-Y.; Li, Z.-H.; Wang, Z.-Q. Effect of Charge on the Structures of Zn(II) Coordination Polymers with Triazole-carboxylate Ligands: Syntheses, Structures, and Luminescent Properties. Crystals 2018, 8, 312. [Google Scholar] [CrossRef] [Green Version]



| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C27H35N9O11Cd | C28H35N9O7Cd | C29H30N10O5Cd |
| Formula weight | 774.0319 | 722.05 | 711.03 |
| Crystal system | orthorhombic | monoclinic | monoclinic |
| Space group | P212121 | C2/c | C2/c |
| a (Å) | 11.281 (2) | 30.639 (6) | 19.825 (4) |
| b (Å) | 13.642 (3) | 12.889 (3) | 13.620 (3) |
| c (Å) | 22.337 (5) | 19.841 (4) | 23.123 (5) |
| α (°) | 90.00 | 90.00 | 90.00 |
| β (°) | 90.00 | 121.18 (3) | 95.43 (3) |
| γ (°) | 90.00 | 90.00 | 90.00 |
| V (Å3) | 3437.7 (12) | 6704 (2) | 6216 (2) |
| Z | 4 | 8 | 8 |
| Dcalc (g·cm−3) | 1.476 | 1.431 | 1.520 |
| μ (mm−1) | 0.703 | 0.707 | 0.758 |
| F(000) | 1544 | 2960 | 2944 |
| R1a, wR2b (I > 2σ(I)) | 0.0758, 0.1916 | 0.0747, 0.1686 | 0.0630, 0.1571 |
| R1, wR2 (all data) | 0.0864, 0.2002 | 0.0912, 0.1800 | 0.0803, 0.1806 |
| GOF | 1.061 | 1.140 | 1.180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Qin, J. Structural Diversities of a Series of Cd(II) Coordination Complexes Based on a Flexible Tripodal N-donor Ligand. Crystals 2022, 12, 53. https://doi.org/10.3390/cryst12010053
Wang H, Qin J. Structural Diversities of a Series of Cd(II) Coordination Complexes Based on a Flexible Tripodal N-donor Ligand. Crystals. 2022; 12(1):53. https://doi.org/10.3390/cryst12010053
Chicago/Turabian StyleWang, Huarui, and Jianhua Qin. 2022. "Structural Diversities of a Series of Cd(II) Coordination Complexes Based on a Flexible Tripodal N-donor Ligand" Crystals 12, no. 1: 53. https://doi.org/10.3390/cryst12010053






