Abstract
Due to the introduction of oxygen atoms, N-oxide energetic compounds have a unique oxygen balance, excellent detonation properties, and a high energy density, attracting the extensive attention of researchers all over the world. N-oxides are classified into two categories based on the structural characteristics of their skeletons: azine N-oxides and azole N-oxides, whose N→O coordination bonds are formed during cyclization. There are six kinds of azine N-oxides, namely 1,2,3,4-tetrazine-1,3-dioxide, 1,2,3,5-tetrazine-2-oxide, 1,2,3-triazine-3-oxide, 1,2,3-triazine-2-oxide, pyridazine-1,2-dioxide, and pyrazine-1-oxide. Azole N-oxides include 1,2,5-oxadiazole-2-oxide, pyrazole-1-oxide, and triazole-1-oxide. Synthetic strategies towards these two categories of N-oxides are fully reviewed. Corresponding reaction mechanisms towards the aromatic N-oxide frameworks and examples that use the frameworks to create high-energy substances are discussed. Moreover, the energetic properties of N-oxide energetic compounds are compared and summarized.
1. Introduction
Research on energetic materials continuously develops energetic materials with higher detonation performance and energy density, taking it as an eternal quest. Since the advent of conventional energetic materials such as trinitrotoluene (TNT), RDX, and HMX, researchers worldwide have been extensively exploring energetic materials with chain, ring, and cage parent nucleus structures composed of C, H, and N as the main elements as well as new energetic parent frameworks such as tetrazine, triazole, and furazan [1,2,3,4,5,6,7,8,9,10,11]. Meanwhile, they strive to improve the performance of the compounds by introducing energetic groups, including nitroamino groups, trinitroethyl groups, fluoroacyl dinitroethyl groups, and azide groups [12,13,14,15,16,17]. As a typical energetic group for improving the detonation performance of compounds, the nitro group (-NO2) could not only increase the energy density of CHON-based compounds but also enhance the oxygen balance of the energetic compounds, which is conducive to energy release. However, excessive introduction of nitro groups could also lead to problems such as increased sensitivity, complex synthetic process and low yield [18]. In addition, there is an upper limit on the energy density theory of CHON-based energetic compounds represented by nitro groups. For example, hexanitrohexaazaisowurtzitane (CL-20) exhibits high detonation performance and energy density. However, to further improve its performance, its synthetic strategy needs to be changed to break through the energy limit of CHON-based energetic materials [19,20].
The energy density of energetic materials can be effectively increased by forming NO coordination bonds through the oxidation of N atom in the heterocyclic aromatic framework. The formation of N→O bonds at a suitable position in the heterocyclic aromatic ring could not only enhance the energy density but could also improve the oxygen balance of energetic compounds. More importantly, the N→O bond with the
characteristics of a coordination double bond could eliminate the electron
repulsion of nitrogen in a N-heterocyclic system and promote σ–π orbital
separation to stabilize molecules [21,22]. To study the effect of an N→O bond on the structures and properties of the
energetic compounds, Lai Weipeng et al. employed the density functional theory
to conduct theoretical calculations of pyrazines, 1,2,3,4-tetrazines,
tetrazinofuranzans, and their N-oxides [23]. The results showed that the N→O bond shortens the length of the C–C bond in the
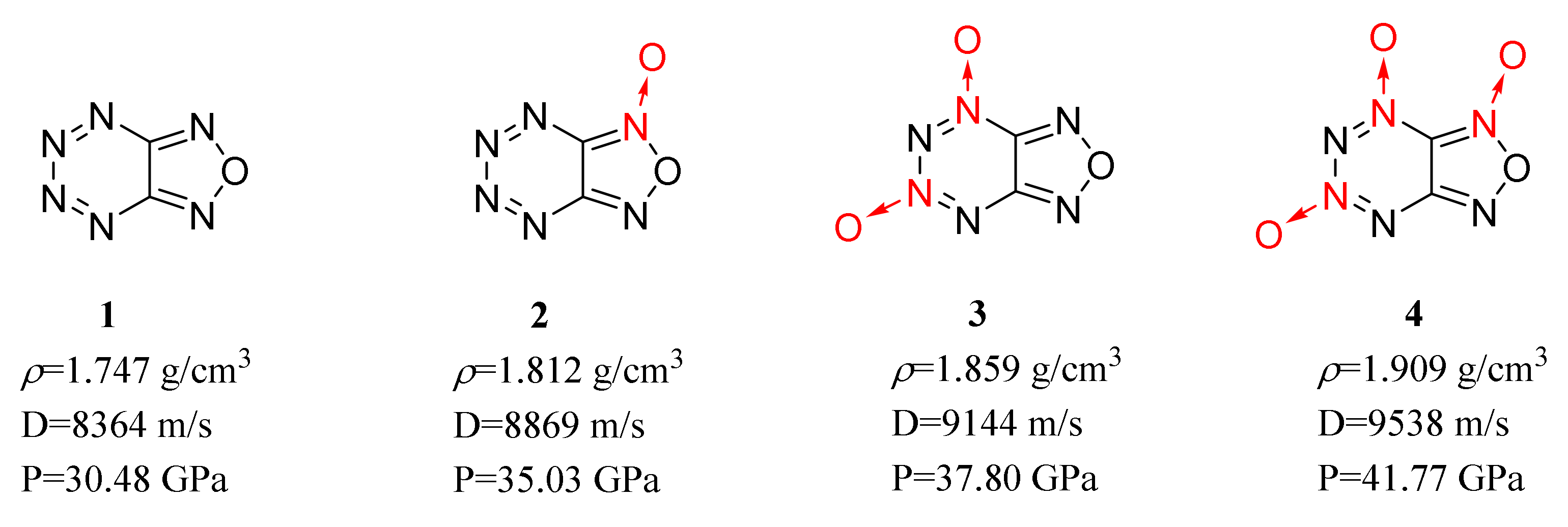
ring, lengthens the C–N bond close to the N→O bond and improves the detonation performance of most energetic compounds. It was also found that the improvement is positively correlated with the number of N→O bonds in the energetic compounds (Figure 1).
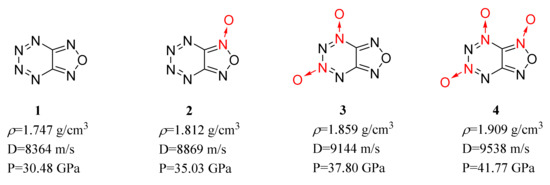
Figure 1.
Calculated performance of compounds 1–4.
Using the N-heterocyclic skeleton as the precursor, an N→O coordination bond could be directly formed by the oxidation of hypochlorous acid (HOF), H2O2/H2SO4 and other oxidation systems. Most of the N-heterocyclic aromatic frameworks could be converted to N-oxides through direct oxidation, yet N-heterocyclic skeletons require certain energy and the corresponding oxidation systems need to be adopted [24]. In the process of constructing heterocyclic skeletons, an N→O coordination bond could be directly generated via the cyclization reaction, thereby bypassing the step of oxidation, shortening the reaction process, and improving production efficiency.
The synthetic methods towards energetic heterocyclic N-oxides, which are simultaneously synthetized during cyclization, are elaborated in this study. The N-oxides could be classified into nine categories based on the structural characteristics of the N-heterocyclic aromatic skeleton. The categories are: 1,2,3,4-tetrazine-1,3-dioxide, 1,2,3,5-tetrazine-2-oxide, 1,2,3-triazine-3-oxide, 1,2,3-triazine-2-oxide, pyridazine-1,2-dioxide, pyrazine-1-oxide, 1,2,5-oxadiazole-2-oxide (furoxan), pyrazole N-oxide, and triazole N-oxide. Some typical cyclization reaction mechanisms are also discussed. The physicochemical properties and detonation performance of the N-oxides are introduced. Direct synthetic strategies towards N-oxides through cyclization reaction would provide a theoretical guidance for the design and development of new energetic compounds.
2. Energetic Heterocyclic N-Oxides via Different Cyclization Reaction
2.1. Azine N-Oxides
2.1.1. 1,2,3,4-Tetrazine-1,3-Dioxide
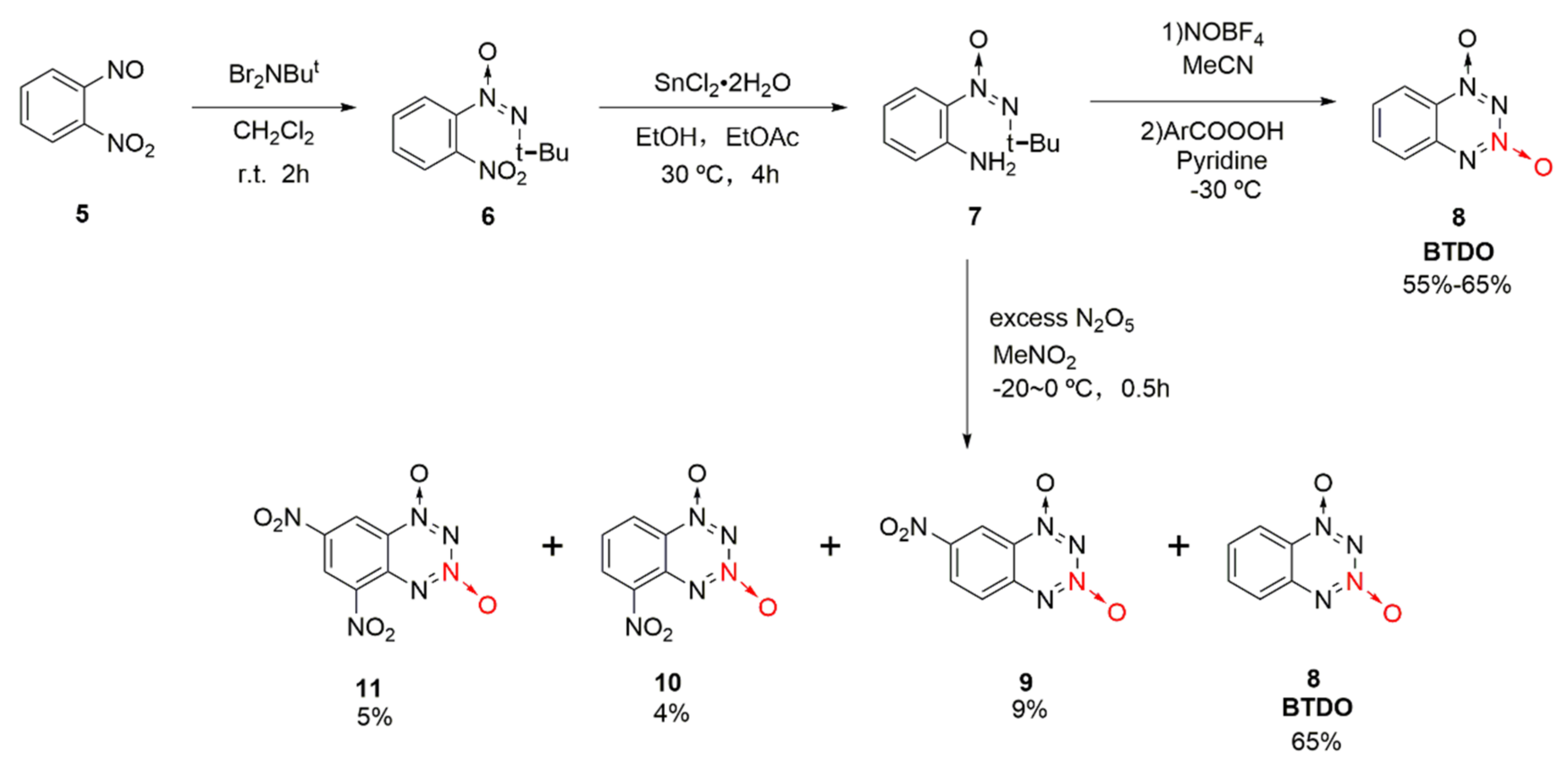
1,2,3,4-Tetrazine-1,3-dioxide was originally derived from 1,2,3,4-tetrazine compounds. According to Churakov et al. [25,26], converting tetrazine into its N-oxide might improve its energetic properties, while the benzene ring was introduced to increase the stability of the tetrazine N-oxide. Benzo-1,2,3,4-tetrazine dioxide (8, BTDO) was synthesized by diazotization or nitration from an intermediate O-tert-butyl-NNO-azoxyaniline (7) [27]. This method is also applicable for the synthesis of 1,2,3,4-tetrazine dioxide. Using o-nitrosonitrobenzene (5) as the raw material, and the intermediate 7 was obtained by a two-step reaction. First, nitroso was transformed into tert-butyl-NNO-azoxy group under the action of N, N-dibromo -t-butylamine. Then, the nitro group was reduced to amino group by SnCl2 to produce the intermediate 7 with a yield of 77%. BTDO was obtained by the diazotization oxidation reaction of intermediate 7 with NOBF4 and benzoic acid peroxide or direct nitrification reaction with excess N2O5. By direct nitration, several nitro-substituted benzo-1,2,3,4-tetrazine dioxides have been obtained via the nitration of benzene in excessive N2O5 (Scheme 1 9–11).
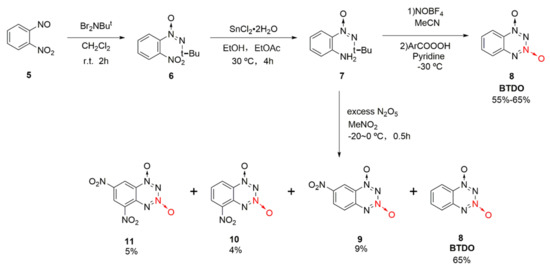
Scheme 1.
Synthetic route for BTDO.
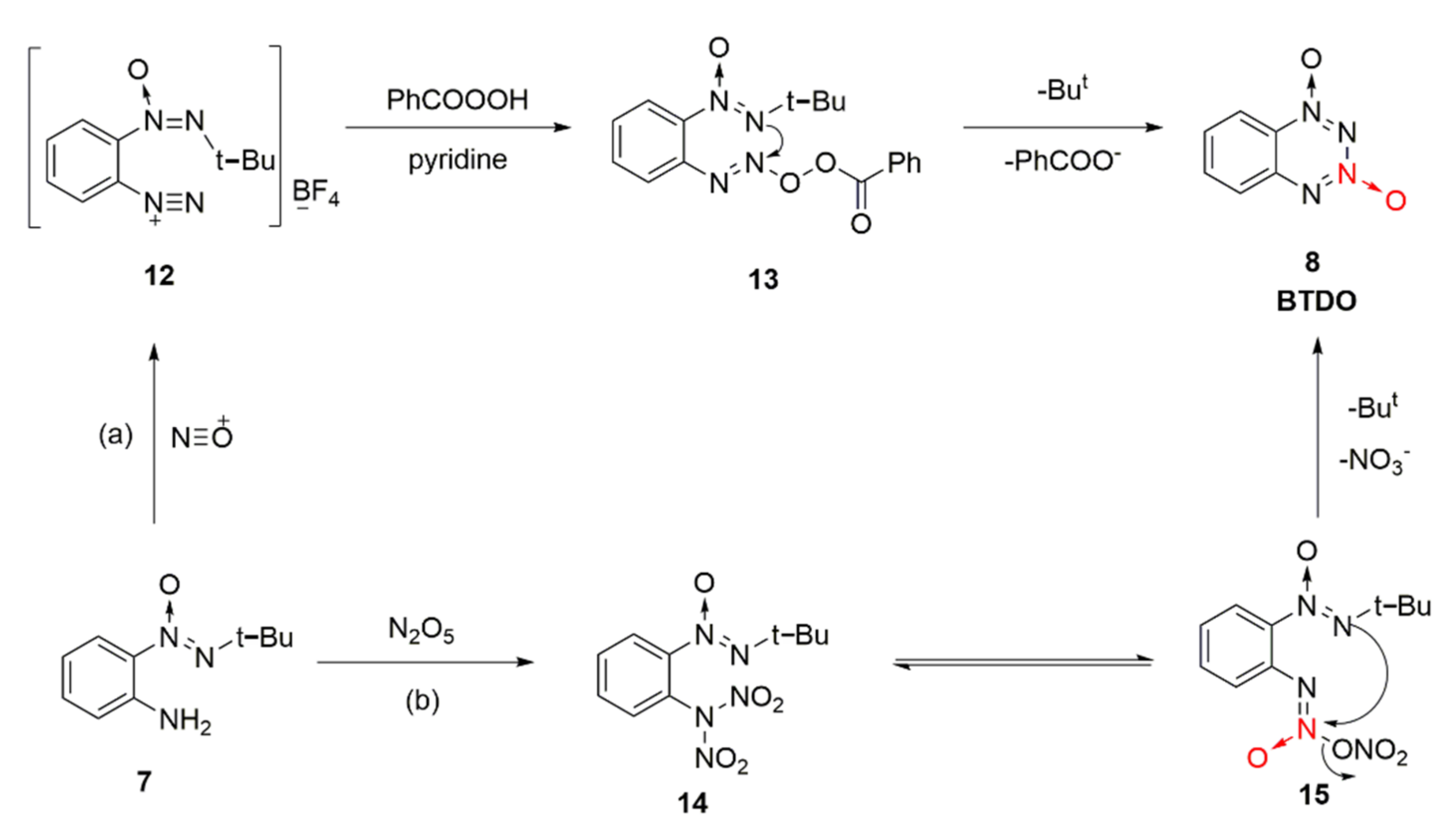
For the synthesis reaction of BTDO by diazotization oxidation and nitration, the reaction mechanism was also examined [25]. Based on the diazotization reaction of nitroso positive ions from NOBF4 and amino group on the benzene ring of intermediate 7, diazonium salt intermediate 12 was generated, and an unstable intermediate 13 formed from 12 and benzoic acid peroxide. The intermediate attacked the N atom connected with tert-butyl to the N atom connected with oxygen atom, and the product BTDO was finally obtained from intramolecular electron transfer after eliminating the benzoic acid anion and tert-butyl cation (Scheme 2a). An intermediate 14 of N, N-dinitroamine was formed by direct nitration of compound 7 using excess N2O5 as the nitrating agent. ONN-azoxy nitrate compound 15 was produced by intramolecular rearrangement. Similarly, the O atom connected with the tert-butyl group attacked the N atom connected with nitrate for addition elimination, and the product BDTO was obtained after removing the tert-butyl cation and nitrate ion (Scheme 2b).
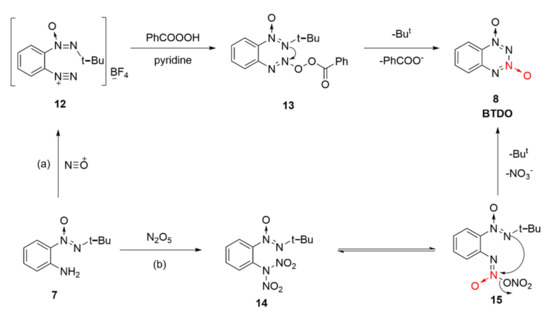
Scheme 2.
Different reaction mechanism (a) and (b) of BTDO.
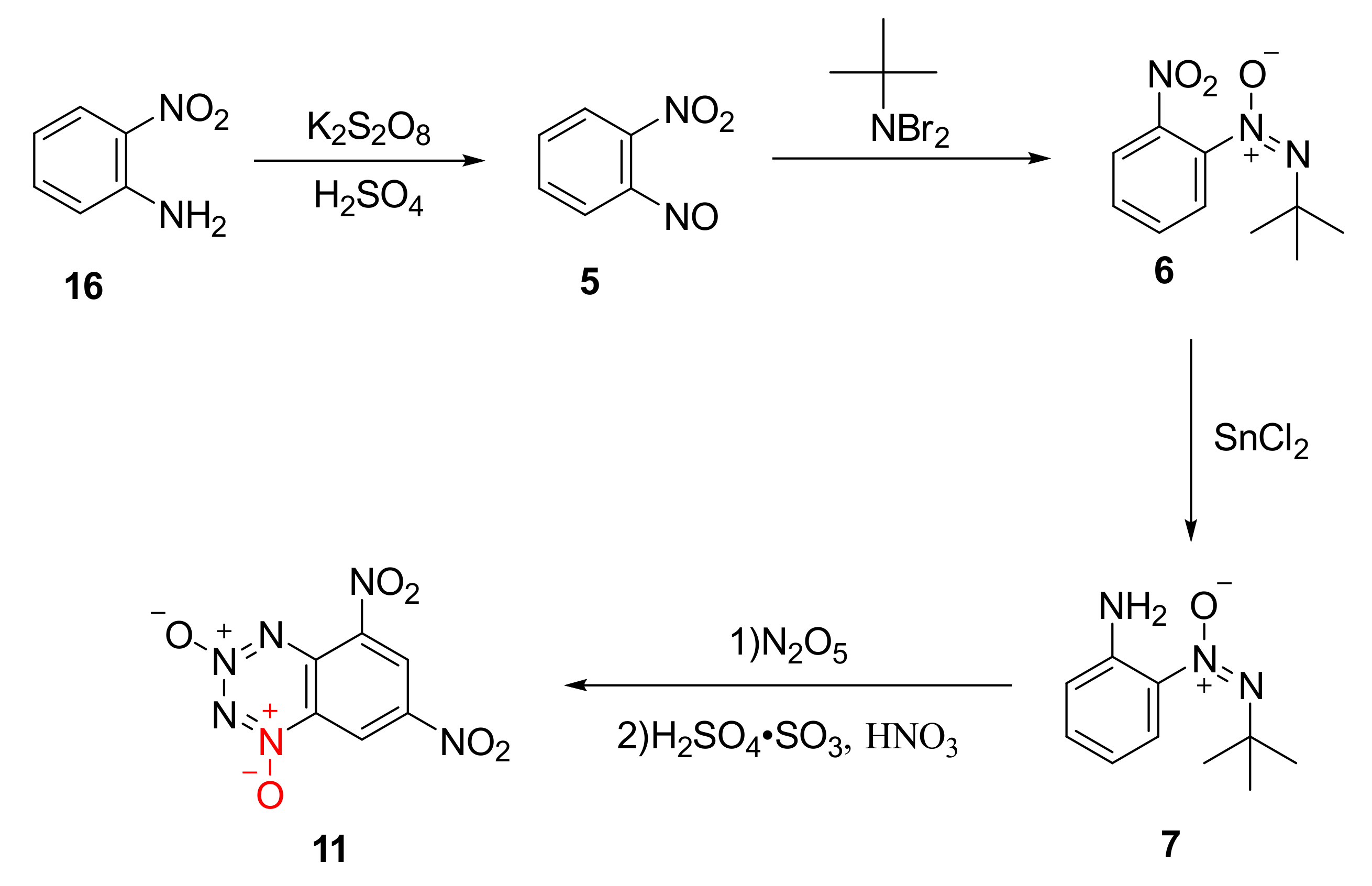
The by-product 11 was produced by nitrating compound 7 with N2O5 for further improving the detonation performance of the compound through introducing two nitro groups into the benzene ring. Using o-nitroaniline (16) as the raw material, compound 7 was synthetized by Klapotke et al. [28] as follows: The compound 5 was first obtained by the nitration of the amino group with potassium persulfate and sulfuric acid, and then compound 7 was formed by the condensation of dibromo-t-butylamine with nitroso group and a further reduction reaction using SnCl2. Using N2O5/dichloromethane as the nitration system, compound 11 as yellow crystal was finally synthetized (Scheme 3). The density of compound 11 was 1.896 g/cm3, and its sensitivity (IS = 5 J) was higher than that of RDX (IS = 7.5 J). Based on theoretical calculation, its detonation performance (D = 8411 m/s, P = 33.0 GPa) was equal to that of RDX (D = 8748 m/s, P = 34.9 GPa).
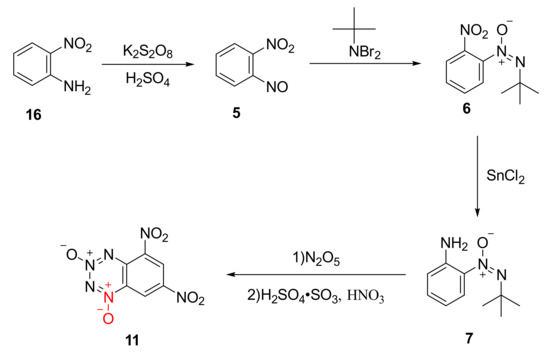
Scheme 3.
Synthetic route for compound 11.
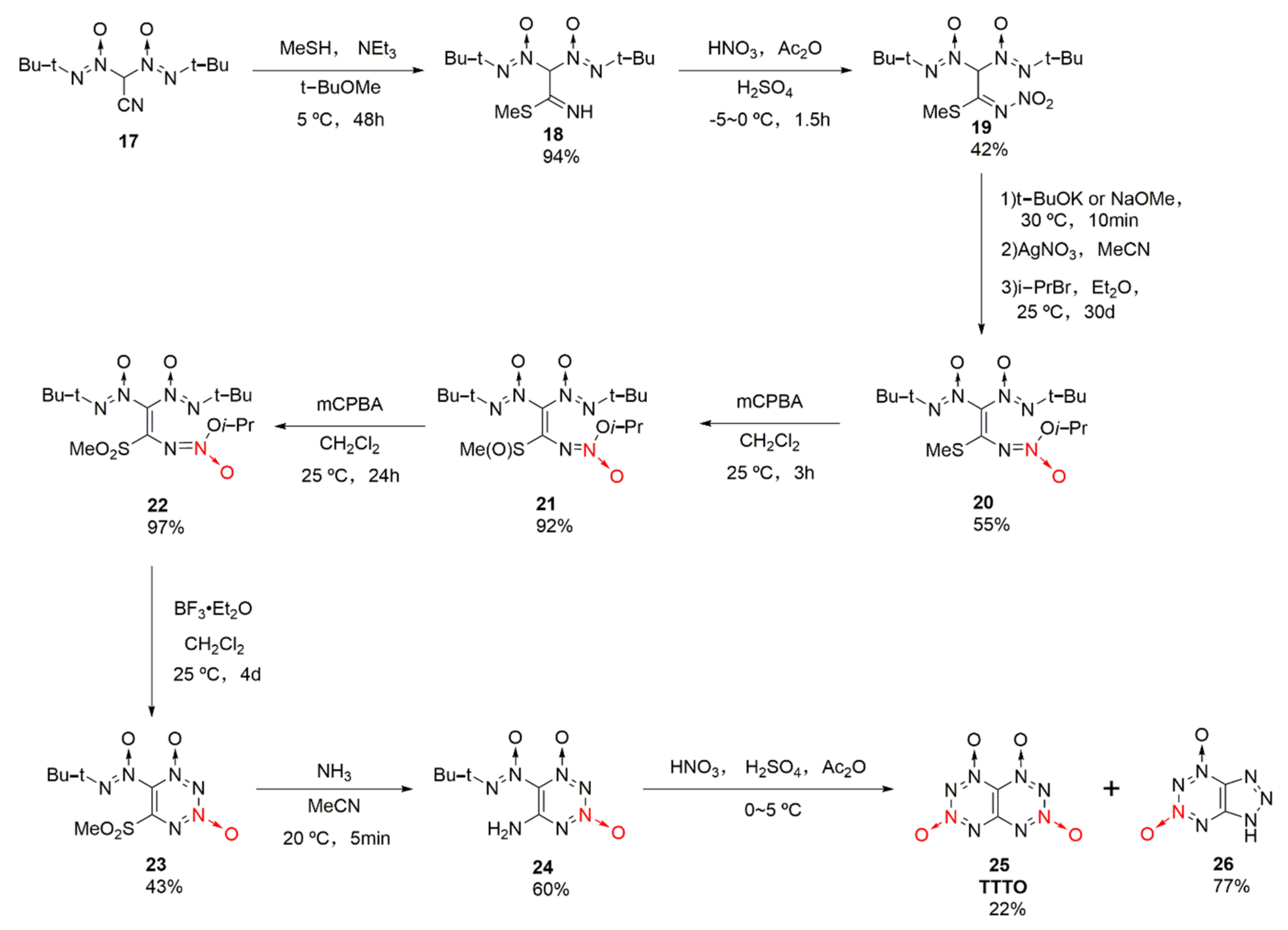
A large number of theoretical calculations [29,30,31,32] showed that [1–4]tetrazino [5,6-e][1–4]tetrazino-1,2,3,4-tetraoxide (25, TTTO) is an energetic material with a high energy density (ρ = 1.899 g/cm3) and high detonation performances (D = 9710 m/s, P= 43.2 GPa), superior to CL-20 (D = 9420 m/s, P= 42.0 GPa). Its symmetrical “butterfly” structure has also attracted extensive attention from researchers. Using 2,2-bis(tert-butyl-NNO-azoxy)acetonitrile (17) as the raw material, TTTO was synthetized by Klenov et al. [33] based on a 10-step synthetic strategy with a total yield of 1.1%(Scheme 4). The synthesis process was too complex and the yield was too low, and chromatographic separation was required for purifying key intermediates. Therefore, yet the synthesis of TTTO has great theoretical value, there is currently no prospect for industrial applications.
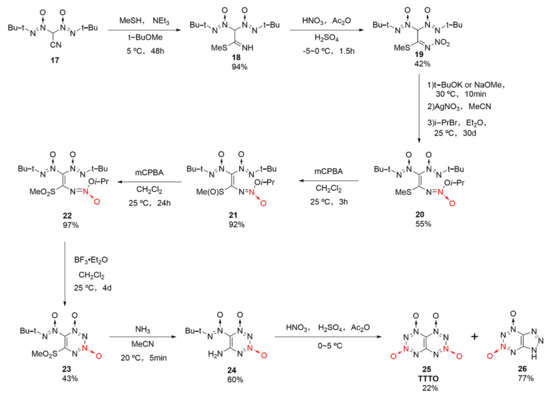
Scheme 4.
Synthetic route for TTTO.
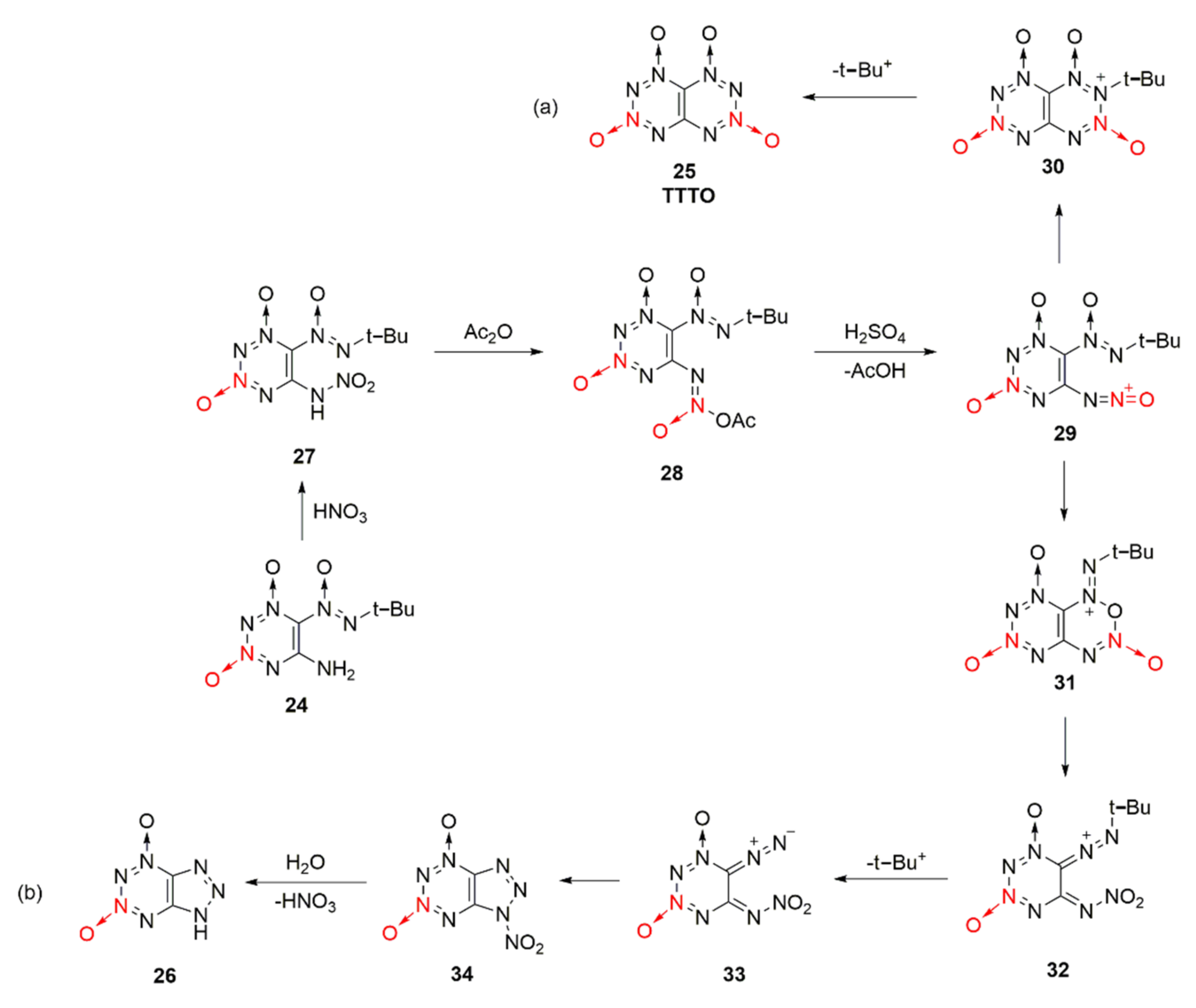
The reaction process of compound 24 forming TTTO and by-product 26 was also reasonably speculated [33]. Under the action of nitric acid, the mononitroamine compound 27 was generated from compound 24, and compound 28 created by further acetylation of the acetic anhydride. Compound 28 was protoned in sulfuric acid after removing an acetic acid molecule to form oxidized diazonium ion intermediate 29, which could be cyclized in two different ways. One was to conduct electron transfer between the oxidized diazo positive ion and the N atom connected with tert-butyl group, and then TTTO was formed by cyclization after removing the tert-butyl positive ion (Scheme 5a). For the other method, compound 31 was obtained by cyclization based on the nucleophilic attack of the O atom in the oxidizing diazo positive ions. However, compound 31 was unstable, so diazo ketone compound 33 was obtained after the ring-opening reaction of 31 and by removing the tert-butyl positive ions. Eventually, by-product 26 was produced by further cyclization and hydrolysis (Scheme 5b)).
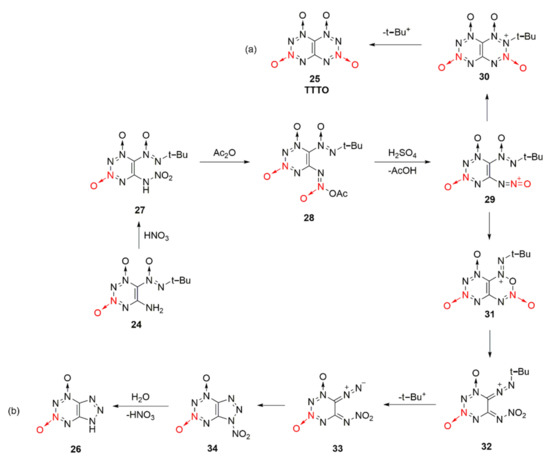
Scheme 5.
Possible synthetic routes (a) and (b) for compound 26 and TTTO.
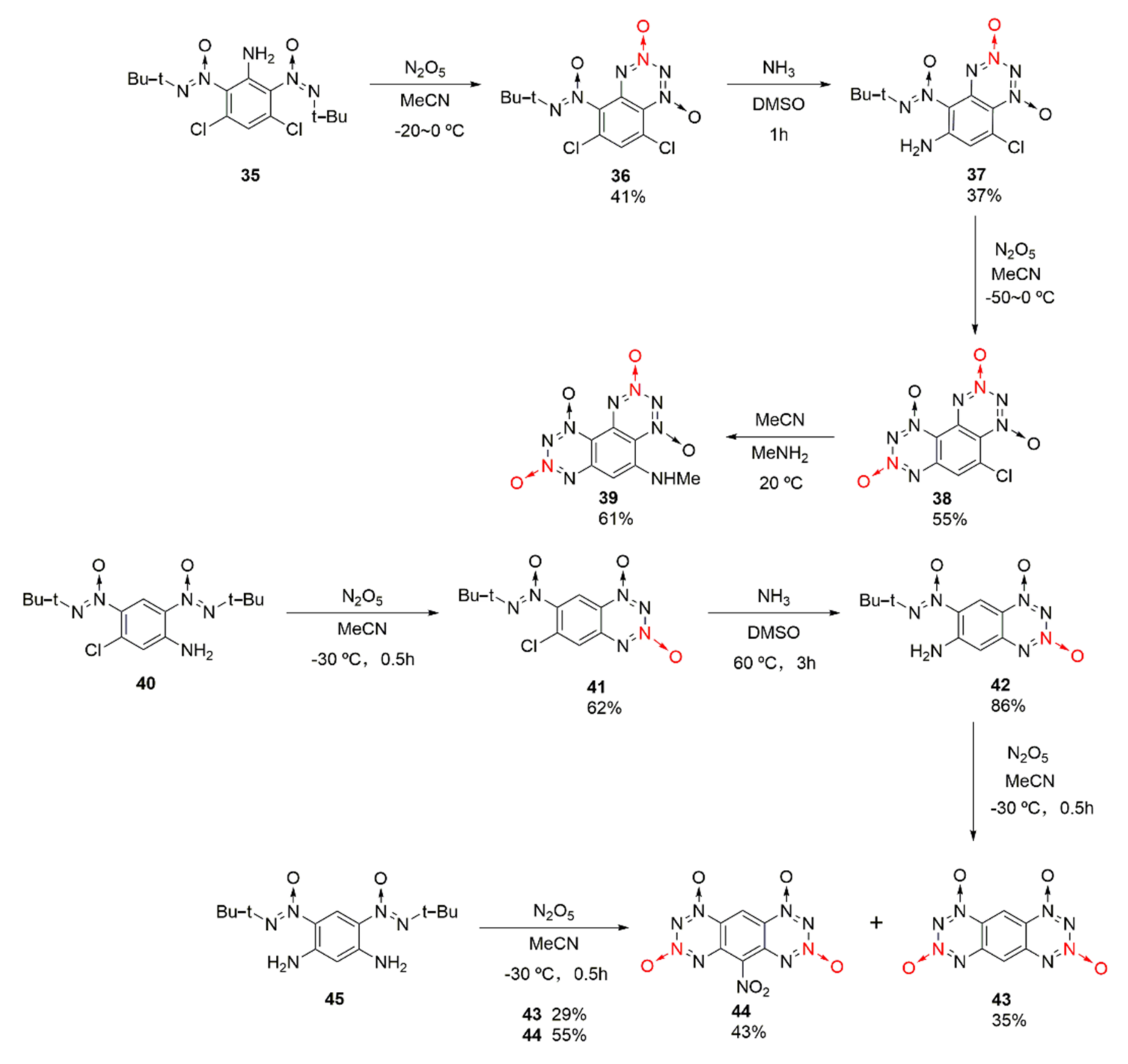
Based on the structuring idea of using anthracene ring and phenanthrene ring, two fused tetrazine dioxides, 1,2,3,4-tetrazino [5,6-f]benzo-1,2,3,4-tetrazino-1,3,7,9-tetraoxide (38) and 1,2,3,4-tetrazino [5,6-g]benzo-1,2,3,4-tetrazino-1,3,7,9-tetraoxide (42), were synthetized by Frumkin et al. [34,35] from different raw materials. Using compound 35 as the starting material, compound 36 was obtained in the first cyclization reaction using nitration with nitric anhydride. Then, compound 38 was obtained by amino substitution and the second cyclization reaction via nitric anhydride. The chlorine atom on compound 38 was easily replaced by some nucleophiles to form other derivatives, such as compound 39, and the properties of the substituted compounds were improved, such as a higher thermal decomposition temperature (Td = 210 °C) than that of compound 38 (Td = 140 °C). Using compound 40 as the starting material, compound 43 was synthetized through nitration using nitric anhydride, amino substitution, and secondary nitration-cyclization. By-product 44 with a nitro group on the benzene ring would also be produced. Meanwhile, compound 43 could be synthesized by one-step nitration from compound 45(Scheme 6).
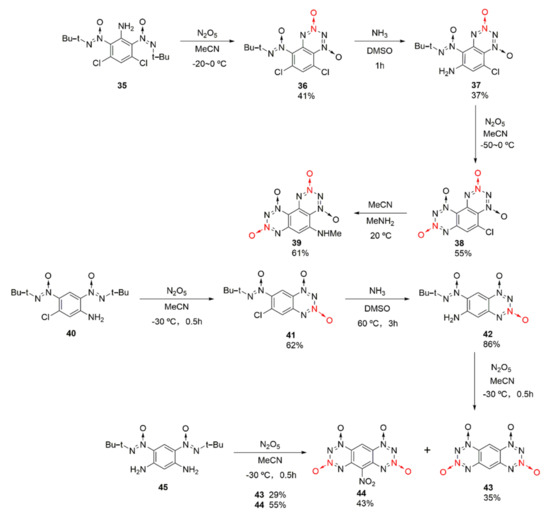
Scheme 6.
Synthetic routes for compounds 38 and 43.
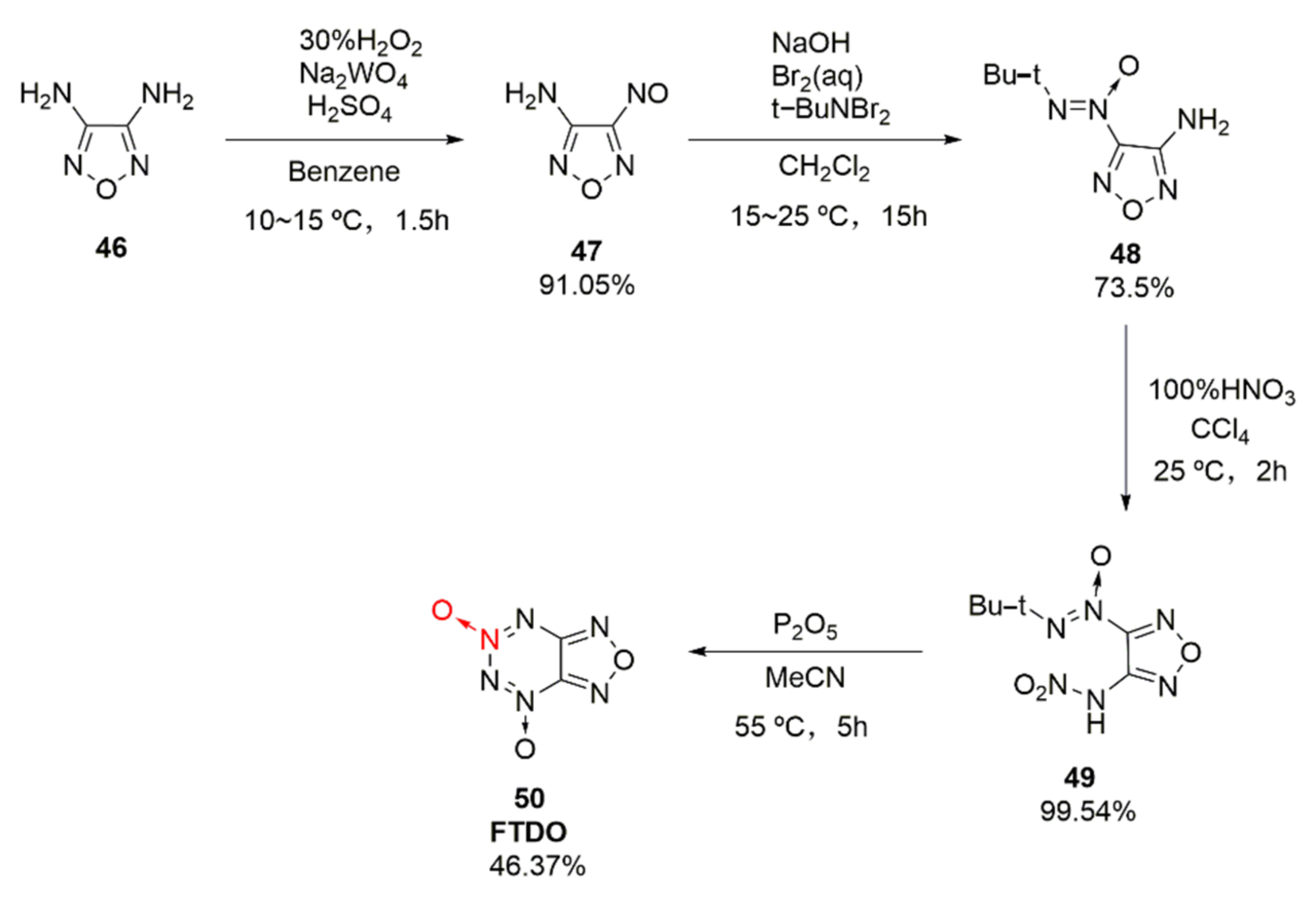
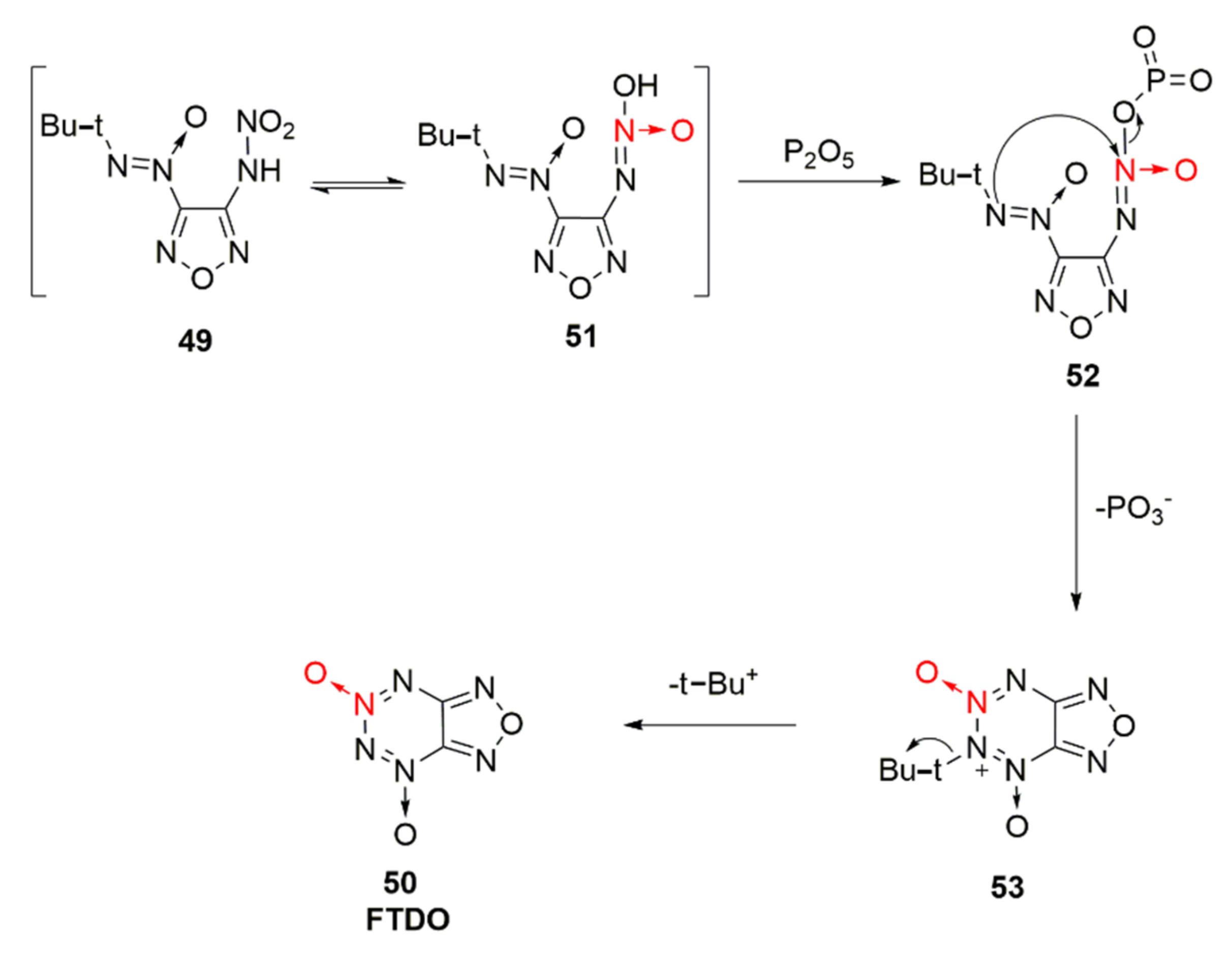
Based on the fusing furazan with tetrazine dioxide, an energetic compound furazano [3,4-e]-1,2,3,4-tetrazine-4,6-dioxide(50, FTDO) was synthetized by Churakov et al. [36] for the first time. FDTO displays a broad prospect with a measured density of 1.85 g/cm3, formation enthalpy of 4.23 MJ·kg, theoretical detonation velocity of 9802 m/s, and explosion pressure of 44.78 GPa, arousing widespread research interests [37,38]. It was synthesized through the nitration-cyclization of compound 48 by using tetrafluoroborate nitrate as the nitration reagent. Yet, the price of tetrafluoroborate nitrate was too expensive, and the nitrated product was difficult to purify. Using 3,4-diaminofurazan (46) as the raw material, compound 48 was obtained by Li Xiangzhi et al. [39] via oxidation and condensation, followed by the nitration of 100% HNO3 and cyclization of P2O5, leading to the high-purity product FDTO (Scheme 7). The effects of N2O5, NO2BF4, 100% HNO3, etc. nitration systems on the product and purity were also compared. It was concluded that using 100% HNO3 as the nitrating agent, a maximum yield of 99.54% and purity of 99.18% could be achieved.
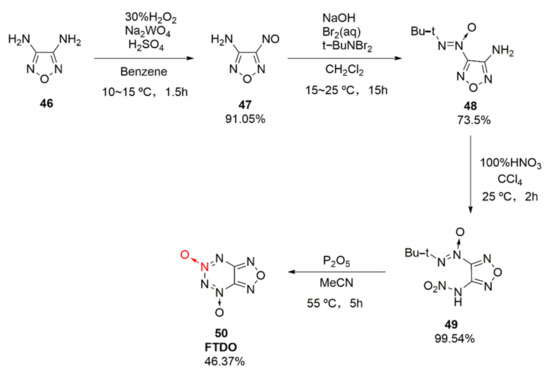
Scheme 7.
Synthetic route for FTDO.
In this reference [39], the cyclization mechanism under the action of P2O5 was discussed (Scheme 8). The nitroamino group in compound 49 was isomerized to produce compound 51 containing a N-hydroxyl group. P2O5 was esterified with the hydroxyl group on compound 51 to produce 3-(tert-butyl-NNO-azoxy)-furazan-4-azoxy alcohol phosphite (52). The N atom connected with tert-butyl group in compound 52 attacked the nucleophilic N atom connected to phosphite. Moreover, the metaphosphate group was removed while electron transfer was performed. The tert-butyl positive ion was then removed to finally obtain FDTO.
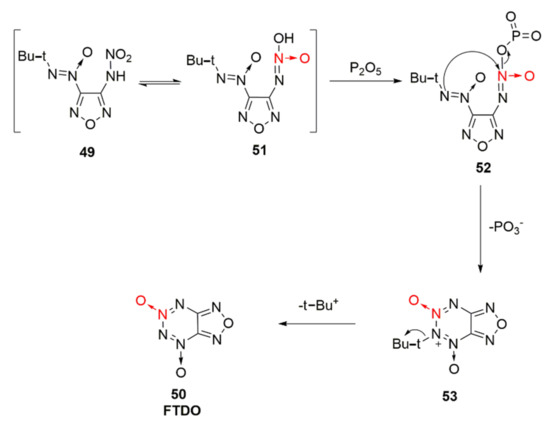
Scheme 8.
Cyclization mechanism of compound 49 under the action of P2O5.
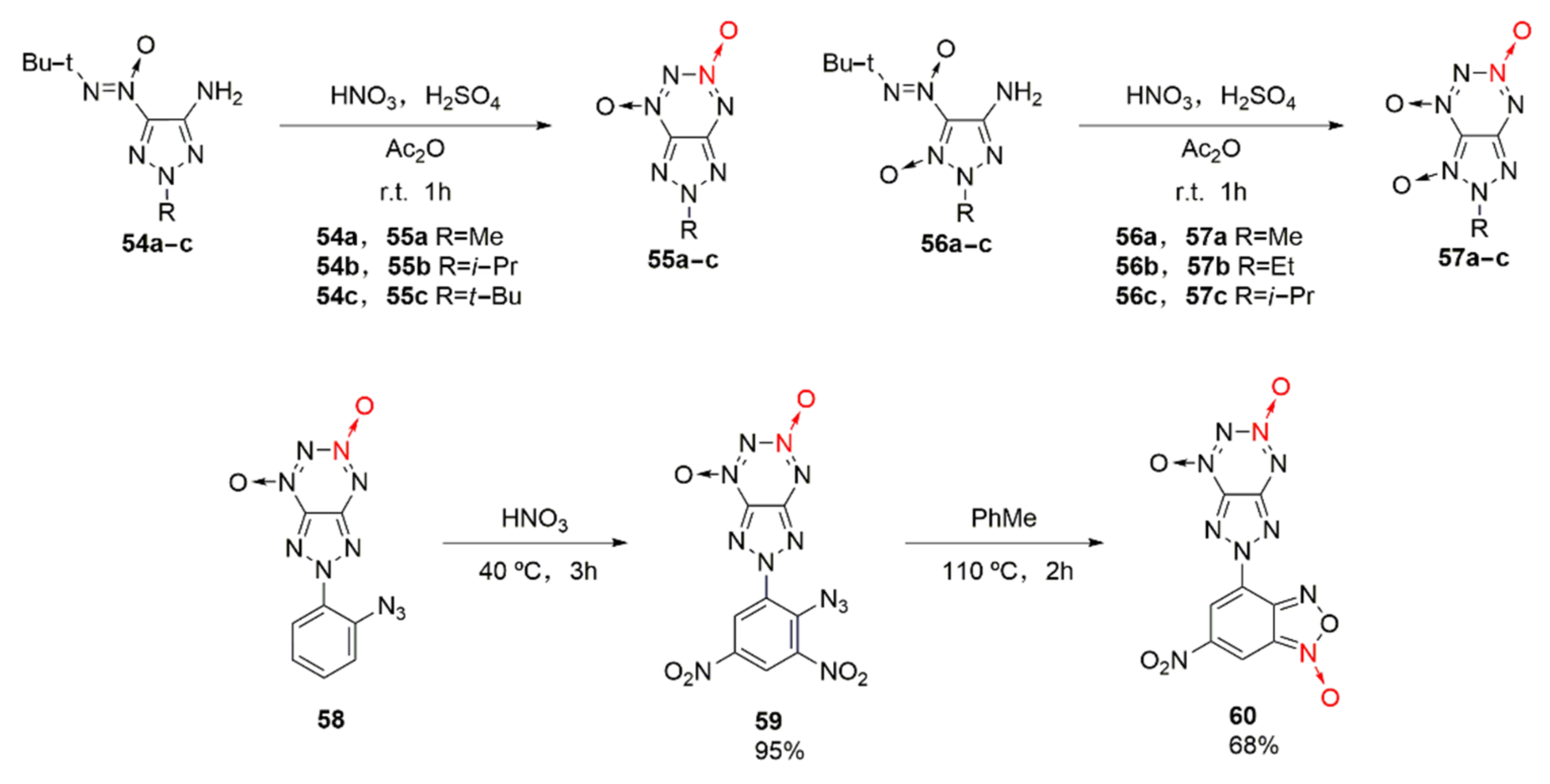
Although the fusion of a five-membered furazan ring with tetrazine dioxide has achieved great success, its low decomposition temperature of 112 °C for FTDO still limits its application as an energetic material [40]. Based on the fusing the five-membered triazole ring with tetrazine dioxide, after introducing alkyl into the triazole ring, a series of triazole tetrazine dioxides (Scheme 9 55a-c, 57a-c) with stable thermal properties were obtained by Voronin et al. [41]. Energetic compounds with excellent detonation properties were also obtained by introducing energetic groups (-N3, -NO2, others) into the triazole ring [42]. Among them, the thermal decomposition temperature of compound 55a is 199 °C, while that of compound 57a-c is within 208 ~ 230°C. Due to the introduction of a benzofurazan ring into the triazole ring, compound 60 as a potential energetic compound was further obtained by Shvets et al. [43]. It exhibits an energy density of 1.84 g/cm3, a thermal decomposition temperature of 190 °C, and a calculated enthalpy of formation of 1005 kJ/mol.
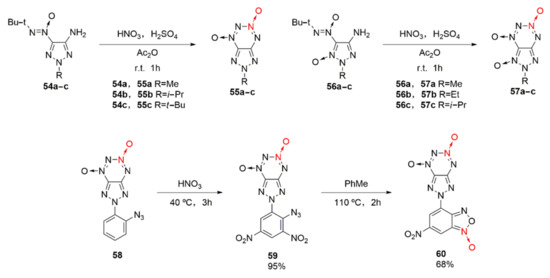
Scheme 9.
Synthetic routes for compounds 55a-c, 57a-c and 60.
2.1.2. 1,2,3,5-Tetrazine-2-Oxides
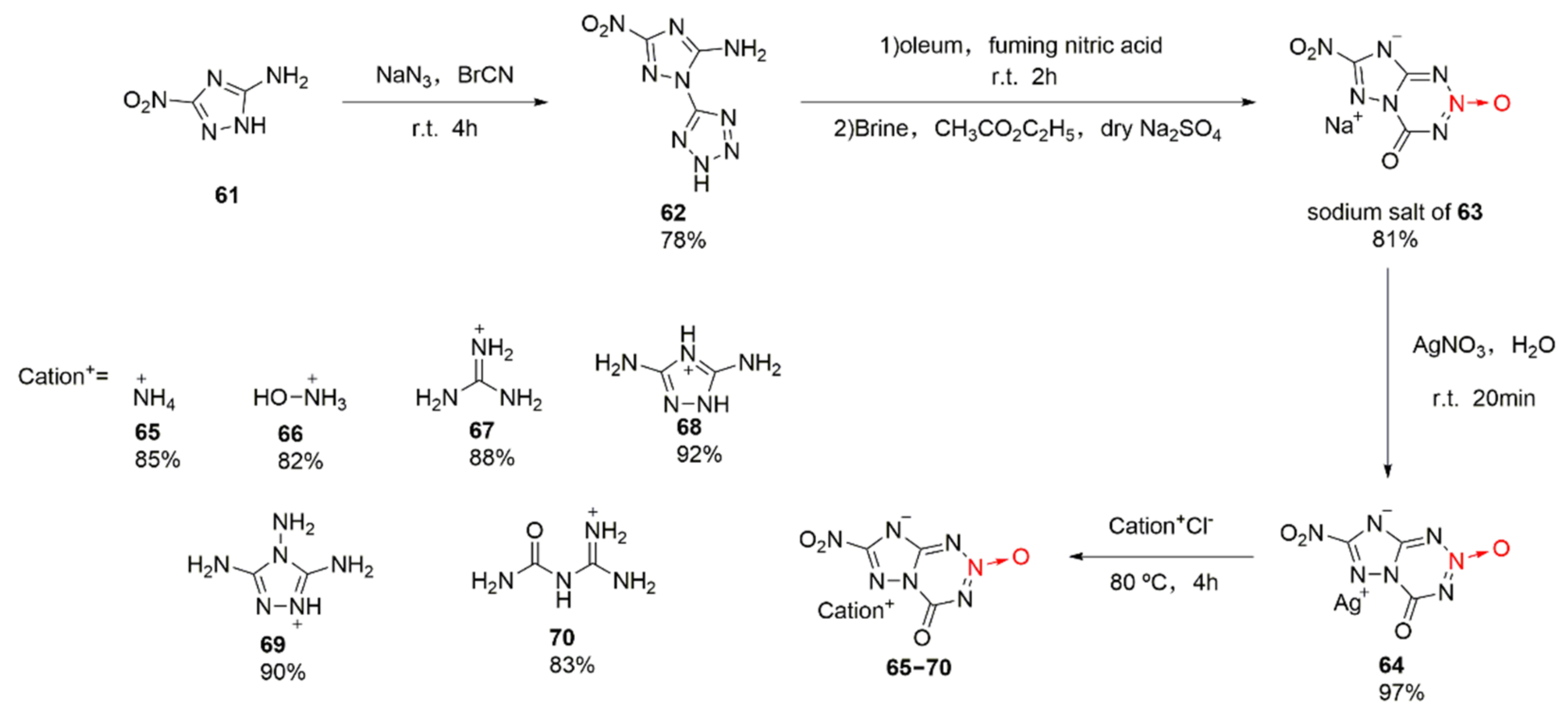
Bian Chengming et al. were the first to study the synthetic methods for 1,2,3,5-tetrazine-2-dioxide [44]. After fusing 1,2,3,5-tetrazine-2-dioxide with triazole, 7-nitro-4-one-4,8-dihydro-1,2,4-triazolo [5,1-d]-1,2,3,5-tetrazine-2-oxide (63), a series of energetic ionic salts were designed and synthetized (Scheme 10 65–70). Using 5-amino-3-nitro-1,2,4-triazole (61) as the raw material [45], a tetrazole ring was introduced under the action of cyanogen bromide and sodium azide to obtain compound 62. Nitric acid and fuming sulfuric acid were subjected to nitration and cyclization, and the sodium salt of compound 63 was obtained after extraction and washing with brine. At last, the energetic ionic salts 65–70 were obtained by an ion exchange reaction for the sodium salt of compound 63 and silver nitrate. Compounds 65–70 display good thermal stability, high density, high detonation performance, and low sensitivity (Table 1). Among them, compound 66 with a hydroxylaminium cation shows the highest potential to be used as an energetic material.
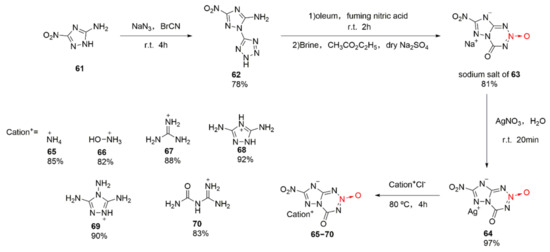
Scheme 10.
Synthetic routes for compounds 65–70.

Table 1.
Physiochemical properties and detonation parameters of energetic salts 65–70.
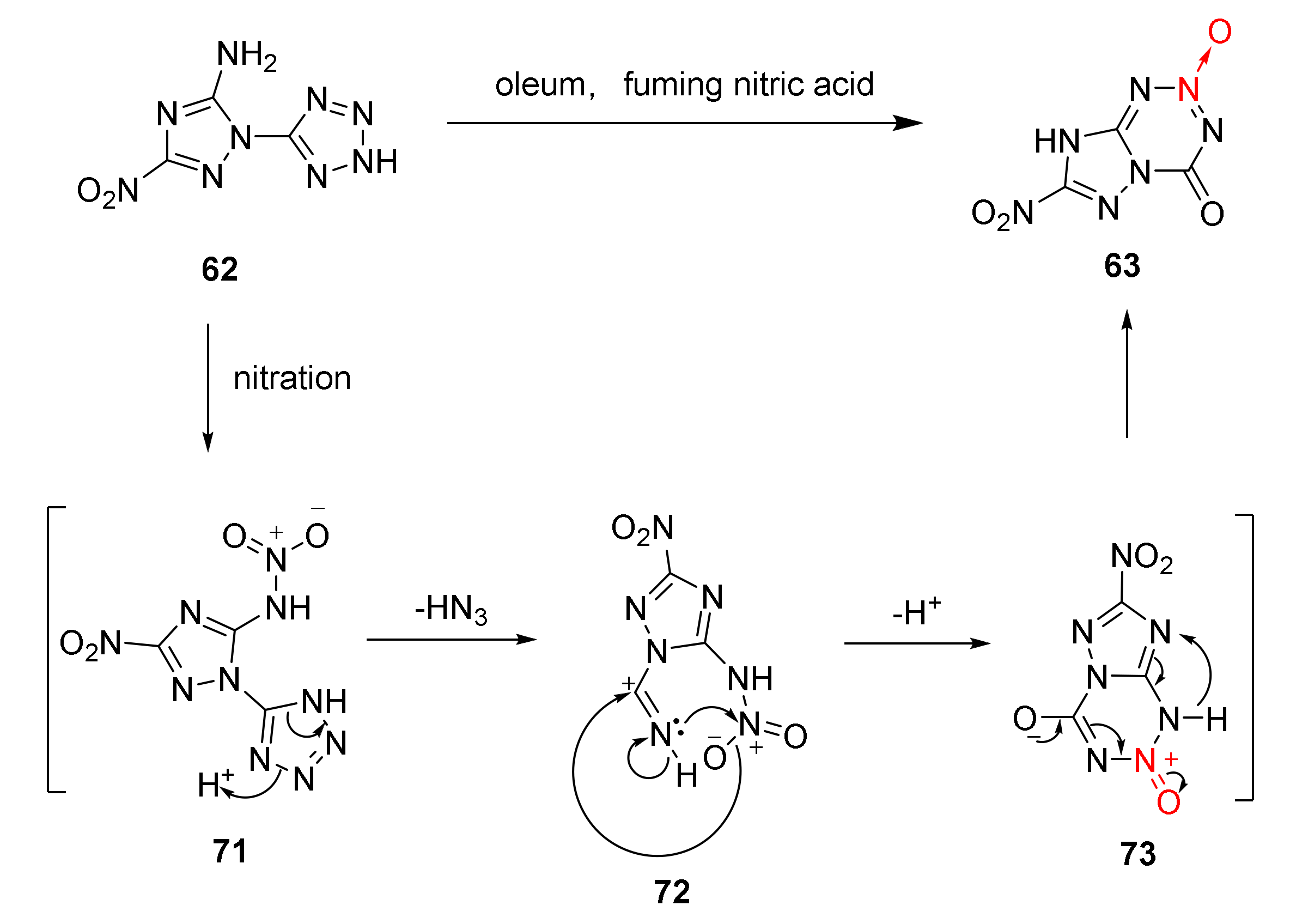
During the nitration reaction of 62, a white precipitate AgN3 was generated from the reaction of AgNO3 and the released gas, which was later verified to be azide acid. According to the infrared spectrum of compound 63, a strong peak appears at 1787 cm−1 and no obvious absorption peak is found at 3600~2500 cm−1. It was verified that the 4-position in compound 63 was acyl group rather than hydroxyl group. Based on the experimental facts, the cyclization mechanism of compound 62 was reasonably speculated [44]: Compound 62 was nitrated in the HNO3/H2SO4 mixed acid system to produce nitramine intermediate 71. The tetrazole in intermediate 71 carried out electron transfer. Then, a molecule of HN3 was removed and a C = N bond was created to form intermediate 72. The O atom on the nitro group initiated a nucleophilic attack on the C atom on the C = N bond, reducing the density of the electron cloud on the N atom of the nitro group to make it appear deficiently electronic. The N atom on the C = N bond attacked the N atom of the nitro group. With the electron transfer and proton removal, a tetrazine ring was basically formed for producing intermediate 73. The intermediate 73 was aromatized to perform electron and proton transfer, and the energetic compound 63 was finally obtained (Scheme 11).
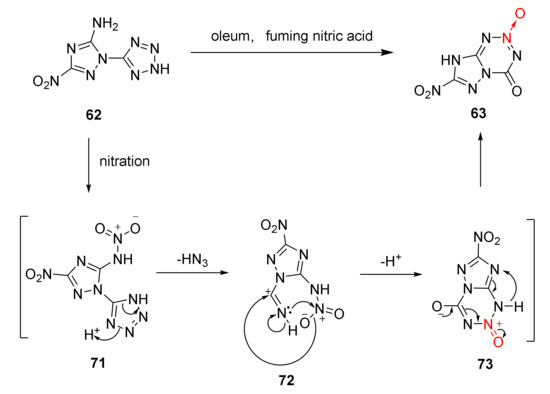
Scheme 11.
Proposed synthetic route for compound 63.
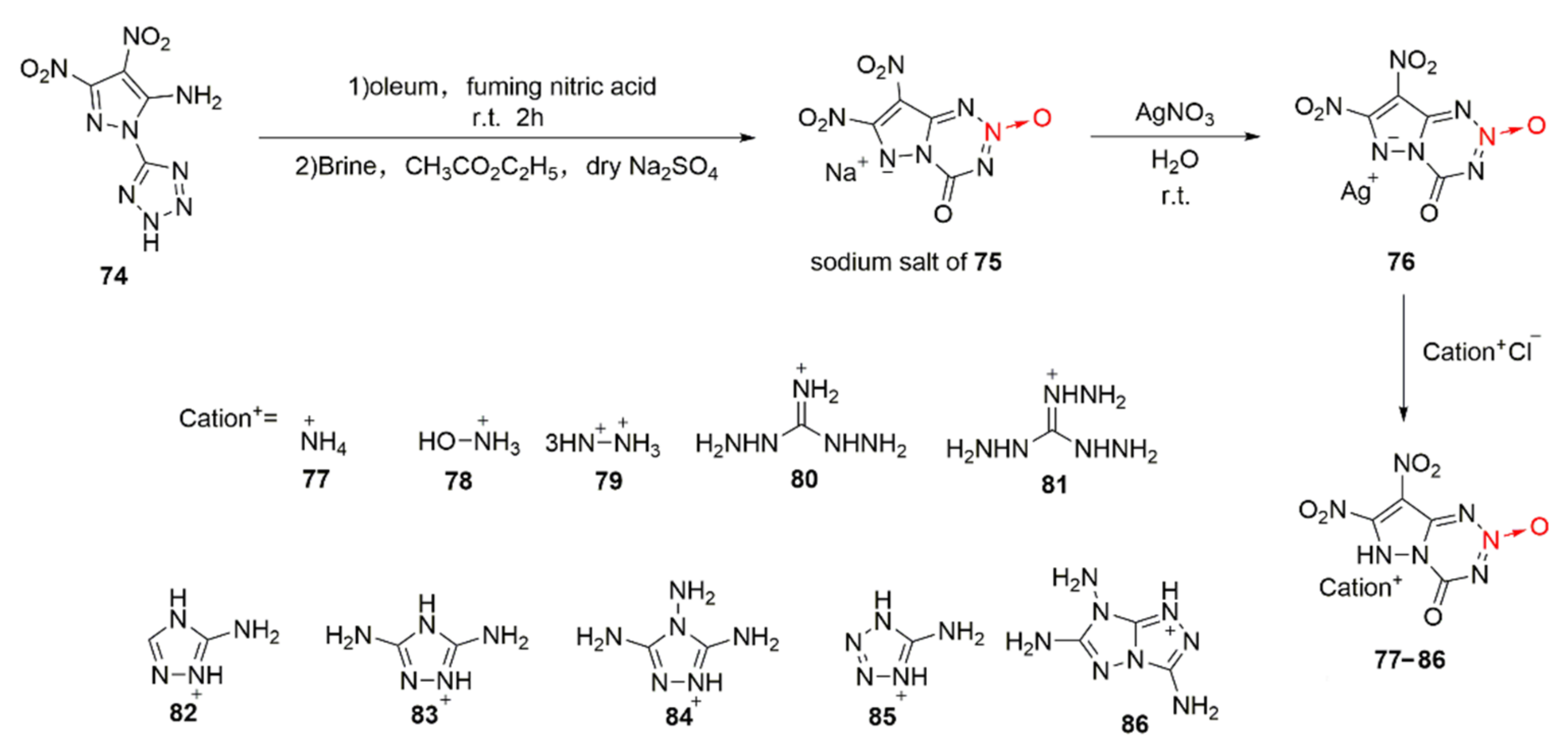
By replacing the triazole ring in compound 63 with a pyrazole ring and introducing another nitro group into the pyrazole ring, Zhao et al. [46] synthesized 7, 8-dinitro-4-keto-4,6-dihydropyrazolo [5,1-d]-1,2,3,5-tetraazine-2-oxide(75). Due to the acidity of 75, a series of energetic ionic salts (Scheme 12 77–86) was synthesized by the same ion exchange method. After analyzing and comparing the measured density with the theoretically calculated detonation performance, it was found that the hydroxylaminium salt of compound 78 also display excellent properties in all aspects. Its density and detonation performance (ρ = 1.95 g/cm3, D = 9228 m/s, P = 39.4 GPa) are similar to those of HMX (ρ = 1.91 g/cm3, D = 9186 m/s, P = 39.7 GPa) and low sensitivity is also presented (IS = 19 J, FS = 360 N).
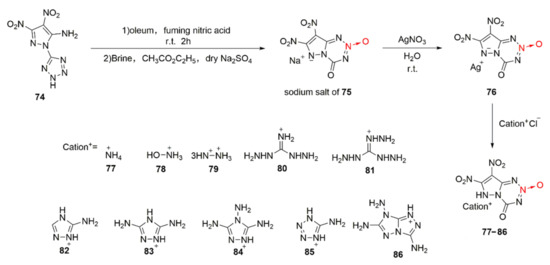
Scheme 12.
Synthetic routes for compounds 77–86.
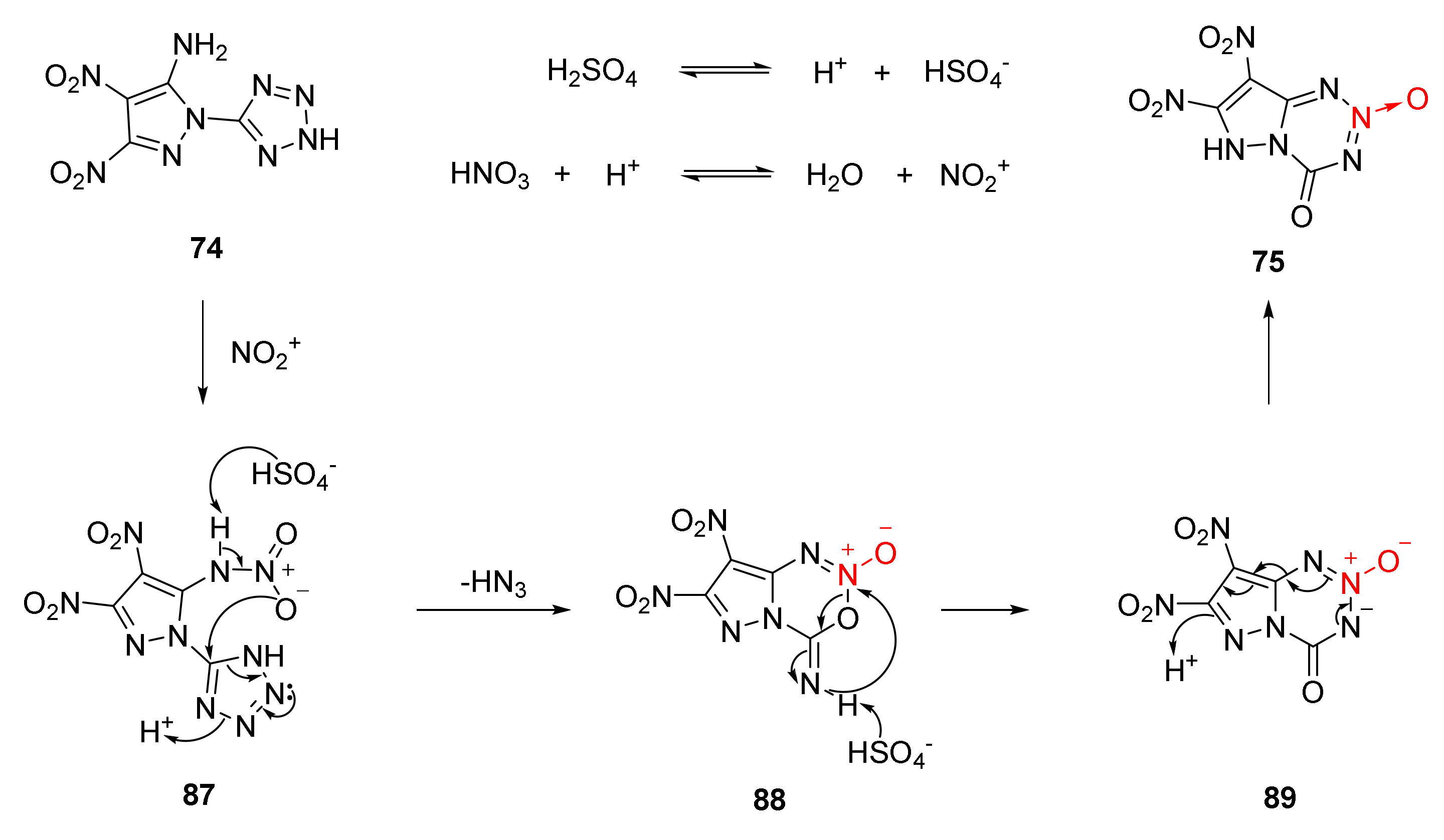
According to the experimental fact that gas was produced when nitration reacted with AgNO3 solution to obtain the white precipitate AgN3[46], the nitration-cyclization mechanism of compound 74 was reasonably described as follows (Scheme 13). The amino group (-NH2) on compound 74 first reacted with the NO2+ to obtain the nitroamine intermediate 87, and the nitroamine intermediate was unstable. After the H atom on the nitroamine group was captured by HSO4- in the system, the O atom on the nitro group attacked the C atom of the tetrazole ring, and the tetrazole ring carried out electron transfer, the intermediate 88 was obtained by removing a molecule of HN3 and cyclization reaction between the remaining C = N unit of the tetrazole ring and the nitro oxygen atom. The intermediate 88 was rearranged under the action of HSO4- to produce a fused ring intermediate 89 containing 1,2,3,5-tetrazine-2-oxide. Further aromatization was carried out to obtain the product compound 75 via intramolecular protons and electron transfer.
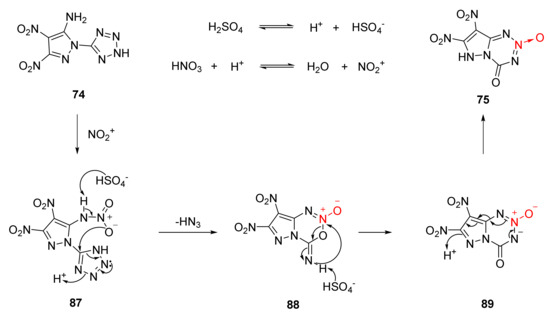
Scheme 13.
Proposed synthetic route for compound 75.
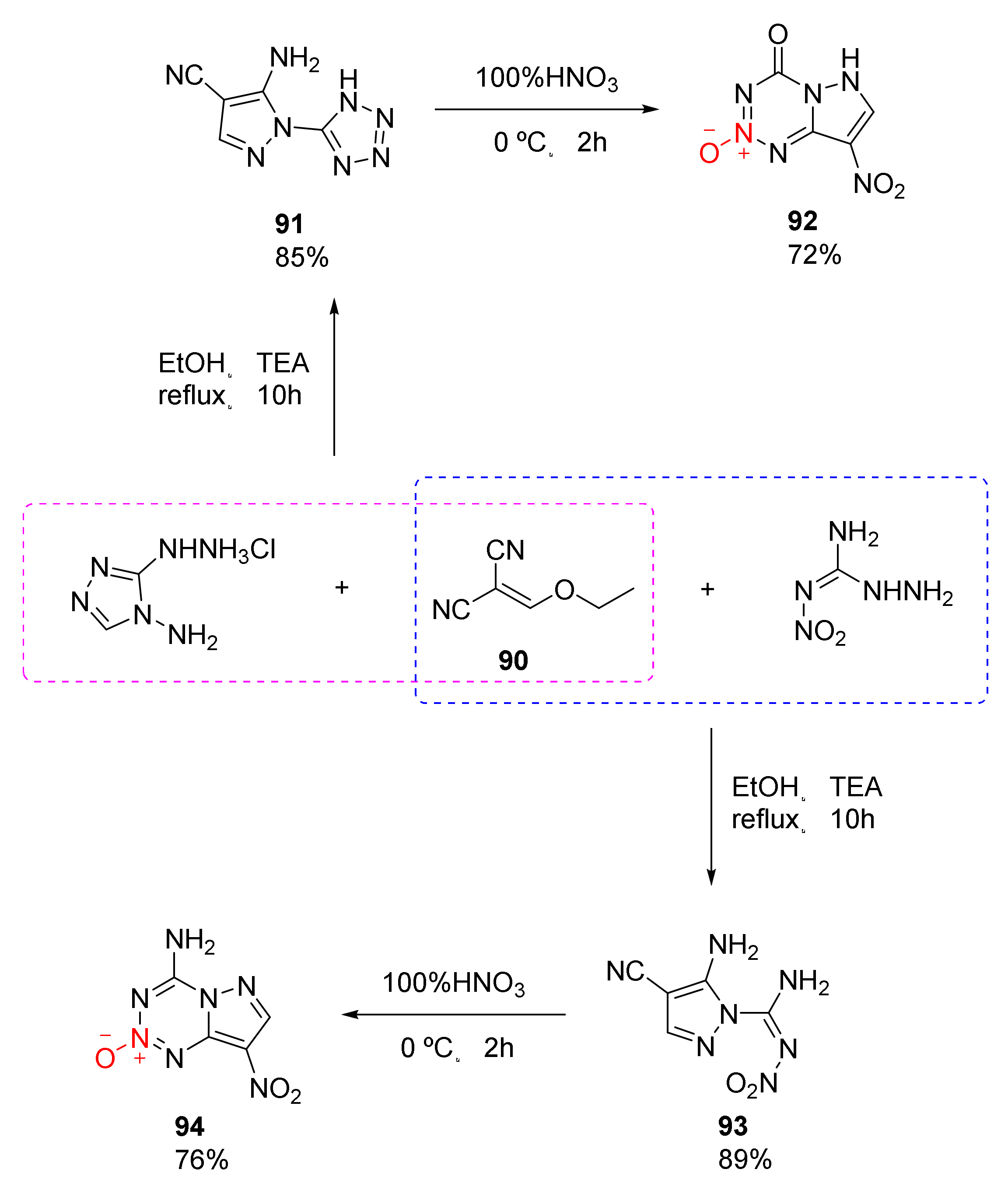
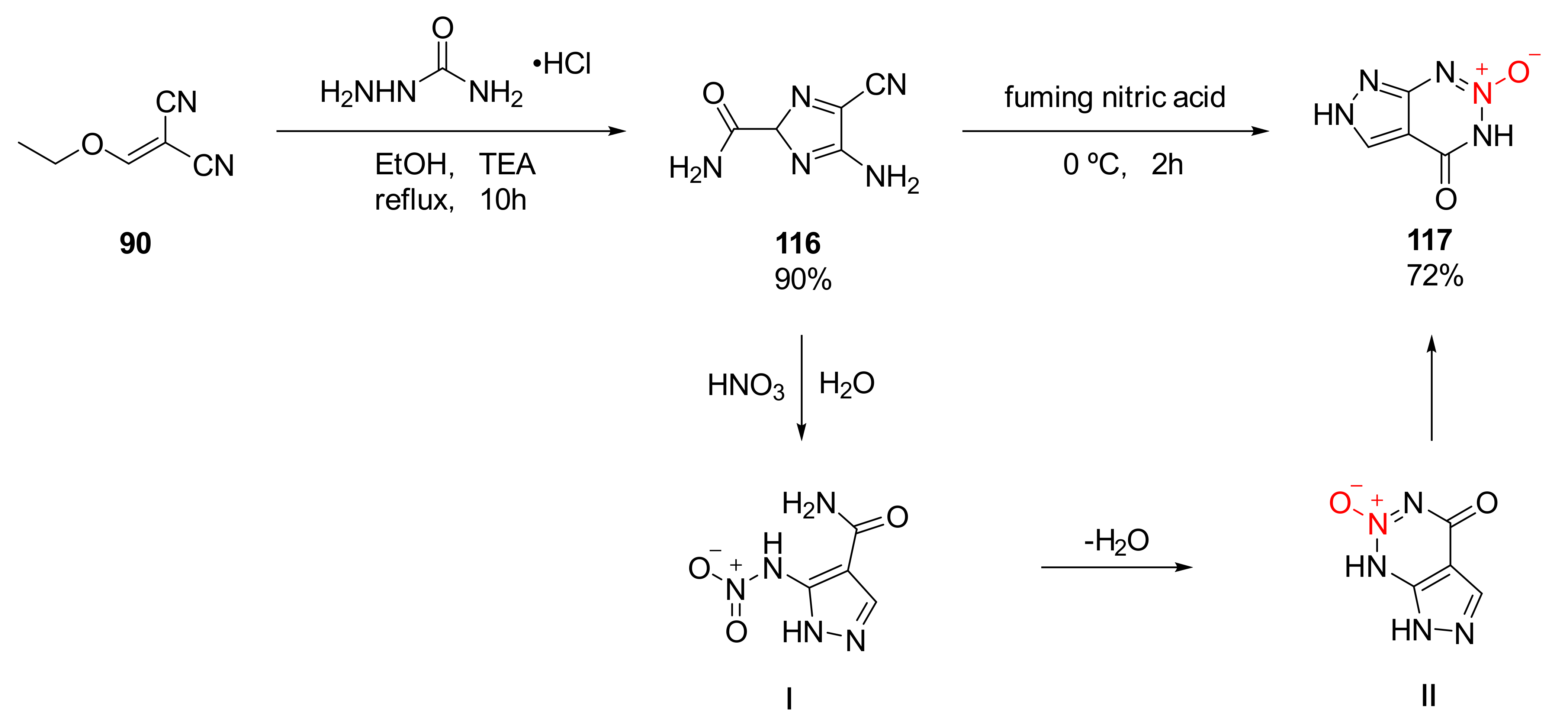
Compound 63 and compound 75 both exhibit excellent detonation properties, but the separation is difficult due to their strong moisture absorption. Moreover, the reagent required for the synthetic process is toxic and the gas produced during reaction is dangerous, limiting their applications to a great extent. An efficient and safe synthesis route for 1,2,3,5-tetrazine-2-oxide was independently developed by Lei et al. [47]. Based on the reactivity of the hydrazino group, two pyrazolo 1,2,3,5-tetrazine-2-oxides with no hygroscopicity, high density, excellent detonation performance, and low sensitivity were obtained (Scheme 14 92, 94). Using ethoxymethylene malononitrile (90) as the raw material, the two compounds 92 and 94 were synthesized via different cyclization and nitration. Based on theoretical calculation and practical analysis, compound 94 display high density (ρ= 1.874 g/cm3), high detonation velocity (D = 8983 m/s), high detonation pressure (P = 34.5 GPa), and low sensitivity (IS = 20 J, FS > 360 N). At the same time, its high thermal decomposition temperature (Td = 302 °C) endows it with the potential to become a heat-resistant explosive.
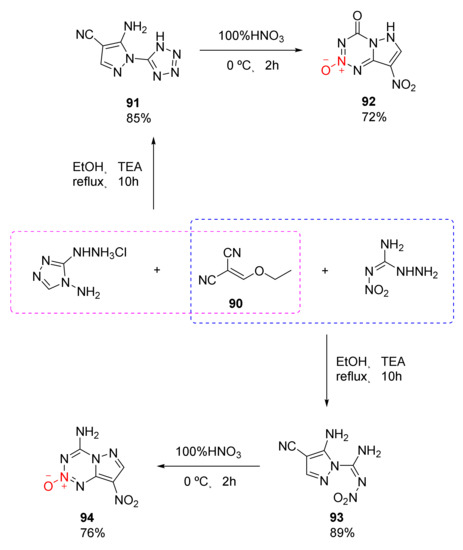
Scheme 14.
Synthetic routes for compounds 92 and 94.
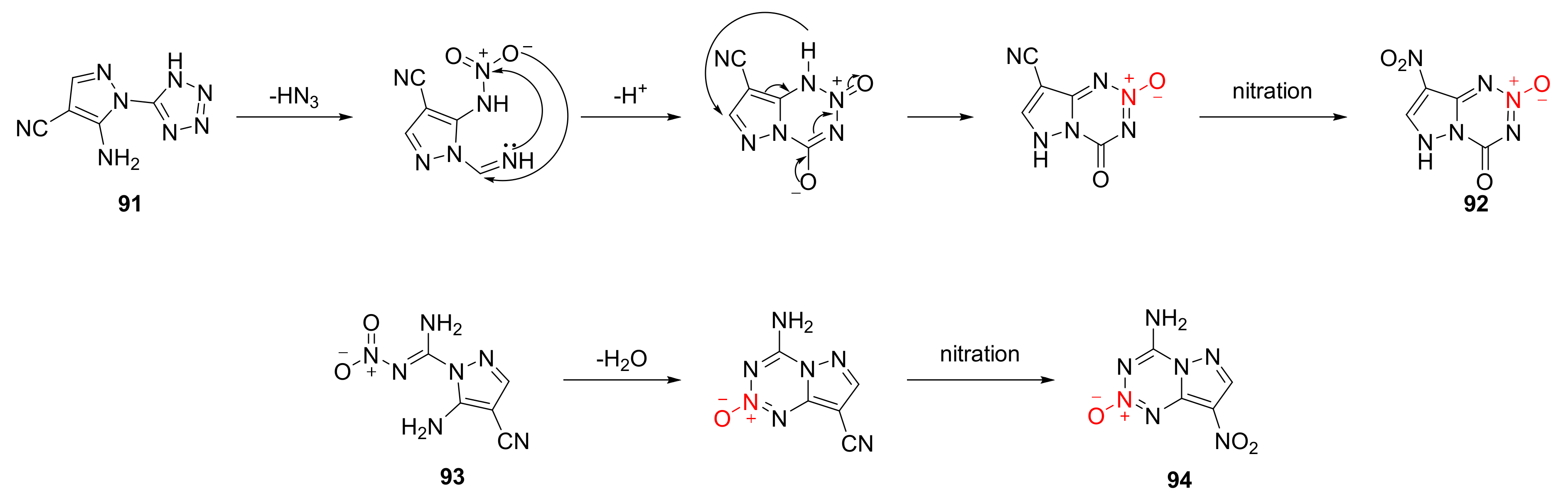
The possible cyclization mechanism for compound 91 and compound 93 was described as follows [47] (Scheme 15). The amino group of compound 91 was nitrated to a nitroamine group, and the o-tetrazole group exposed C = N moiety after removing a molecule of HN3 by electron transfer. A cyclization was carried out after the C = N moiety and nitro group had a nucleophilic reaction. With the electron induction of ring, the cyano group was replaced by a nitro group in the nitration reaction to produce compound 92. Compound 93 was cyclized by the dehydration condensation of nitro and amino groups in the imine structure, also under the induction effect of 1,2,3,5-tetrazine-2-oxycycle. Then, the cyano group was substituted with nitro to obtain compound 94.
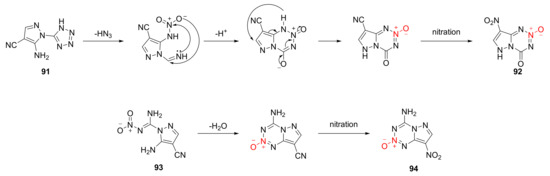
Scheme 15.
Possible synthetic routes for compounds 92 and 94.
2.1.3. 1,2,3-Triazine-3-Oxides
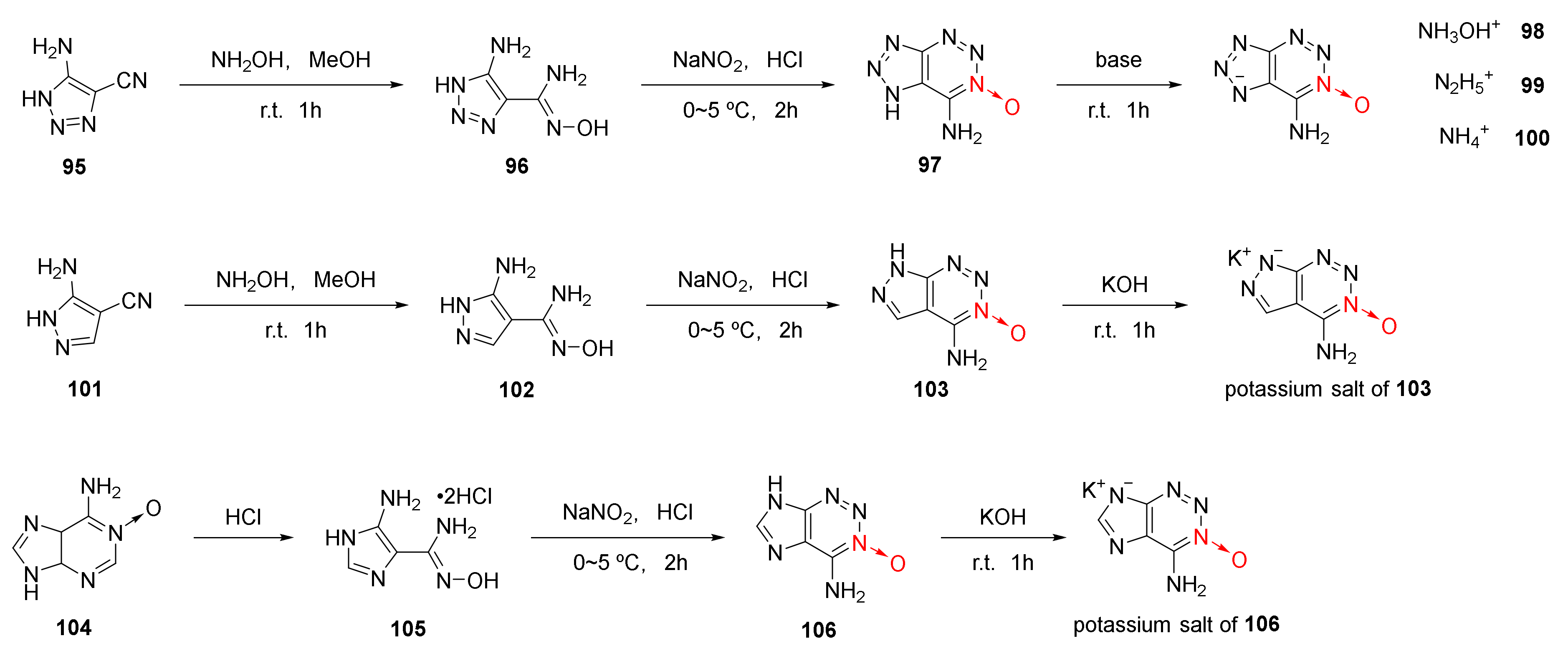
Using o-cyano N-heterocyclic aromatic amine as the starting material, 1,2,3-triazine-3-oxide skeleton was formed by the addition, diazotization and cyclization reaction via the reactivity of an amino group with the o-cyano group. Based on the above design ideas of 1,2,3-triazine-3-oxide skeleton, three energetic compounds (Scheme 16 97, 103, 106) in which 1,2,3-triazine-3-oxide was fused with triazole, imidazole, and pyrazole, were synthetized by Tang et al. [48] for the first time. Since triazole and pyrazole are acidic under the action of strong electron absorbing groups, five energetic ionic salts (98–100, 103 and 106) were obtained by the neutralization reaction from three fused energetic compounds, namely 97, 103, and 106. Based on theoretical calculation and experimental study, it was found that compound 99 displayed the best detonation performance (D = 9358 m/s, P = 33.6 GPa), even exceeding RDX (D = 8795 m/s) in detonation velocity (Table 2).
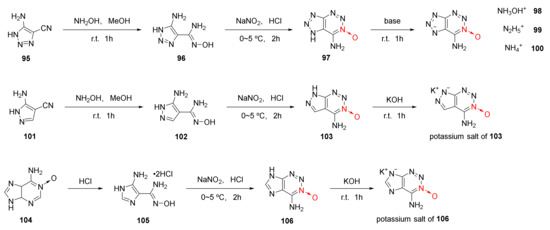
Scheme 16.
Synthetic routes for ionic salts 98–100 and compounds 97, 103, 107.

Table 2.
Energetic properties and detonation parameters of ionic salts 98–100 and compounds 97, 103, 107.
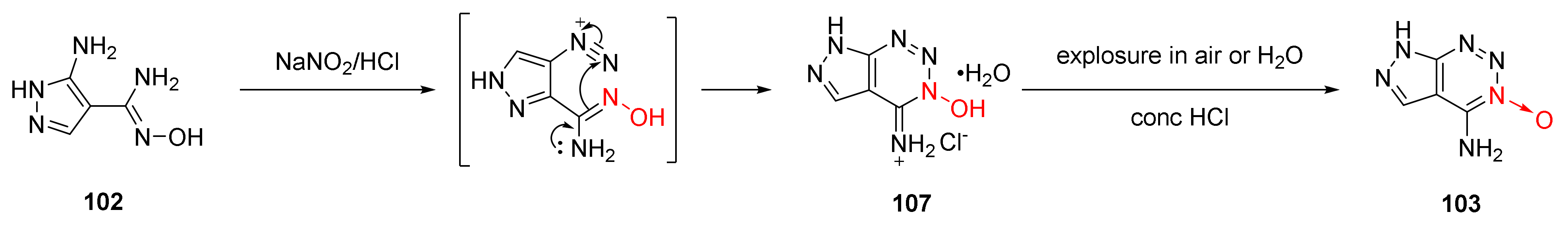
In the nitrosation reaction of compound 102 with dilute hydrochloric acid, a white precipitate was firstly produced, which turned yellow after being exposed to air for several days or being washed with water. Its crystal structure was confirmed by X-ray single-crystal diffraction. It was a hydrochloride intermediate (107) formed after amino diazotization and cyclization with a hydroxime group. According to the experimental results of the synthetic compound 103, the cyclization mechanism of 1,2,3-triazine-3-oxide was proposed as follows [48] (Scheme 17): The diazonium positive ions was generated by the nitrosation reaction of the amino group. The hydrochloride intermediate 107 was obtained by further cyclization with a hydroxime group. Finally, compound 103 was obtained after removing HCl and H2O.
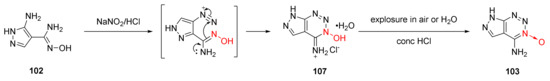
Scheme 17.
Proposed synthetic route for compound 103.
2.1.4. 1,2,3-Triazine-2-Oxides
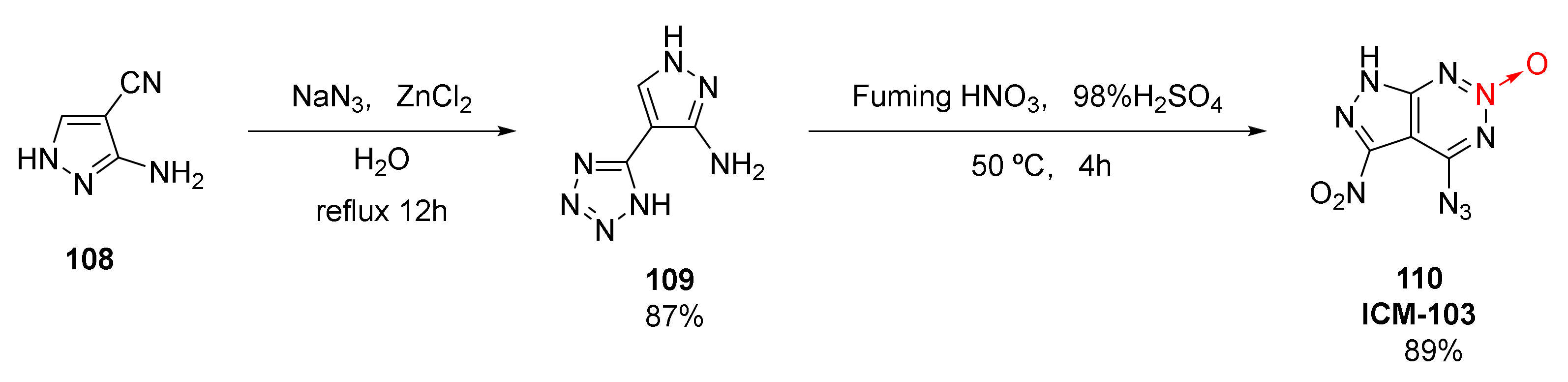
High detonation performance, appropriate mechanical sensitivity, and high chemical and thermal stability are the basic characteristics of green detonators. A fused ring energetic compound 6-nitro-7-azido-pyrazolo [3,4-d]-1,2,3-triazine-2-oxide (110, ICM-103) was synthetized by Deng Mucong et al. [49,50] from industrial 3-amino-4-cyanopyrazole(108). First, under the action of the catalyst ZnCl2, the cyano group on the pyrazole ring reacted with azide anion to form a tetrazole ring. Then, the nitration reaction was cyclized to form 1,2,3-triazine-2-oxide(Scheme 18). The total yield of the two-step reaction was 77.4%. Especially, by only one nitration, azido and nitro groups were formed and cyclized, laying a foundation for the industrial mass production of ICM-103. Additionally, a high measured density and excellent calculated detonation performance (ρ= 1.86 g/cm3, D = 9111 m/s, P = 35.14 GPa), ICM-103 shows an appropriate mechanical sensitivity (IS = 4 J, FS = 60 N), and its flame sensitivity (Flame S > 60 cm) even exceeds that of the widely used azide dinitrophenol (DDNP) (Flame S = 17 cm). More importantly, ICM-103 does not contain metal components and is environmentally friendly. Thus, its synthesis is of great significance for the research of green primary explosives.
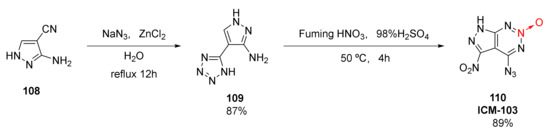
Scheme 18.
Synthetic route for ICM-103.
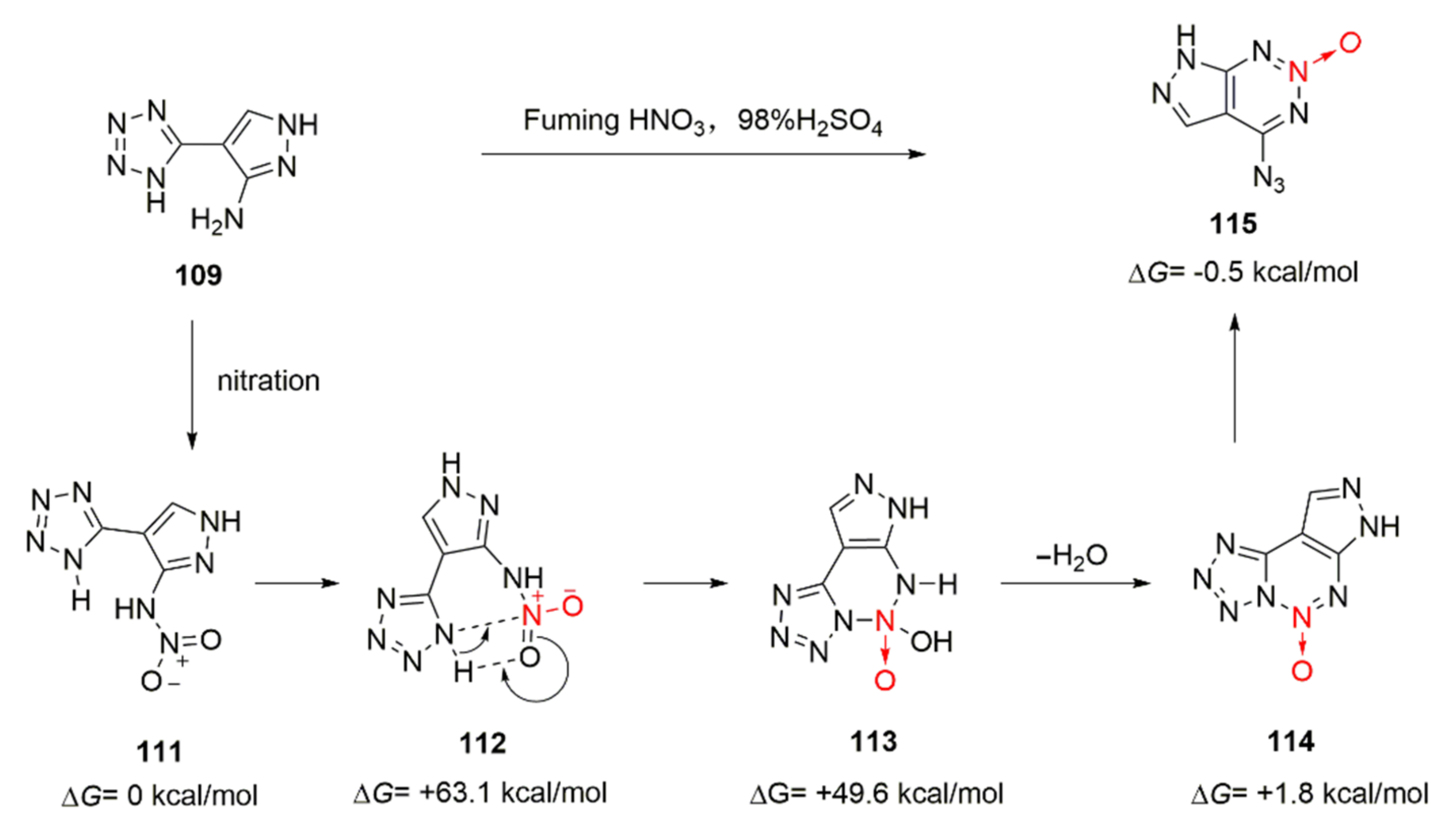
Deng Mucong proposed a reasonable cyclization mechanism for compound 109 based on the theoretical calculation of the Gibbs free energy of various intermediates encountered in the reaction process [49] (Scheme 19). Compound 109 was first nitrated with mixed acid to produce an unstable nitroamine intermediate 111, and its Gibbs free energy was defined as 0 (ΔG= 0 kcal.mol−1). With the induction of the intramolecular hydrogen bond, the N-H in the tetrazole ring reacted with the N = O in the nitro group and intermediate 113 was generated via the transition state 112. After an intramolecular elimination reaction was conducted, a fused ring intermediate 114 was obtained by removing a molecule of water. The tetrazole ring on the intermediate 114 was isomerized to form a azido group and obtain 7-azido-pyrazolo-[3,4-d]-1,2,3-triazine-2-oxide(115). IMC-103 was obtained by further nitration and introduction of a nitro group at the position 6 of 115.
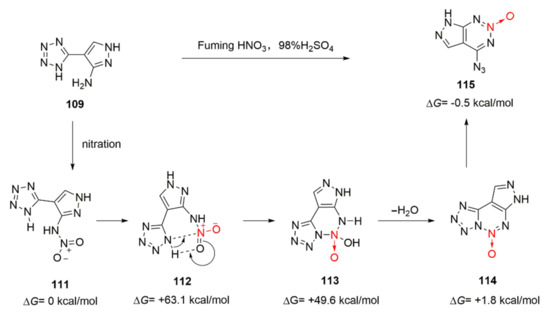
Scheme 19.
Possible synthetic route for ICM-103.
Using ethoxymethylene malononitrile (90) and carbamoyl hydrazide as raw materials, compound 116 was obtained by the condensation cyclization reaction in the solution of triethylamine and ethanol, and pyrazolo-1,2,3-triazine-2-oxide(117)(ρ= 1.825 g/cm3, D = 8323 m/s, P = 27.7 GPa, IS > 20 J, FS > 360 N) was synthetized by Lei et al. [47] via further nitration and cyclization using fuming nitric acid. Due to the high thermal decomposition temperature (Td = 275 °C), compound 117 could be used as a heat-resistant explosive. The cyclization mechanism of compound 116 was basically the same as that of compound 109: By removing the amide group on the pyrazole ring using compound 116 under the action of nitric acid, while hydrolyzing the cyano group on the ring to an amide group, the amino group was nitrated to a nitroamino group. Similarly, with the induction of a hydrogen bond, the nitro group and the amino group underwent electrophilic addition and elimination reaction to remove a molecule of water. Then, further intramolecular electron transfer was performed to obtain compound 117(Scheme 20).
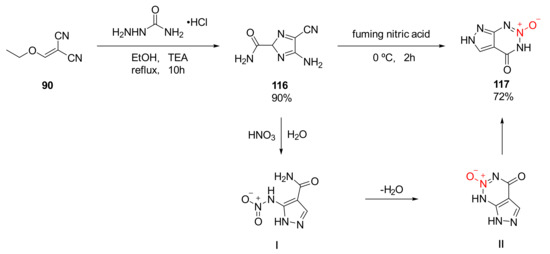
Scheme 20.
Possible synthetic route for compound 117.
2.1.5. Pyridazine-1,2-Dioxide
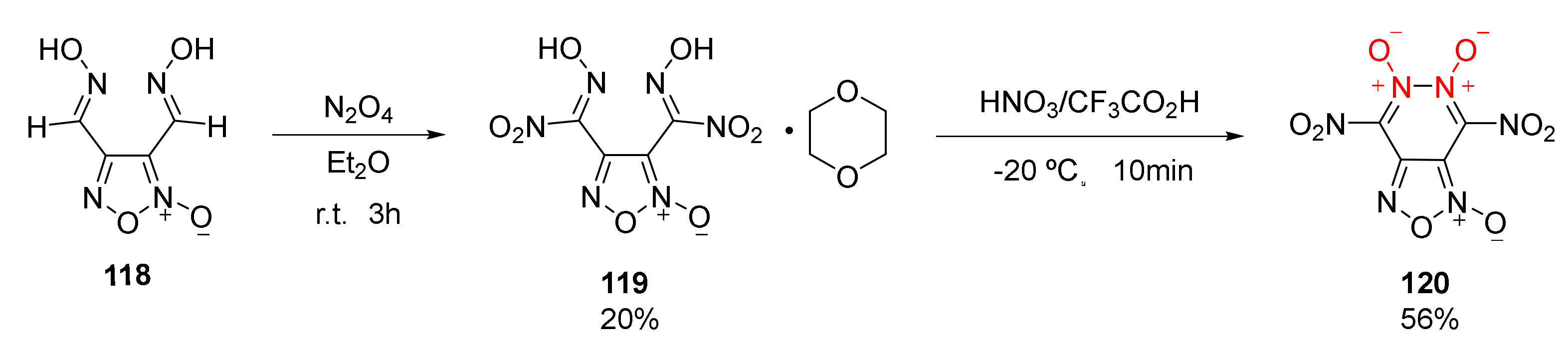
According to the characteristics of NO donor drugs, 4,6-dinitro-furazan [3,4-d]-1,5,6-trioxide(120) was obtained by Ogurstov et al. [51] by fusing furoxan with pyridazine-1,2-dioxide. Using 3,4-dihydroxyfurazan(118) as the raw material, a 1:1 stable complex of furazan dinitroxime acid (119) with 1,4-dioxane was obtained by the nitration of N2O4 in Et2O solution. Then, compound 120 (m.p. = 50–52 °C) was synthetized in the nitration system of HNO3 and CF3COOH(Scheme 21). As compound 120 could not exist stably in organic solvents such as CH2Cl2 and MeCN, it could not be detected by elemental analysis and mass spectrometry. Its structure, however, could still be observed by infrared spectrum in KBr and carbon spectrum in deuterated acetone. Compound 120 has a density as high as 1.98 g/cm3 and a calculated detonation velocity of 9.52 km/s, making it comparable to CL-20. Yet, its applications as an energetic material are largely limited by its poor thermal stability.
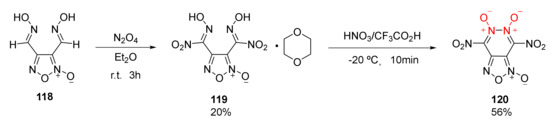
Scheme 21.
Synthetic route for compound 120.
2.1.6. Pyrazine-1-Oxide
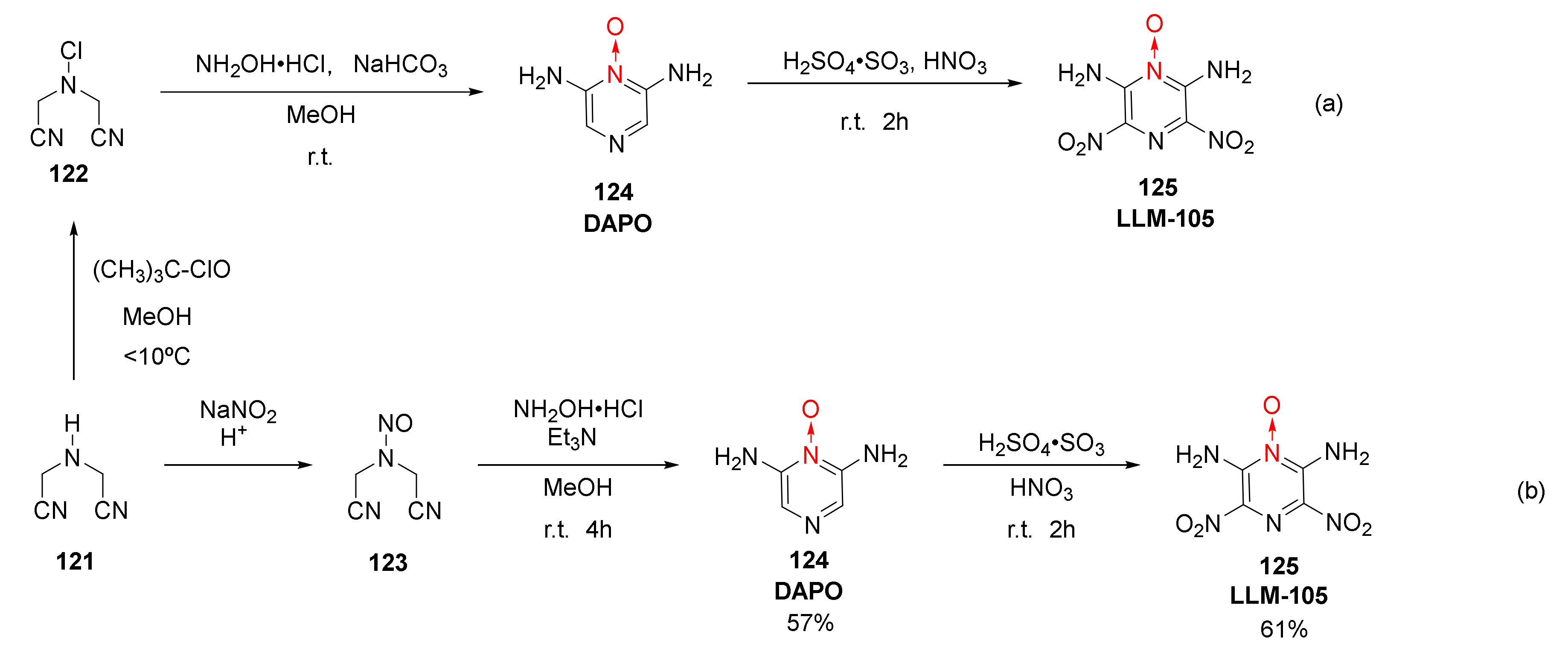
The 2,6-diamino-3,5-dinitro-1-oxide (125, LLM-105) has a crystal density of 1.918 g/cm3, experimental detonation velocity [52] of 8560 m/s, and DSC exothermic peak of 342 °C. It exhibits good thermal stability, an impact sensitivity of 117 cm, high sensitivity, and excellent comprehensive properties, thus being widely used in the field of energetic materials. It could not only be used as the main charge and insensitive initiator of weapons but it also has potential in civil engineering, such as oil exploration. Jing Suming et al. used iminodiacetonitrile (121) as the raw material to synthetize LLM-105 by a three-step process of substitution, condensation, and nitration (Scheme 22a) [52]. LLM-105 was also synthetized by Zhao Xiaofeng et al. [53] using a novel synthesis method of nitrosation, condensation, and nitration (Scheme 22b), which shows potential for industrial applications.
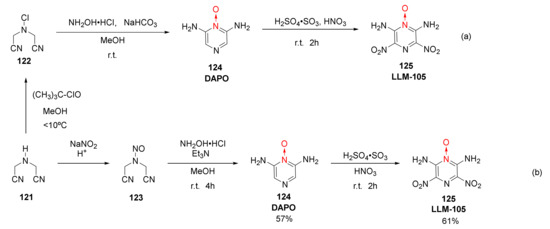
Scheme 22.
Synthetic routes (a) and (b) for LLM-105.
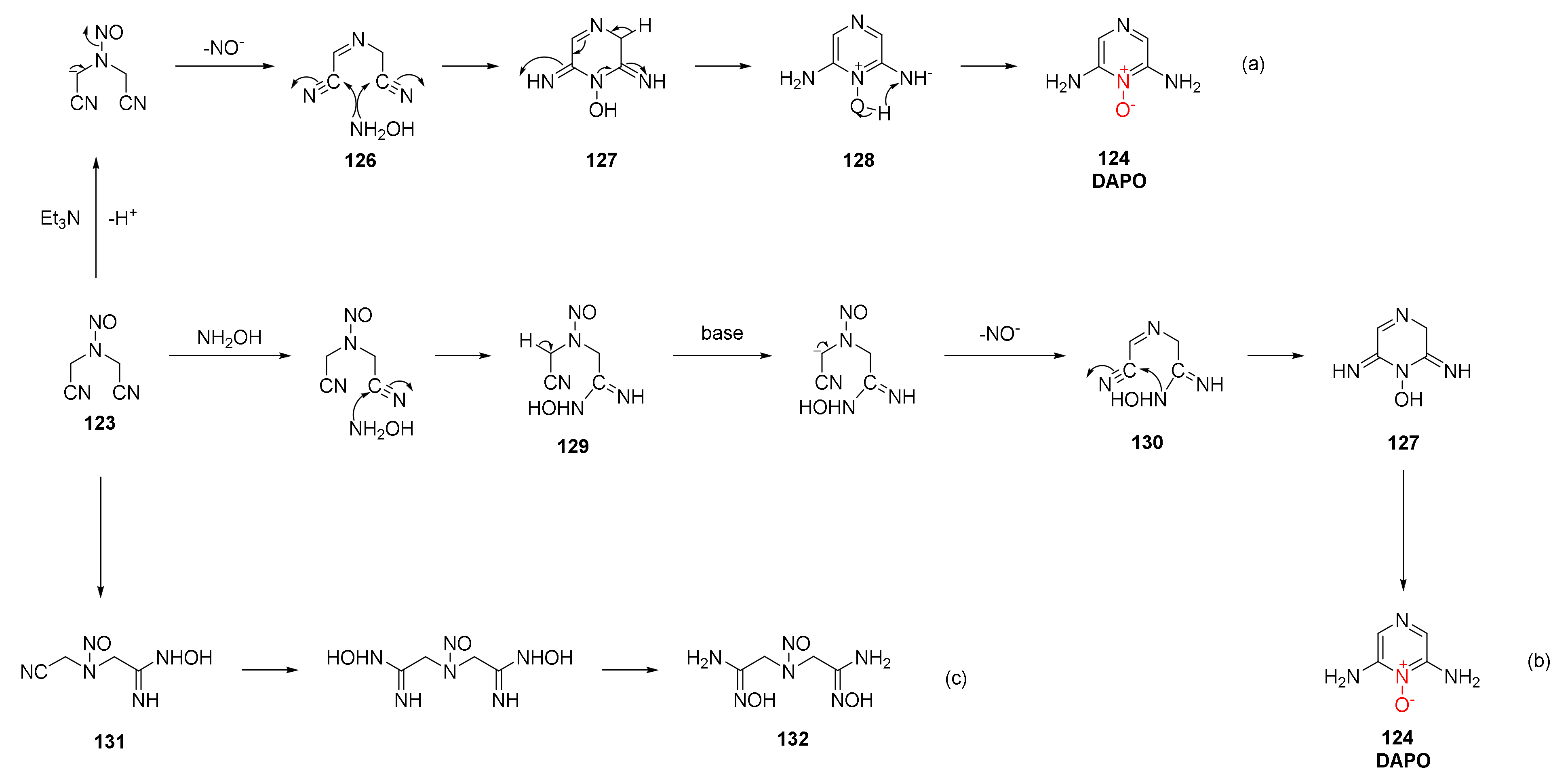
Zhao Xiaofeng et al. discussed the reaction mechanism for synthesizing DAPO [54]. Compound 123 was stripped of a proton under the alkaline action of Et3N, and the nitrogen oxide anion (NO-) was removed after electron transfer to form intermediate 126. Intermediate 126 was then condensed and cyclized with free hydroxylamine to obtain compound 127, which was further aromatized to obtain compound 128. Finally, the product DAPO was obtained by intramolecular proton transfer (Scheme 23a). On such basis, a new possible reaction mechanism was proposed by Wang Jinmin et al. [55], who used sodium hydroxide as alkaline medium for the preparation of DAPO. A cyano group in compound 123 first reacted with hydroxylamine to obtain compound 129. Under the alkaline condition of NaOH, HNO (H+ and NO−) was removed to obtain intermediate 130, and the hydroxylamino group of the intermediate condensed with another cyano group to obtain compound 127. After further intramolecular electron and proton transfer, the product DAPO was obtained (Scheme 23b). Li et al. [56] found that compound 131 and compound 134 (Scheme 23c) could be separated from the product by changing the ratio of compound 123, hydroxylamine hydrochloride, and alkaline medium. Then, the cyclization mechanism of DAPO was divided into two stages. In the first stage, the basic reagent reacted with hydroxylamine hydrochloride to obtain free hydroxylamine and condensed with compound 123 to form compound 129. In the second stage, Et3N grabbed protons to separate nitrogen oxide anions and then condensed and cyclized to obtain the product DAPO.
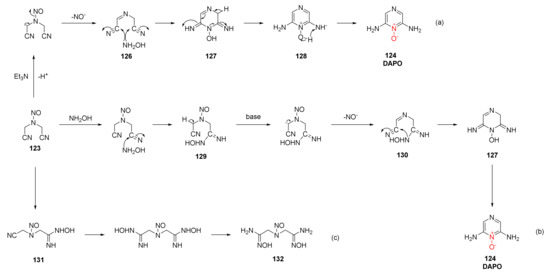
Scheme 23.
Synthetic routes (a), (b) and (c) for DAPO.
2.2. Oxazole N-Oxide Energetic Compounds
2.2.1. 1,2,5-Oxadiazole-2-Oxides
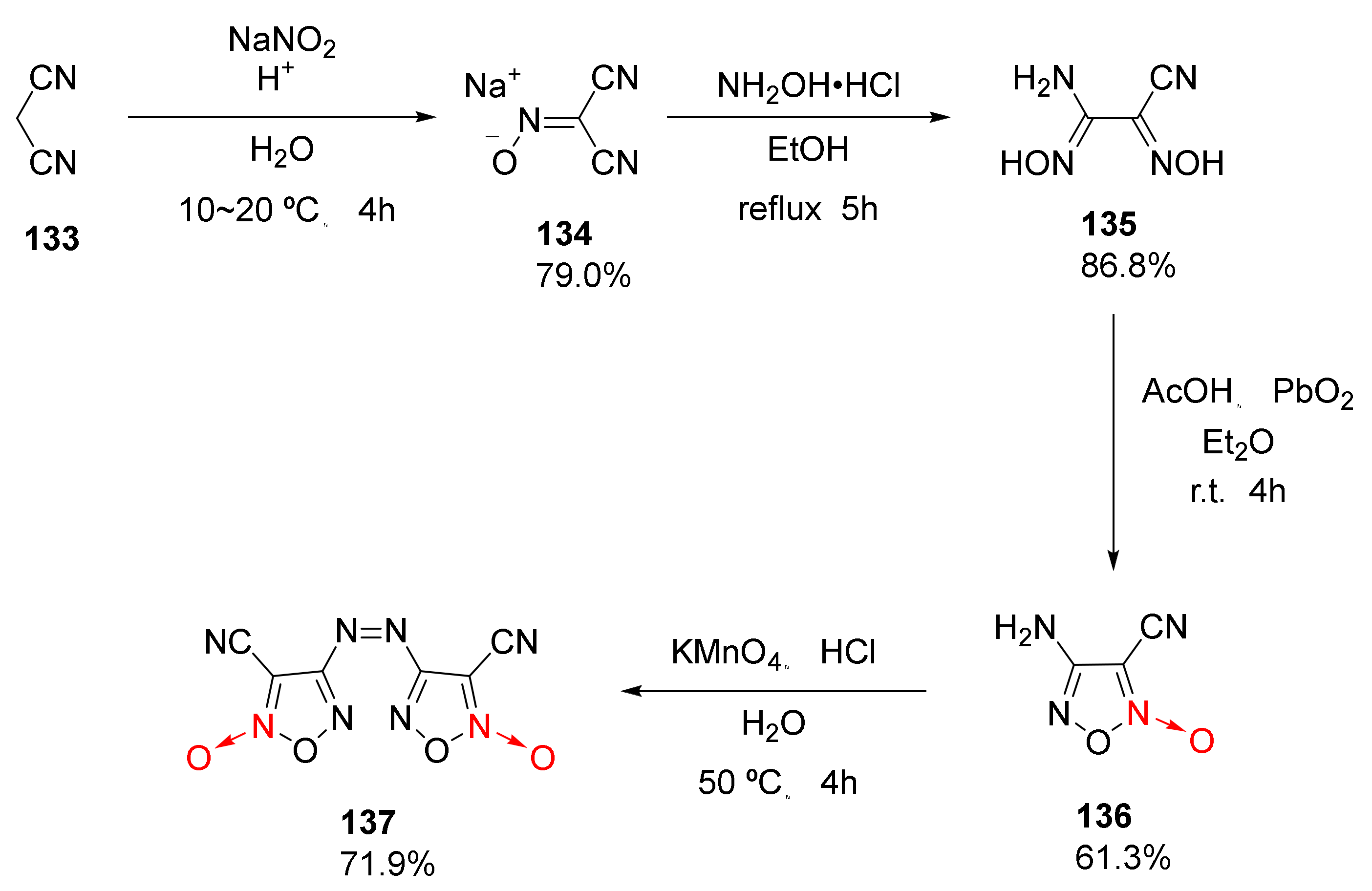
1,2,5-Oxadiazole-2-oxide is also called furoxan. Due to the coordinated oxygen atom on its ring, it has a unique “potential nitro” structure, and it can be used as an energetic group to effectively improve the detonation performance and energy density of compounds, thus attracting wide research interests. Using malondinitrile (133) as the starting material, 3,3′-dicyano-4,4′-azofuroxan was synthetized by Luo Yifen et al. [57] via nitrosation, NH2OH addition, and PbO2 and KMnO4 secondary oxidation. The four-step reaction had a yield of 30.2% (Scheme 24).
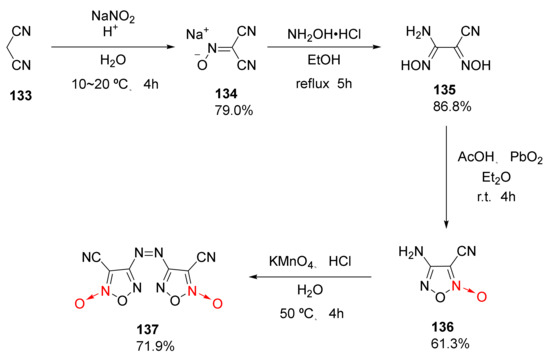
Scheme 24.
Synthetic route for compound 137.
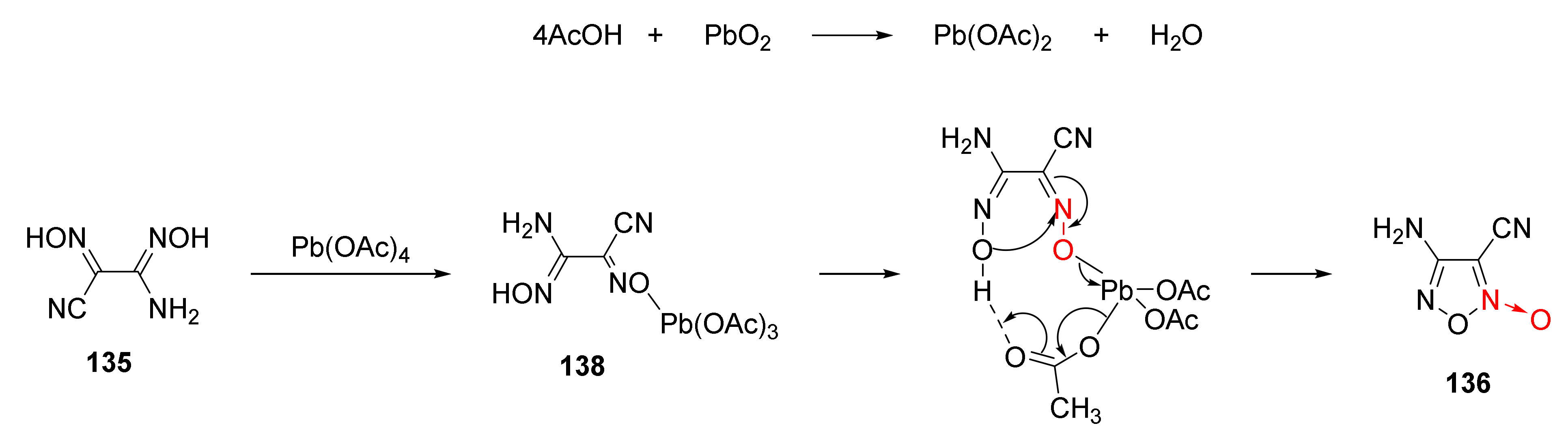
The oxidation-cyclization mechanism under the action of lead peroxide was analyzed [57] as follows (Scheme 25). First, lead peroxide reacted with acetic acid to obtain lead tetraacetate. Then, the hydroxyl in compound 135 reacted with lead tetraacetate to obtain intermediate 138. Under the action of the intramolecular hydrogen bond, intermediate 138 experienced intramolecular coupling and intramolecular electron transfer via a macrocyclic transition state. Compound 136 was obtained by the cyclization reaction after removing a molecule of lead acetate and a molecule of acetic acid.
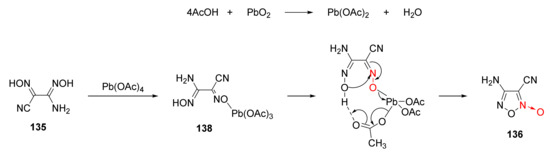
Scheme 25.
Possible synthetic route for compound 136.
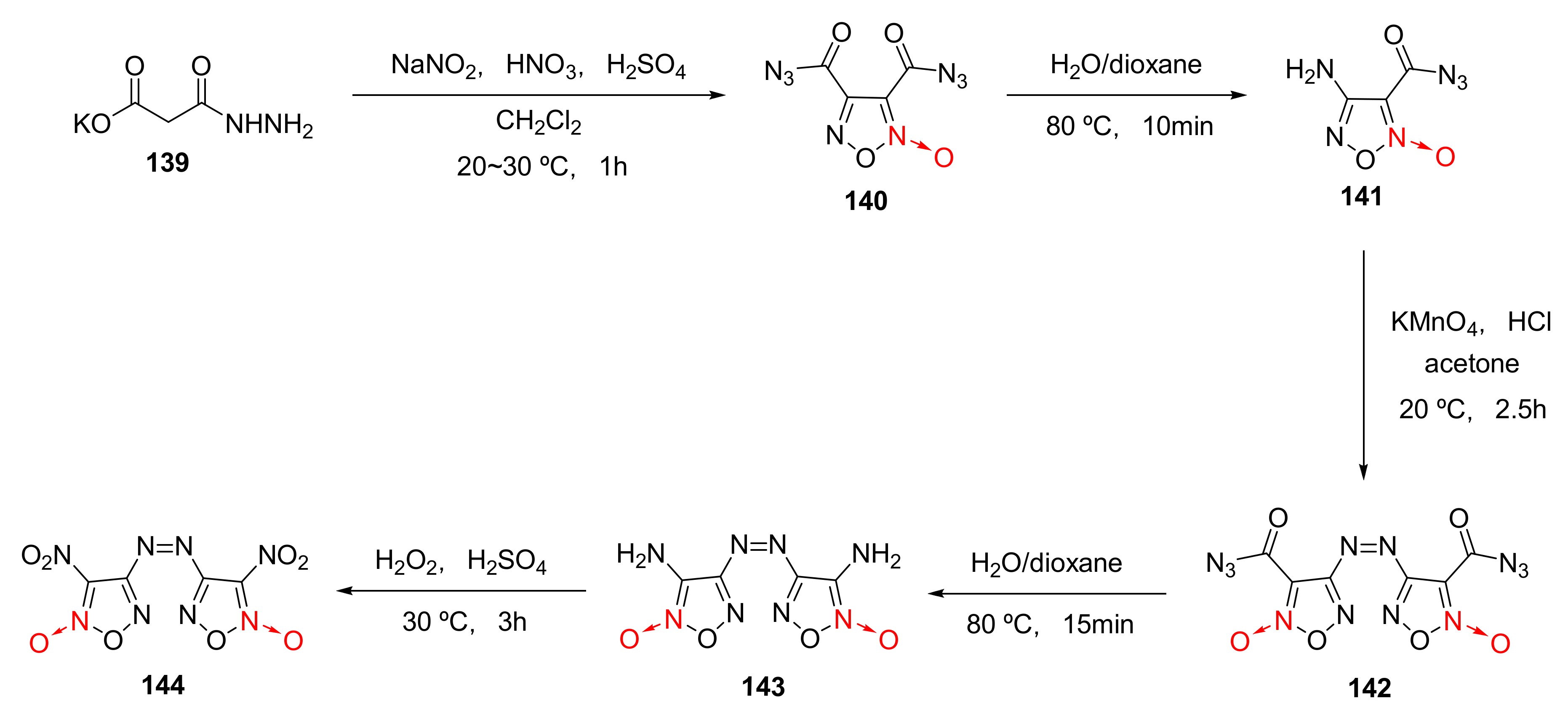
Using malonyl monohydrazide monopotassium salt (139) as the raw material, dinitroazofurazan (144) was synthetized by nitrosation-nitration, first hydrolysis, oxidative coupling, second hydrolysis and oxidation [58] (Scheme 26). Dinitroazofurazan has a density as high as 2.002 g/cm3 and a measured detonation velocity of 10 km/s. Such characteristics as high density and high detonation velocity make it an important component for energetic composite solid propellants.
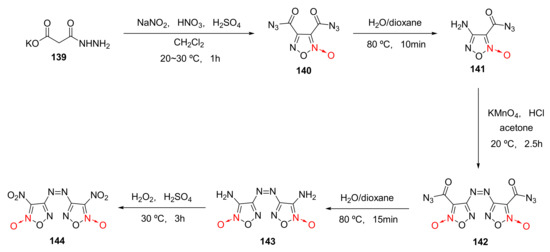
Scheme 26.
Synthetic route for compound 144.
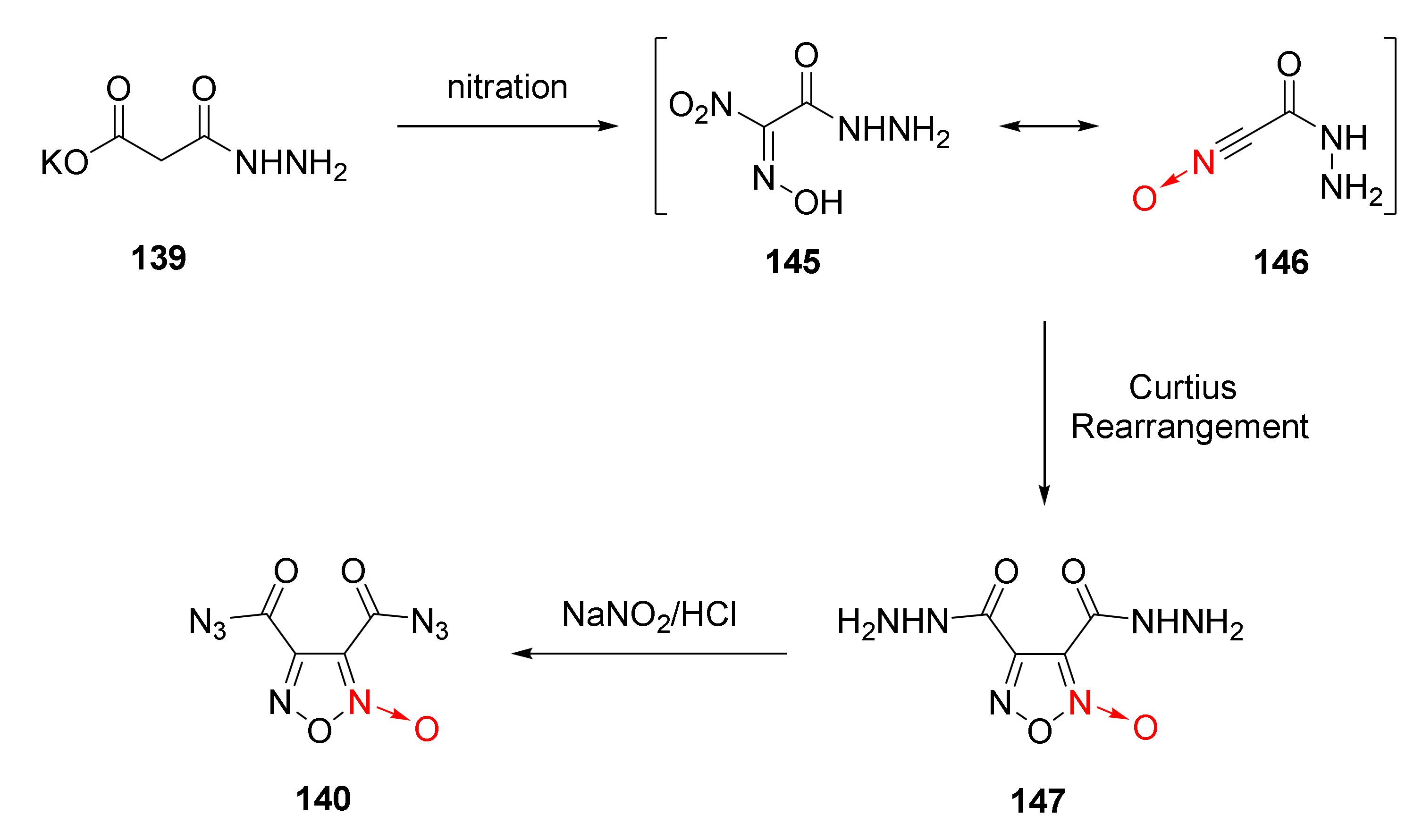
The reaction mechanism of compound 140 was discussed as follows [58] (Scheme 27). Compound 139 was first nitrated to produce intermediate 145 with a resonance structure 146. The intermolecular hydrogen bond of compound 146 increased the stability of the compound. After Curtius rearrangement, compound 147 was generated. The hydrazido group in compound 147 was transformed into an azido group after nitrosation to obtain compound 140.
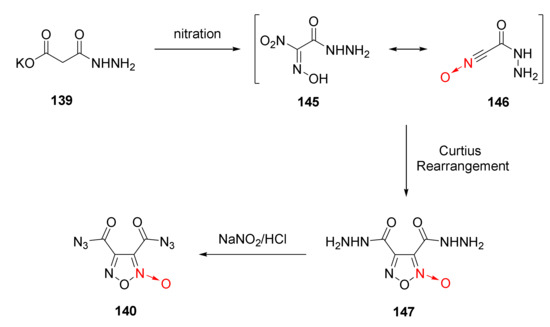
Scheme 27.
Synthetic route for compound 140.
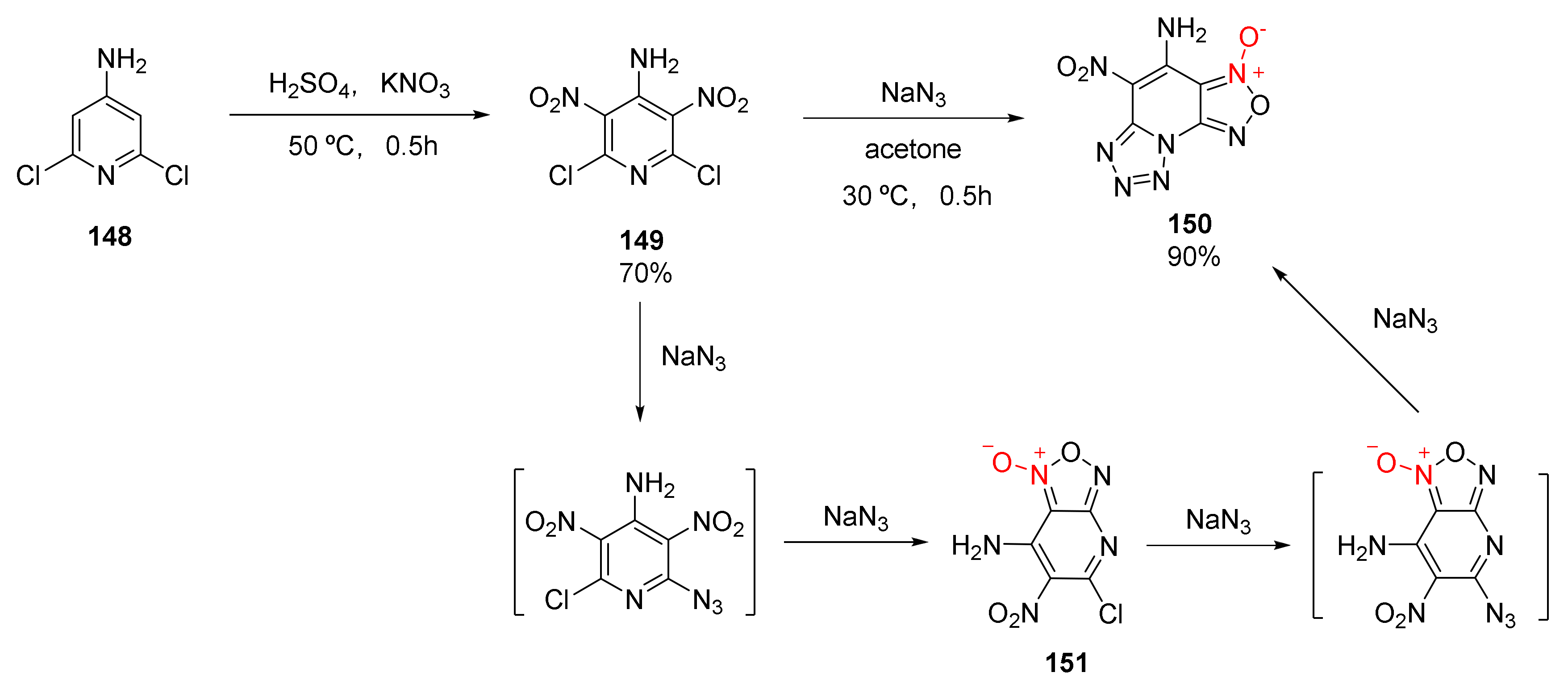
Ma et al. synthesized a fused ring energetic compound 4-amino-5-nitro-1,2,5-oxadiazolo [3,4-e]tetrazolo [1,5-a]pyridine-3-oxide (150) from industrial 4-amino-2,6-dichloropyridine (148) via two steps, nitration and substitution-cyclization [59] (Scheme 28). The existence of compound 151 could be detected during the second step of the reaction, indicating that the azide group was not introduced into the pyridine ring at one time but was divided into two steps. The first introduced azide group was cyclized with a nitro group and a molecule of N2 was removed to generate compound 151. After the second azide group was introduced, the tautomerization of azide-tetrazole occurred due to the electron deficiency of the pyridine ring, resulting in compound 150. With a density of 1.921 g/cm3, a calculated detonation velocity of 8838 m/s, and a detonation pressure of 36.01 GPa, compound 150 could be an energetic material with an excellent detonation performance.
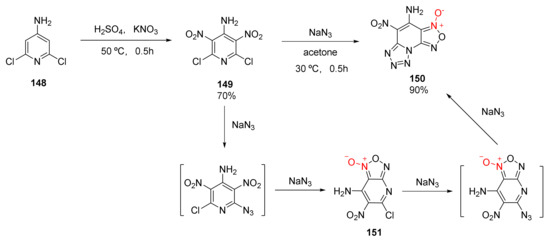
Scheme 28.
Synthetic route for compound 150.
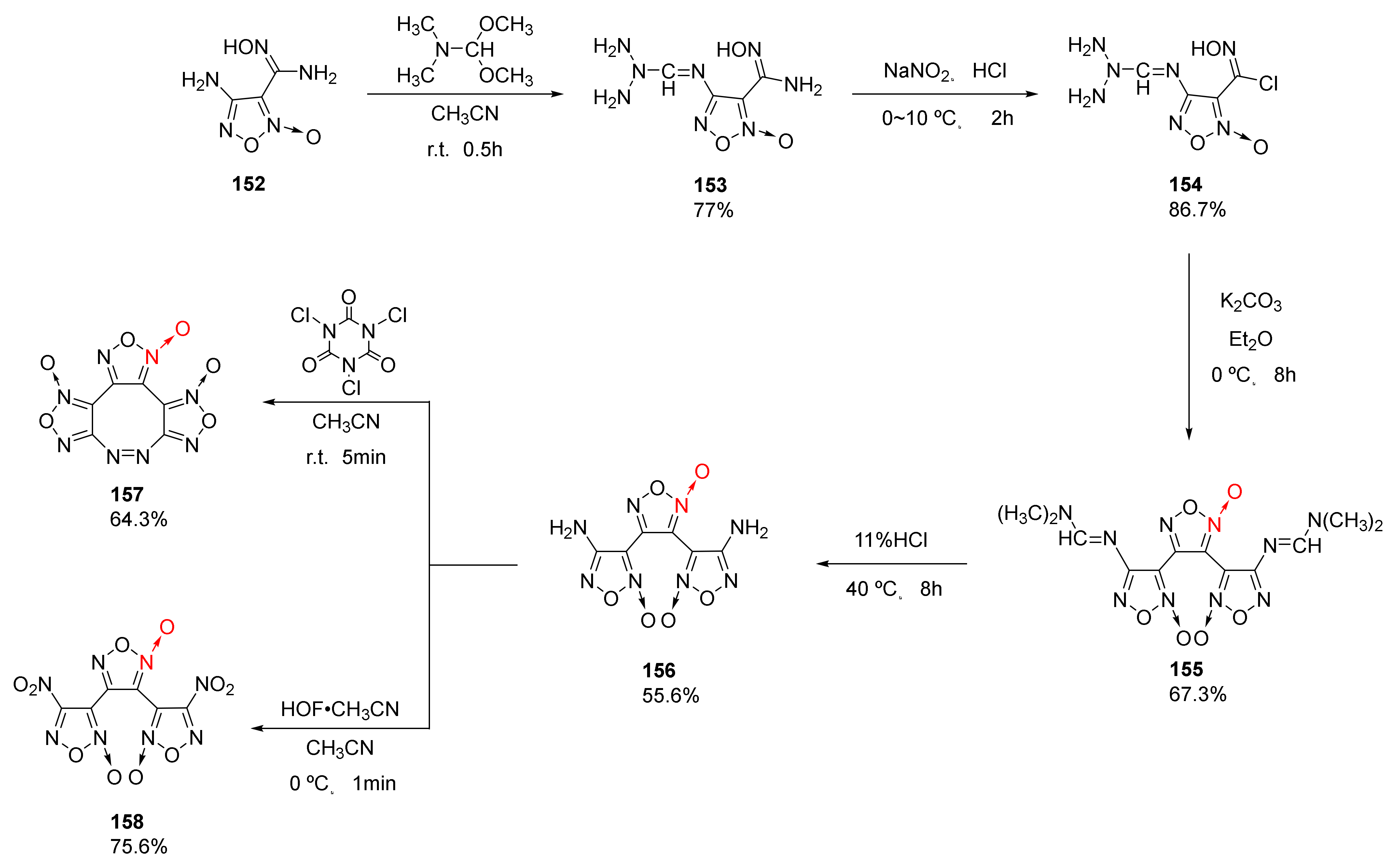
Using 4-amino-3-amidoximo furoxan(152) as a starting material, 3,4-bis (4’-aminofuroxan-3’-yl)furoxan(156) (ρ = 1.787 g/cm3, D = 8480 m/s, P = 31.0 GPa) was synthetized by He et al. [60] via amino protection, diazotization-chlorination, intermolecular condensation cyclization, and hydrolysis. Then, compounds 157 (ρ = 1.895 g/cm3, D = 9417 m/s, P = 39.6 GPa), and 158 (ρ = 1.914 g/cm3, D = 9503 m/s, P = 40.8 GPa) were obtained through oxidative coupling or oxidation (Scheme 29). The three compounds show good detonation performance and energy density. Compound 158 exhibit a positive oxygen balance (OB = +18.6%) with good detonation performance, and its sensitivity (IS = 3 J, FS = 40 N) is close to that of CL-20. Due to its outstanding properties, it could be used as an energetic component in solid rocket propellants.
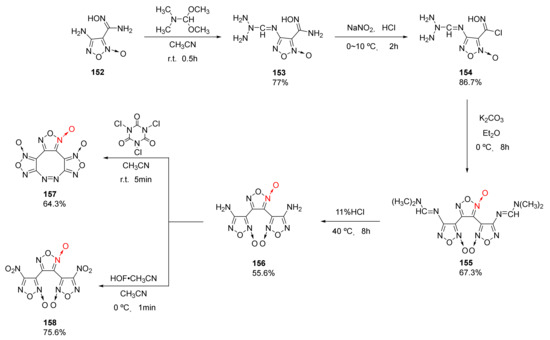
Scheme 29.
Synthetic routes for compounds 156–158.
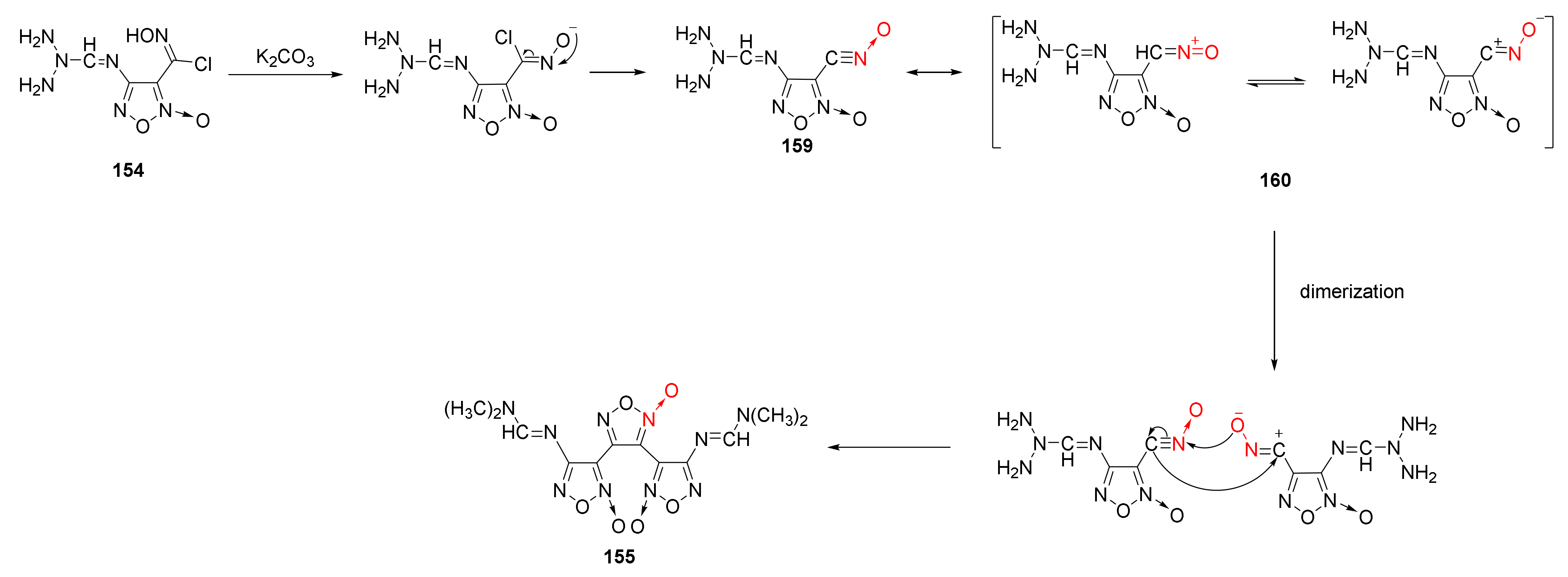
Using on-line infrared technology to monitor the synthesis of 3,4-bis(4’- aminofuroxan-3’-yl)furoxan, Sun Kunlun et al. observed the infrared spectrum of the pure substance of the intermediate component produced in the reaction to deduce the reasonable reaction mechanism of furoxan construction from chloro-oxime via dimerization and cyclization [61] (Scheme 30). Under alkaline conditions, the O–H bond and C–Cl bond of the chloro-oxime group broke and removed a molecule of HCl. Then, intramolecular electron transfer was carried out to obtain intermediate 159. As the reaction progressed, intermediate 159 generated the resonance structure of intermediate 160, which was dimerized with intermediate 159 to obtain product 156.
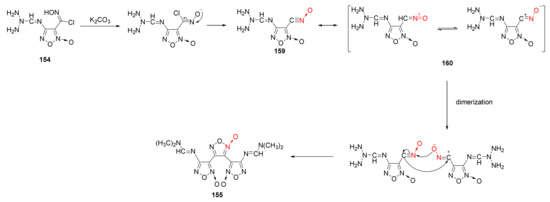
Scheme 30.
Synthetic route for compound 155.
2.2.2. Pyrazole-1-Oxide
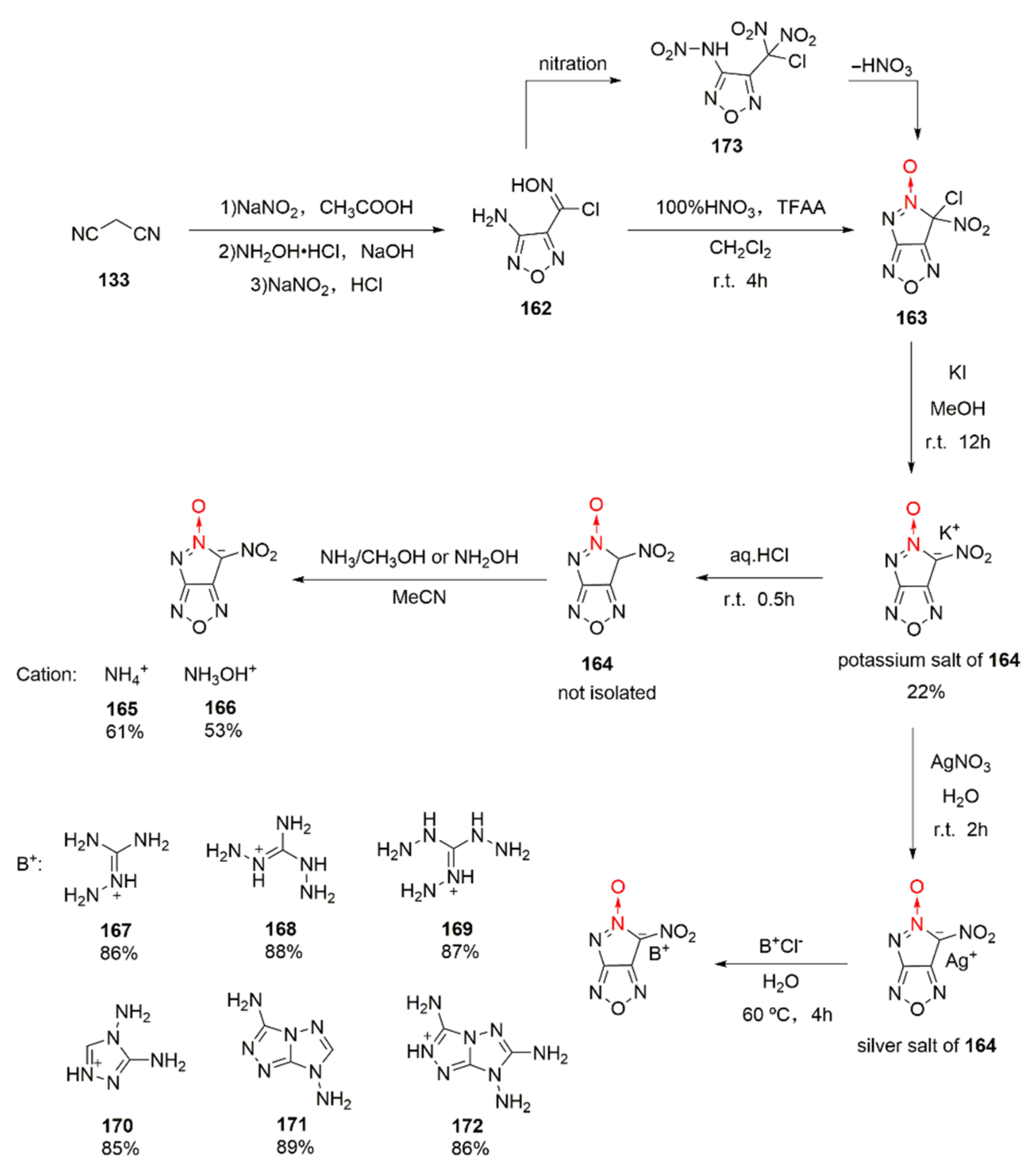
4-Amino-3-chlorooximofurazan (162) can be oxidized to be the dipotassium salt of 3-dinitromethyl-4-nitroaminofurazan. Yet, when Li et al. used this synthetic approach, the potassium salt of 6-nitro-pyrazolo [3,4-c]furazan-5-oxide (164) was accidentally isolated [62]. This unique reaction process was thoroughly studied by Tang et al., [63]. Using malonic nitrile as the starting material, 4-amino-razan-3-chlorooxime (162) was obtained by three steps, namely nitration, addition, and cyclization. Then, the 100% HNO3/TFAA system was used for nitration. Compound 162 was first nitrated to be intermediate 173 during nitration, and intermediate 173 experienced an intramolecular electron transfer to remove HNO3 for generating compound 163. Compound 163 was dissolved in methanol and was ion-exchanged with KI to obtain the potassium salt of compound 164. Based on the reaction characteristics of 164, a series of energetic ionic salts (165–172) were designed and synthesized by the neutralization or ion exchange method (Scheme 31). Through theoretical calculation and experimental measurement, the potassium salt of compound 164 was found as high as 2.036 g/cm3, the sensitivity of compound 172 was satisfying (IS = 20 J, FS = 360 N), and the calculated detonation velocity and detonation pressure of hydroxylaminium salt (166) were the highest among the products (9174 m/s and 39.1 GPa). The hydroxylaminium salt 166 with high sensitivity (IS = 2 J, FS = 40 N) also has potential for becoming a green primary explosive.
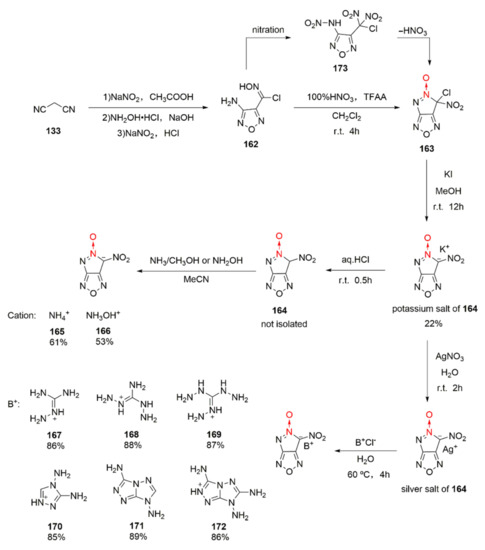
Scheme 31.
Synthetic routes for energetic salts 165–172.
2.2.3. Triazole-1-Oxides
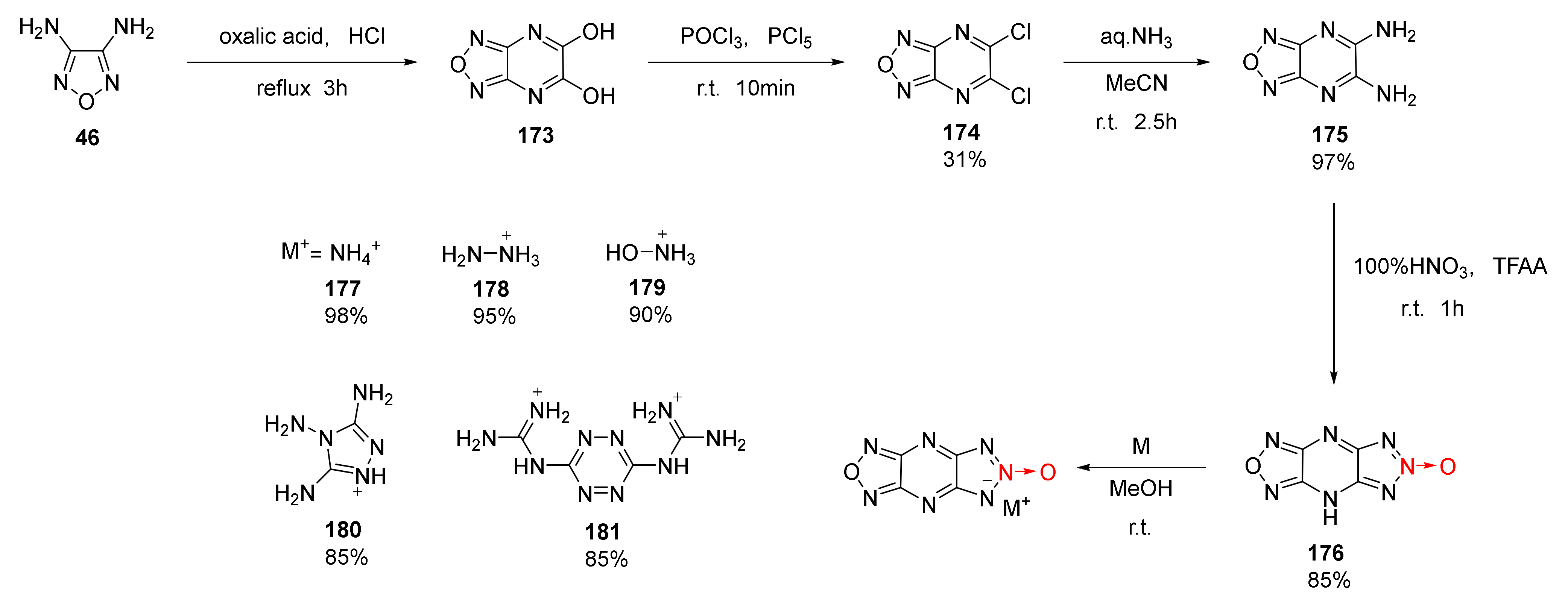
The synthesis of 1,2,3-Triazolo [4,5-e]furazano [3,4-b]pyrazine-6-oxide(177) and its energetic ionic salts (179–183) was reported by Thottempudi et al. [64]. Using 3,4-diaminofurazan (46) as the starting material, diaminofurazano [3,4-b]pyrazine 175 was obtained by a three-step process of condensation-cyclization, chlorination, and ammoniation. Then, triazole-1-oxide (176) was synthetized using the nitration-cyclization of HNO3/TFAA. Due to the acidity of compound 176, a series of energetic ionic salts (177–181) were synthesized by the neutralization reaction with a yield ranging between 85% and 98%(Scheme 32). Compound 176 and ionic salts 177–181 display outstanding energy density and detonation properties (Table 3). Moreover, these compounds exhibit extremely low impact sensitivity, making them suitable for insensitive explosives.
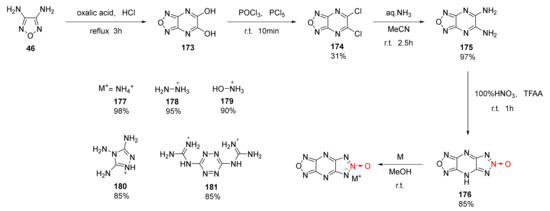
Scheme 32.
Synthetic routes for compounds 177–181.

Table 3.
Physical and detonation properties of compounds 177–181.
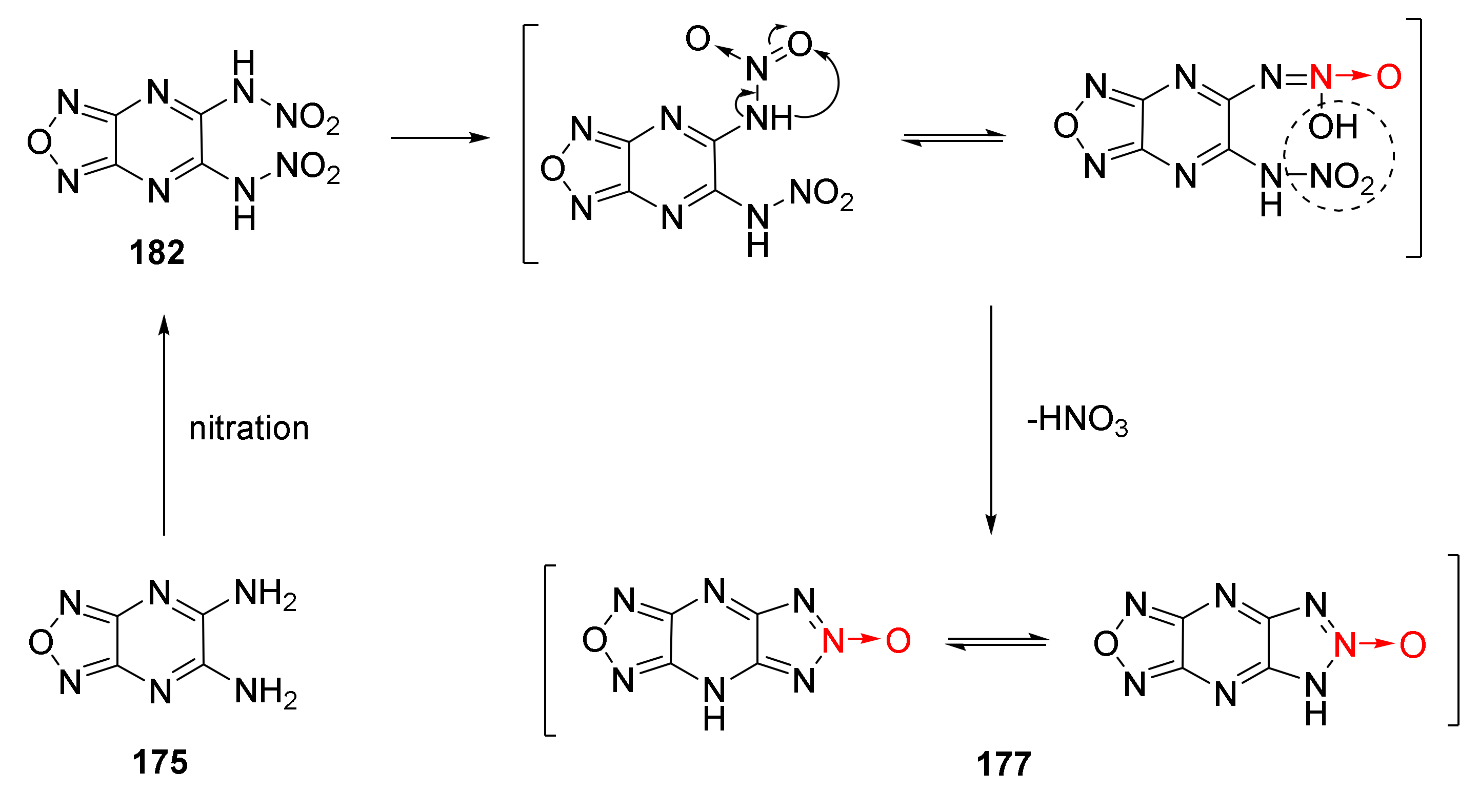
Li Yanan et al. discussed the nitration-cyclization reaction mechanism of triazole-1-oxide 175 in 100% HNO3/TFAA system [65] (Scheme 33). Two nitro groups in compound 175 were first converted into nitroamino groups through nitration to obtain intermediate 182. One nitroamino group in intermediate 182 was rearranged into a hydroxyloxyazo group, and the rearranged intermediate 182 was stripped of a molecule of HNO3 to produce compound 177.
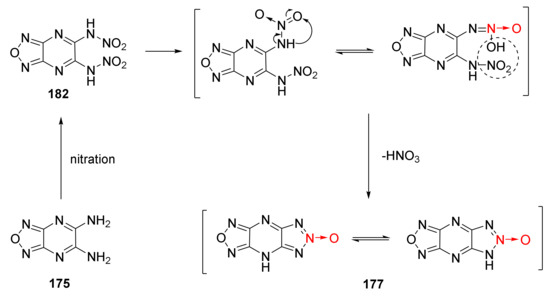
Scheme 33.
Synthetic route for compound 177.
3. Conclusions
N-oxides can be synthesized by either direct N-oxidation or cyclization with N→O coordination bonds. As for the second approach, the N→O coordination bonds in energetic N-oxides could be introduced into the cyclization process, avoiding the pollution of the N-oxidation reaction, shortening the synthetic route, and improving efficiency. In this study, the synthetic methods towards two kinds of N-oxides (nine energetic N-oxides) are introduced in detail. The reaction mechanisms towards cyclization reaction are also discussed to clarify the formation of N→O coordination bonds. The physicochemical properties and detonation performances of typical N-oxides are also introduced, laying a foundation for future applications.
The synthesis of N-oxides by cyclization is one of the most important directions of energetic materials. At present, the industrialized manufacturing process of heat-resistant explosive LLM-105 has been found based on its synthetic method. With improved synthetic methods and technological advancements, new N-oxides with excellent performance are expected to emerge, and the manufacturing process for more N-oxide energetic materials will be improved to meet the need of mass production.
Author Contributions
W.S. completed the main content of the article; Z.X., L.Z., J.Z. and J.H. assisted with literature research and scheme drawing; W.P. and B.W. designed the main content and scope of the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 22105155).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, H.X.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, D.M.; Talawar, M.B.; Asthana, S.N.; Mahulikar, P.P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Song, S.W.; Yang, Z.J.; Qi, X.J.; Wang, K.C.; Liu, Y.; Zhang, Q.H.; Tian, Y. Accelerating the discovery of insensitive high-energy-density materials by a materials genome approach. Nat. Commun. 2018, 9, 2444. [Google Scholar] [CrossRef]
- Tang, Y.X.; Kumar, D.; Shreeve, J.M. Balancing excellent performance and high thermal stability in a dinitropyrazole fused 1,2,3,4-tetrazine. J. Am. Chem. Soc. 2017, 139, 13684–13687. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.J.; Bi, F.Q.; Huo, H.; Luo, Y.F.; Li, X.Z.; Chen, S.P.; Wang, B.Z. The ingenious synthesis of a nitro-free insensitive high-energy material featuring face-to-face and edge-to-face π-interactions. Front. Chem. 2019, 7, 559. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Zhang, J.L.; Dong, J.; Zhai, L.J.; Guo, T.; Wang, Z.J.; Bi, F.Q.; Wang, B.Z. A promising insensitive energetic material based on a fluorodinitromethyl explosophore group and 1,2,3,4-tetrahydro-1,3,5-triazine: Synthesis, crystal structure and performance. RSC Adv. 2020, 10, 11816–11822. [Google Scholar] [CrossRef]
- Xu, Y.G.; Shen, C.; Lin, Q.H.; Wang, P.C.; Jiang, C.; Lu, M. 1-Nitro-2-trinitromethyl substituted imidazoles: A new family of high performance energetic materials. J. Mater. Chem. A 2020, 4, 17791–17800. [Google Scholar] [CrossRef]
- Barton, L.M.; Edwards, J.T.; Johnson, E.C.; Bukowski, E.J.; Sausa, R.C.; Byrd, E.F.C.; Orlicki, J.A.; Sabatini, J.J.; Baran, P.S. Impact of stereo-and regiochemistry on energetic materials. J. Am. Chem. Soc. 2019, 141, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.X.; Chen, X.; Zhang, C.; Zheng, W.W.; Tian, H.W.; Bai, Y. Reaserch on the S-Tetrazine-based Energetic Compounds. Chin. J. Explos. Propellants 2021, 44, 407–419. (In Chinese) [Google Scholar]
- Han, Y.Z.; Yang, Y.Z.; Du, Z.M.; Zhang, Y.H.; Yao, Q. Progress of Study on Thermal Behaviors of Nitrogen-rich Compounds as Azole, Triazine and Furazan. Chin. J. Explos. Propellants 2016, 39, 1–11. (In Chinese) [Google Scholar]
- Liu, Y.; Gong, L.S.; Yi, X.Y.; He, P.; Zhang, J.G. Tunable 1,2,3-triazole-N-oxides towards high energy density materials: Theoretical insight into structure-property correlations. New J. Chem. 2022, 46, 11741–11750. [Google Scholar] [CrossRef]
- Tang, Y.X.; Zhang, J.H.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Taming of 3, 4-Di(nitramino)furazan. J. Am. Chem. Soc. 2015, 137, 15984–15987. [Google Scholar] [CrossRef] [PubMed]
- Chavez, D.; Klapötke, T.M.; Parrish, D.; Piercey, D.G.; Stierstorfer, J. The Synthesis and Energetic Properties of 3, 4-Bis(2,2,2-trinitroethylamino)furazan (BTNEDAF). Propellants Explos. Pyrotech. 2010, 35, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Ma, Q.; Tang, S.H.; Fan, G.J. Crystal Structure and Thermal Decomposition Properties of N,N’-Bis(2-fluoro-2,2’-dinitroethyl)-3,4’-dinitraminefurazan. Chin. J. Energ. Mater. 2019, 27, 41–46. (In Chinese) [Google Scholar]
- Xiao, X.; Ge, Z.X.; Wang, W.; Liu, Q.; Su, H.P.; Li, T.Q.; Bi, F.Q.; Ji, X.T. Progress of 3-Azido-1,2,4-Triazole and Its Derivatives. Chin. J. Energ. Mater. 2014, 22, 100–107. (In Chinese) [Google Scholar]
- Lai, Y.; Liu, Y.; Huang, W.; Zeng, Z.Z.; Yang, H.W.; Tang, Y.X. Synthesis and Characterization of Pyrazole- and Imidazole- Derived Energetic Compounds Featuring Ortho Azido/nitro Groups. FirePhysChem 2022, 2, 160–164. [Google Scholar] [CrossRef]
- Wu, B.; Yang, L.F.; Zhai, D.D.; Ma, G.M.; Pei, C.H. Facile Synthesis of 4-Amino-3,5-dinitropyrazolated Energetic Derivatives via 4-Bromopyrazole and Their Performances. FirePhysChem 2021, 1, 76–82. [Google Scholar] [CrossRef]
- Vishnevskiy, Y.V.; Tikhonov, D.S.; Schwabedissen, J.; Stammler, H.G.; Moll, R.; Krumm, B.; Klapötke, T.M.; Mitzel, N.W. Tetranitromethane: A nightmare of molecular flexibility in the gaseous and solid States. Angew. Chem. Int. Edit. 2017, 56, 9619–9623. [Google Scholar] [CrossRef]
- Zhai, L.J.; Zhang, J.L.; Zhang, J.R.; Wu, M.J.; Bi, F.Q.; Wang, B.Z. Progress in Synthesis and Properties of High Energy Density Compounds Regulated by N-F Bond. Chin. J. Org. Chem. 2020, 40, 1484–1501. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.L.; Wang, B.Z.; Qiu, L.L.; Xu, R.Q.; Sheremete, A.B. Recent Synthetic Efforts towards High Energy Density Materials: How to Design High-Performance Energetic Structures? FirePhysChem 2022, 2, 83–139. [Google Scholar] [CrossRef]
- Yuan, J.; Long, X.P.; Zhang, C.Y. Influence of N-Oxide Introduction on the Stability of Nitrogen-Rich Heteroaromatic Rings: A Quantum Chemical Study. J. Phys. Chem. A 2016, 120, 9446–9457. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Murray, J.S. Computational analysis of relative stabilities of polyazine N-oxides. Struct. Chem. 2013, 24, 1965–1974. [Google Scholar] [CrossRef]
- Lai, W.P.; Lian, P.; Ge, Z.X.; Liu, Y.Z.; Yu, T.; Lv, J. Theoretical study of the effect of N-oxides on the performances of energetic compounds. J. Mol. Model. 2016, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, W.; Wang, Y.; Zhang, Q.H. Research Progress of Nitrogen⁃rich Fused⁃ring N⁃oxides. Chin. J. Energ. Mater. 2021, 29, 567–578. (In Chinese) [Google Scholar]
- Zhu, J.P.; Ren, J.; Han, X.Y.; Li, Y.X.; Wang, J.L.; Cao, R.L. Study on Structures and Detonation Performance for Polynitropyrazine-N-oxides by Density Functional Theory. Chin. J. Explos. Propllants 2010, 33, 47–51. (In Chinese) [Google Scholar]
- Churakov, A.M.; Ioffe, S.L.; Strelenko, Y.A.; Tartakovskii, V.A. 1,2,3,4-tetrazine 1,3-dioxides-a new class of heterocyclic compounds. Bull. Acad. Sci. USSR Div. Chem. Sci. 1990, 39, 639–640. [Google Scholar] [CrossRef]
- Bi, F.Q.; Wang, B.Z.; Li, X.Z.; Fan, X.Z.; Xu, C.; Ge, Z.X. Progress in the Energetic MateriaIs Based on 1,2,3,4-Tetrazine 1,3-Dioxid. Chin. J. Energ. Mater. 2012, 20, 630–637. (In Chinese) [Google Scholar]
- Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J.; Weyrauther, M. The Synthesis and Energetic Properties of 5,7-Dinitrobenzo-1,2,3,4-tetrazine-1,3-dioxide (DNBTDO). Propellants Explos. Pyrotech. 2012, 37, 527–535. [Google Scholar] [CrossRef]
- Christe, K.O.; Dixon, D.A.; Vasiliu, M.; Wagner, R.I.; Haiges, R.; Boatz, J.A.; Ammon, H.L. Are DTTO and iso-DTTO Worthwhile Targets for Synthesis? Propellants Explos. Pyrotech. 2015, 40, 463–468. [Google Scholar] [CrossRef]
- Khakimov, D.V.; Dzyabchenko, A.V.; Pivina, T.S. Computer simulation of the crystal structure of tetrazino-tetrazine tetraoxide (TTTO) isomers with one and two independent molecules in the unit cell. Russ. Chem. Bull. 2020, 69, 212–217. [Google Scholar] [CrossRef]
- Mendoza-Cortes, J.L.; An, Q.; Goddard, W.A., III; Ye, C.; Zybin, S. Prediction of the crystal packing of di-tetrazine-tetroxide (DTTO) energetic material. J. Comput. Chem. 2016, 37, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Li, J.C.; Hou, H.; Wang, B.S. Extensive theoretical studies of a new energetic material: Tetrazino-tetrazine-tetraoxide (TTTO). J. Comput. Chem. 2009, 30, 1816–1820. [Google Scholar] [CrossRef]
- Klenov, M.S.; Guskov, A.A.; Anikin, O.V.; Churakov, A.M.; Strelenko, Y.A.; Fedyanin, I.V.; Lyssenko, K.A.; Tartakovsky, V.A. Synthesis of Tetrazino-tetrazine 1,3,6,8-Tetraoxide (TTTO). Angew. Chem. Int. Ed. 2016, 55, 11472–11475. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, A.E.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Synthesis of 1,2,3,4-tetrazino[5, 6-g]benzo-1,2,3,4-tetrazine-1,3,7,9-tetraoxides. Russ. Chem. Bull. 2006, 55, 1654–1658. [Google Scholar] [CrossRef]
- Frumkin, A.E.; Churakov, A.M.; Strelenko, Y.A.; Kachala, V.V.; Tartakovsky, V.A. Synthesis of 1,2,3,4-Tetrazino[5, 6-f]benzo-1,2,3,4-tetrazine-1,3,7,9-Tetra-N-oxides. Org. Lett. 1999, 1, 721–724. [Google Scholar] [CrossRef]
- Churakov, A.M.; Loffe, S.L.; Tartakovsky, V.A. Synthesis of [1,2,5]Oxadiazolo [3,4-e][1,2,3,4]tetrazine-4,6-Di-N-oxide. Mendeleev Commun. 1995, 6, 227–228. [Google Scholar] [CrossRef]
- Dong, L.L.; Zhang, G.Q.; Chi, Y.; Fan, G.J.; He, L.; Tao, G.H.; Huang, M. Synthesis of Furazano[3,4-e]-1,2,3,4-tetrazine-1,3-dioxide. Chin. J. Energ. Mater. 2012, 20, 690–692. (In Chinese) [Google Scholar]
- Zelenov, V.P.; Lobanova, A.A.; Sysolyatin, S.V.; Sevodina, N.V. New syntheses of [1,2,5]oxadiazolo[3,4-e][1,2,3,4]tetrazine 4,6-dioxide. Russ. J. Org. Chem. 2013, 49, 455–465. [Google Scholar] [CrossRef]
- Li, X.Z.; Wang, B.Z.; Li, H.; Li, Y.A.; Bi, F.Q.; Huo, H.H.; Fan, X.Z. Novel Synthetic Route and Characterization of [1,2,5]oxadiazolo-[3,4-e][1,2,3,4]tetrazine 4,6-Di-N-oxide (FTDO). Chin. J. Org. Chem. 2012, 32, 1975–1980. [Google Scholar] [CrossRef][Green Version]
- Voronin, A.A.; Zelenov, V.P.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Alkylation of 1-hydroxy-1H-[1,2,3]triazolo[4,5-e][1,2,3,4]tetrazine 5,7-dioxide. Russ. Chem. Bull. 2014, 63, 475–479. [Google Scholar] [CrossRef]
- Voronin, A.A.; Zelenov, V.P.; Churakov, A.M.; Strelenko, Y.A.; Fedyanin, I.V.; Tartakovsky, V.A. Synthesis of 1,2,3,4-tetrazine 1,3-dioxides annulated with 1,2,3-triazoles and 1,2,3-triazole 1-oxides. Tetrahedron 2014, 70, 3018–3022. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zheng, C.M.; Liu, Y.; Gong, X.D.; Xia, M.Z. Theoretical studies of the structure, stability, and detonation properties of vicinal-tetrazine 1,3-dioxide annulated with a five-membered heterocycle. 1. Annulation with a triazole ring. J. Mol. Model. 2015, 21, 1–9. [Google Scholar]
- Shvets, A.O.; Konnov, A.A.; Klenov, M.S.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Synthesis of 2-(6-nitrobenzofuroxan-4-yl)-2H-[1,2,3]triazolo-[4,5-e][1,2,3,4]tetrazine 4,6-dioxide. Russ. Chem. Bull. 2020, 69, 739–741. [Google Scholar] [CrossRef]
- Bian, C.M.; Dong, X.; Zhang, X.H.; Zhou, Z.M.; Zhang, M.; Li, C. The unique synthesis and energetic properties of a novel fused heterocycle: 7-nitro-4-oxo-4, 8-dihydro-[1,2,4]triazolo[5,1-d][1,2,3,5]tetrazine 2-oxide and its energetic salts. J. Mater. Chem. A 2015, 3, 3594–3601. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Bian, C.M. Energetic ionic salts of 7-nitryl-4-ketone-4,8-dihydro-[1,2,4]triazole[5,1-d][1,2,3,5]tetrazine-2-oxide and preparation method of energetic ionic salt. CN Patent 104447762, 25 March 2015. (In Chinese). [Google Scholar]
- Zhao, B.J.; Wang, P.P.; Fu, W.; Li, C.; Zhou, Z.M. High Density of a New Fused Heterocycle: 7,8-Dinitro-4-oxo-4,6-dihydropyrazolo[5,1-d][1,2,3,5]tetrazine 2-oxide and Its Energetic Salts. ChemistrySelect 2018, 3, 4797–4803. [Google Scholar] [CrossRef]
- Lei, C.J.; Cheng, G.B.; Yi, Z.X.; Zhang, Q.H.; Yang, H.W. A facile strategy for synthesizing promising pyrazole-fused energetic compounds. Chem. Eng. J. 2021, 416, 129190. [Google Scholar] [CrossRef]
- Tang, Y.X.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Energetic and fluorescent azole-fused 4-amino-1,2,3-triazine-3-N-oxides. ACS Appl. Energy Mater. 2019, 2, 8871–8877. [Google Scholar] [CrossRef]
- Deng, M.C.; Feng, Y.G.; Zhang, W.Q.; Qi, X.J.; Zhang, Q.H. A green metal-free fused-ring initiating substance. Nat. Commun. 2019, 10, 1339. [Google Scholar] [CrossRef]
- Deng, M.C.; Feng, Y.A.; Zhang, Q.H. Green environmental protection type primary explosive and preparation method thereof. CN Patent 108752349, 11 June 2018. (In Chinese). [Google Scholar]
- Ogurtsov, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Fakhrutdinov, A.N.; Zlotin, S.G.; Rakitin, O.A. [1,2,5]Oxadiazolo[3,4-d] pyridazine 1,5,6-trioxides: Efficient synthesis via the reaction of 3,4-bis (hydroxyimino) methyl)-1,2,5-oxadiazole 2-oxides with a mixture of concentrated nitric and trifluoroacetic acids and structural characterization. Tetrahedron Lett. 2018, 59, 3143–3146. [Google Scholar] [CrossRef]
- Liu, Y.G.; Huang, Z.; Yu, X.J. Progress of research of new insensitive energetic material LLM-105. Expl. Shock Wave 2004, 24, 465–469. (In Chinese) [Google Scholar]
- Jing, S.M.; Liu, Y.C.; Yuan, J.M. New Synthesis of LLM-105 and Experimental Study of Replacement Process. Initiat. Pyrotech. 2012, 2, 34–36. (In Chinese) [Google Scholar]
- Zhao, X.F.; Liu, Z.L.; Yao, Q.Z.; Chen, J.; Dong, Y. Synthesis of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Chin. J. Explos. Propellants 2012, 35, 15–17. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.M.; Du, Y. Optimization of Synthetic Method of 2,6-Diamino-pyrazine-1-oxide. Chin. J. Explos. Propellants 2017, 40, 25–27. (In Chinese) [Google Scholar]
- Li, Y.H.; Wang, Y.; Liao, L.Y.; Guo, Z.C.; Chen, L.P.; Wu, W.Q. Thermal Risk Analysis Based on Reaction Mechanism: Application to the 2,6-Diaminopyrazine-1-oxide Synthesis Process. Org. Process Res. Dev. 2021, 25, 849–857. [Google Scholar] [CrossRef]
- Luo, Y.F.; Ma, L.; Wang, B.Z.; Zhou, Y.S.; Huo, H.; Jia, S.Y. Synthesis and Characterization of 3,3’-Dicyano-4,4’-azofuroxan. Chin. J. Energ. Mater. 2010, 18, 538–540. (In Chinese) [Google Scholar]
- He, J.X.; Lu, Y.H.; Lei, Q.; Cao, Y.L. Synthesis and Properties of High Energetic Compound 3,3’-Dinitro-4,4’-azofuroxan. Chin. J. Energ. Mater. 2011, 34, 9–12. (In Chinese) [Google Scholar]
- Ma, C.M.; Pan, Y.; Jiang, J.C.; Liu, Z.L.; Yao, Q.Z. Synthesis and thermal behavior of a fused, tricyclic pyridine-based energetic material: 4-amino-5-nitro-[1,2,5]oxadiazolo[3,4-e]tetrazolo[1,5-a]pyridine-3-oxide. New J. Chem. 2018, 42, 11259–11263. [Google Scholar] [CrossRef]
- He, C.L.; Gao, H.X.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Boosting energetic performance by trimerizing furoxan. J. Mater. Chem. A 2018, 6, 9391–9396. [Google Scholar] [CrossRef]
- Sun, K.L.; Wu, N.; Yang, H.; Yang, X.F.; Li, H. Independent Component Analysis Combined with On-line Infrared Spectroscopy for Researching the Synthesis Reaction Mechanism of 3,4’-Bis(4’-aminofurazano-3’)furoxan. Chem. J. Chin. Univ. 2014, 35, 244–249. (In Chinese) [Google Scholar]
- Li, Y.; Huang, H.F.; Shi, Y.M.; Yang, J.; Pan, R.M.; Lin, X.Y. Potassium nitraminofurazan derivatives: Potential green primary explosives with high energy and comparable low friction sensitivities. Chem. Eur. J. 2017, 23, 7353–7360. [Google Scholar] [CrossRef]
- Tang, Y.X.; He, C.L.; Shreeve, J.M. A furazan-fused pyrazole N-oxide via unusual cyclization. J. Mater. Chem. A 2017, 5, 4314–4319. [Google Scholar] [CrossRef]
- Thottempudi, V.; Yin, P.; Zhang, J.H.; Parrish, D.A.; Shreeve, J.M. 1,2,3-Triazolo [4,5,-e]furazano[3,4-b]pyrazine 6-Oxide—A Fused Heterocycle with a Roving Hydrogen Forms a New Class of Insensitive Energetic Materials. Chem. Eur. J. 2014, 20, 542–548. [Google Scholar] [CrossRef]
- Li, Y.N.; Hu, J.J.; Chen, T.; Wang, B.; Chang, P.; Wang, B.Z. Synthesis and Properties of 4-Amino-1,2,3-triazolo[4,5-e]furazano[3,4-b]pyrazine 6-oxide. Chin. J. Energ. Mater. 2020, 28, 664–669. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).