Spin-Polarized Study of the Structural, Optoelectronic, and Thermoelectric Properties of the Melilite-Type Gd2Be2GeO7 Compound
Abstract
:1. Introduction
2. Methodology and Calculation Settings
3. Results and discussion
3.1. Structural Properties
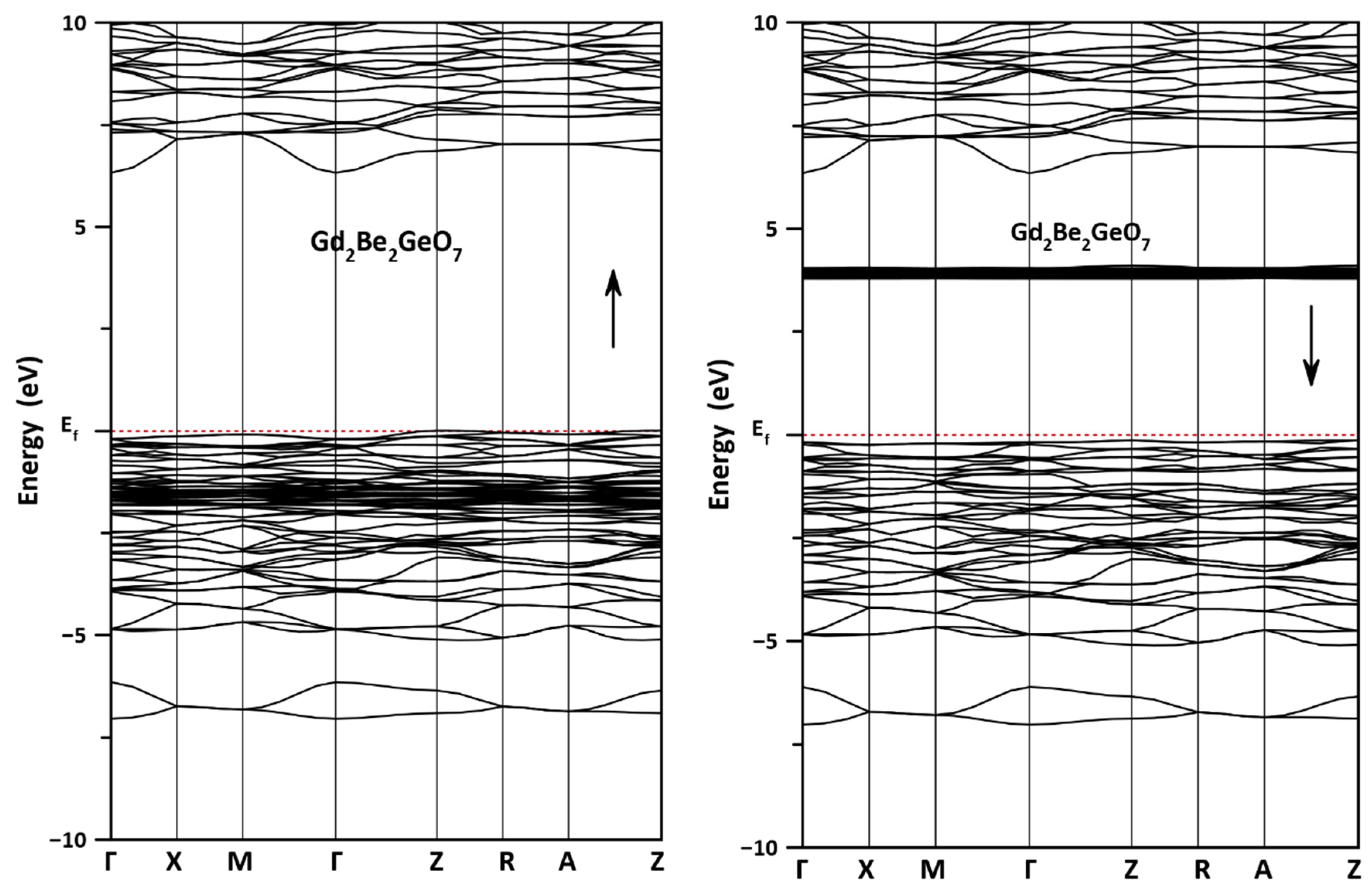
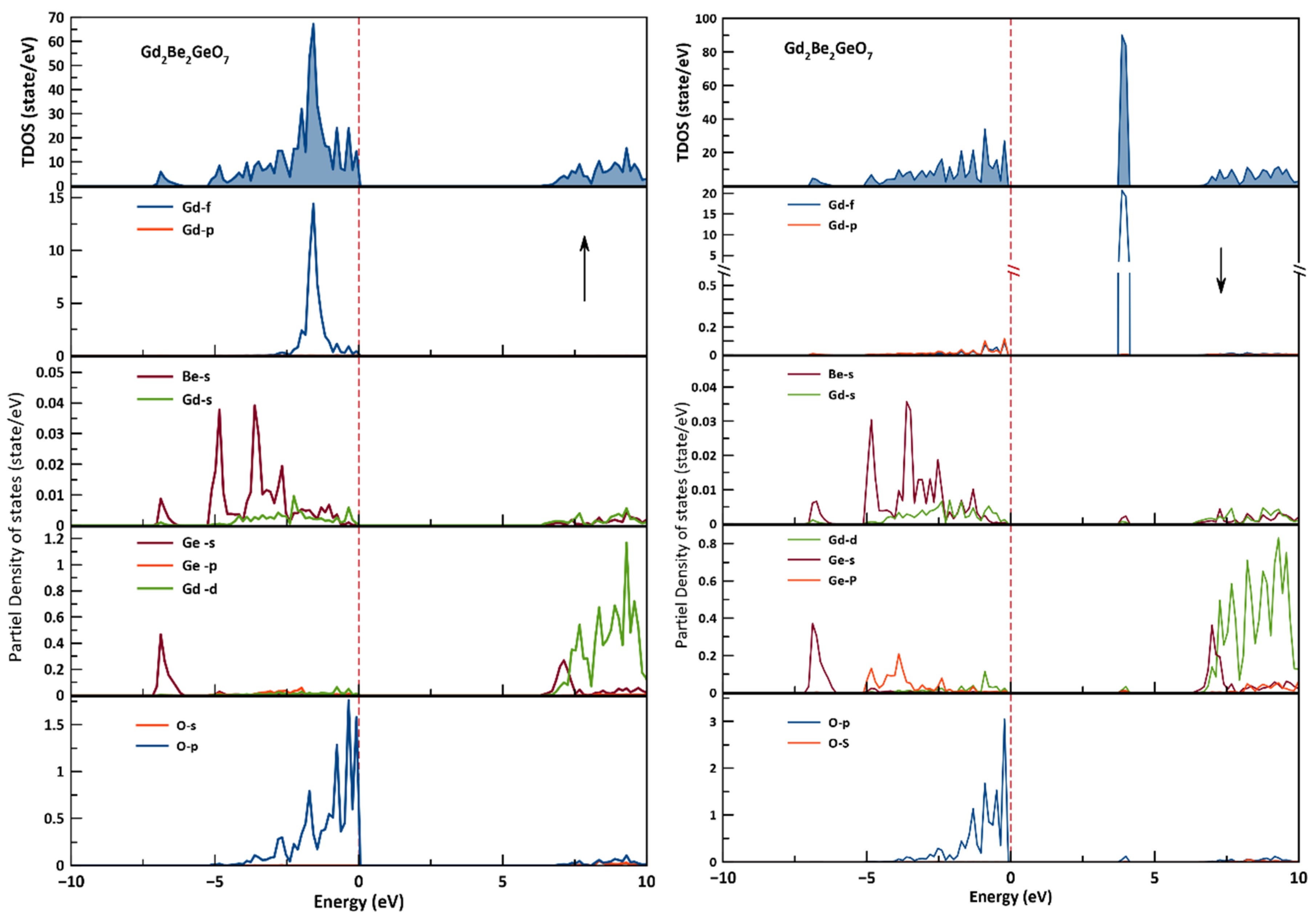
3.2. Electronic Properties
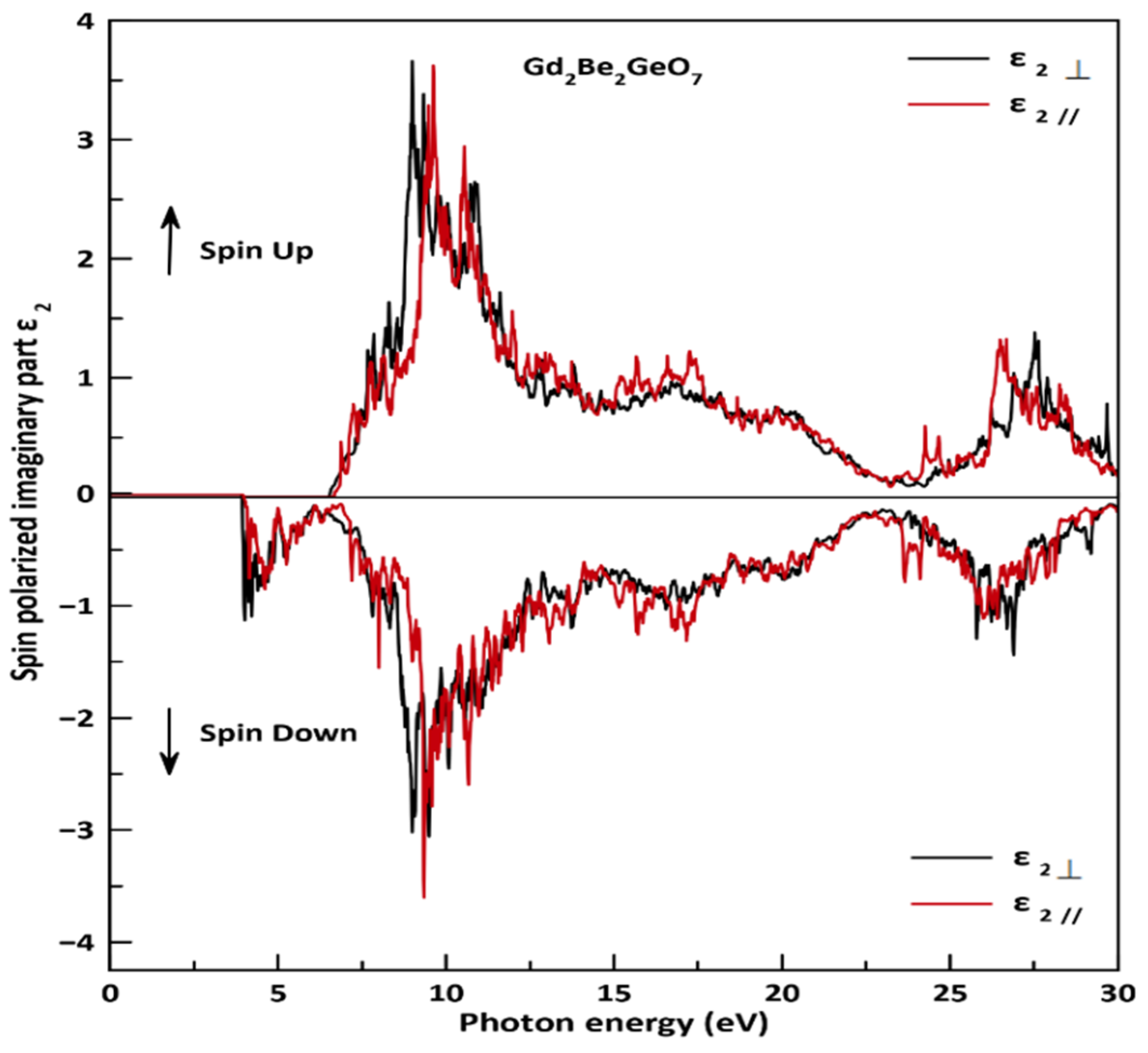
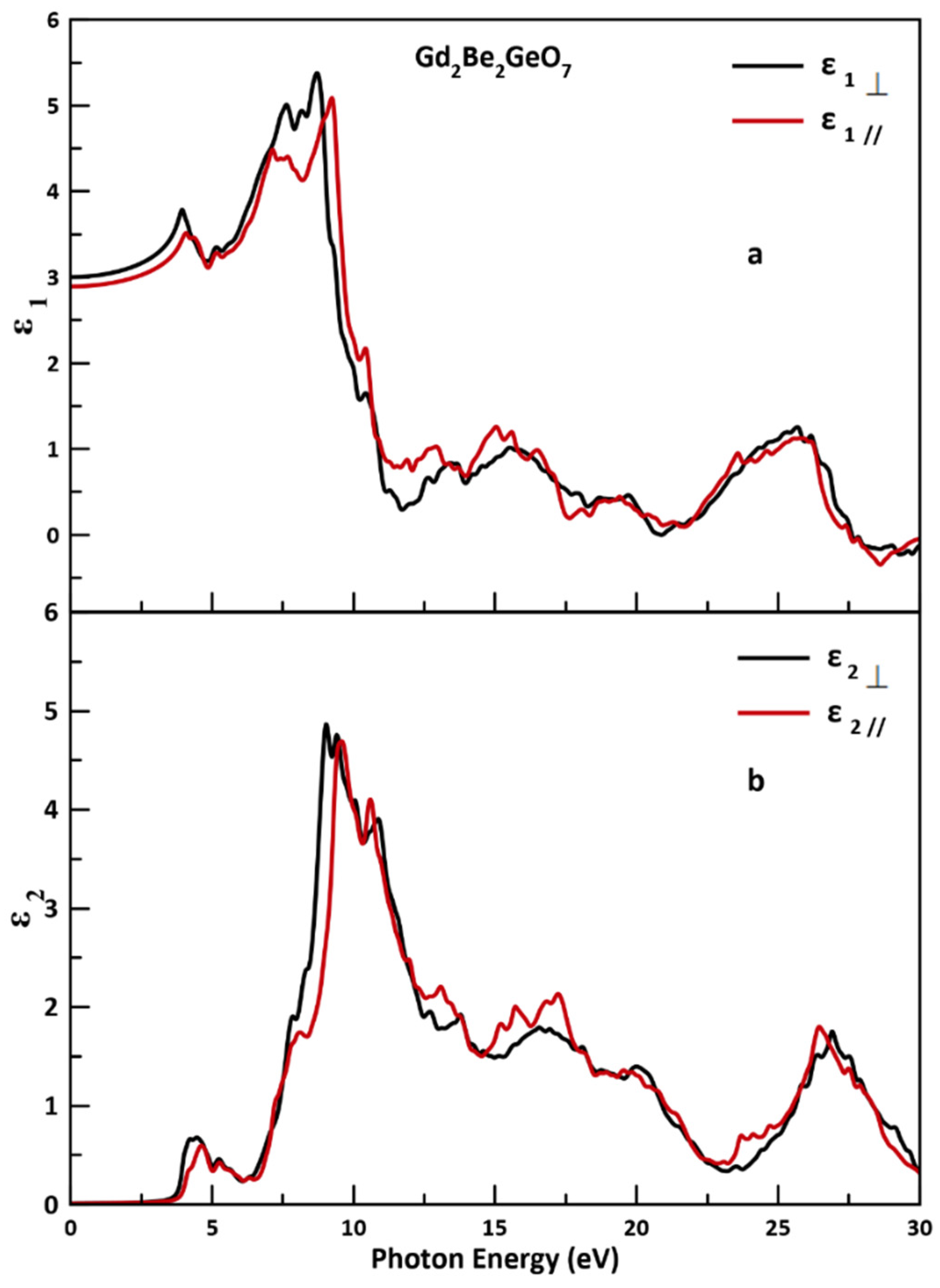
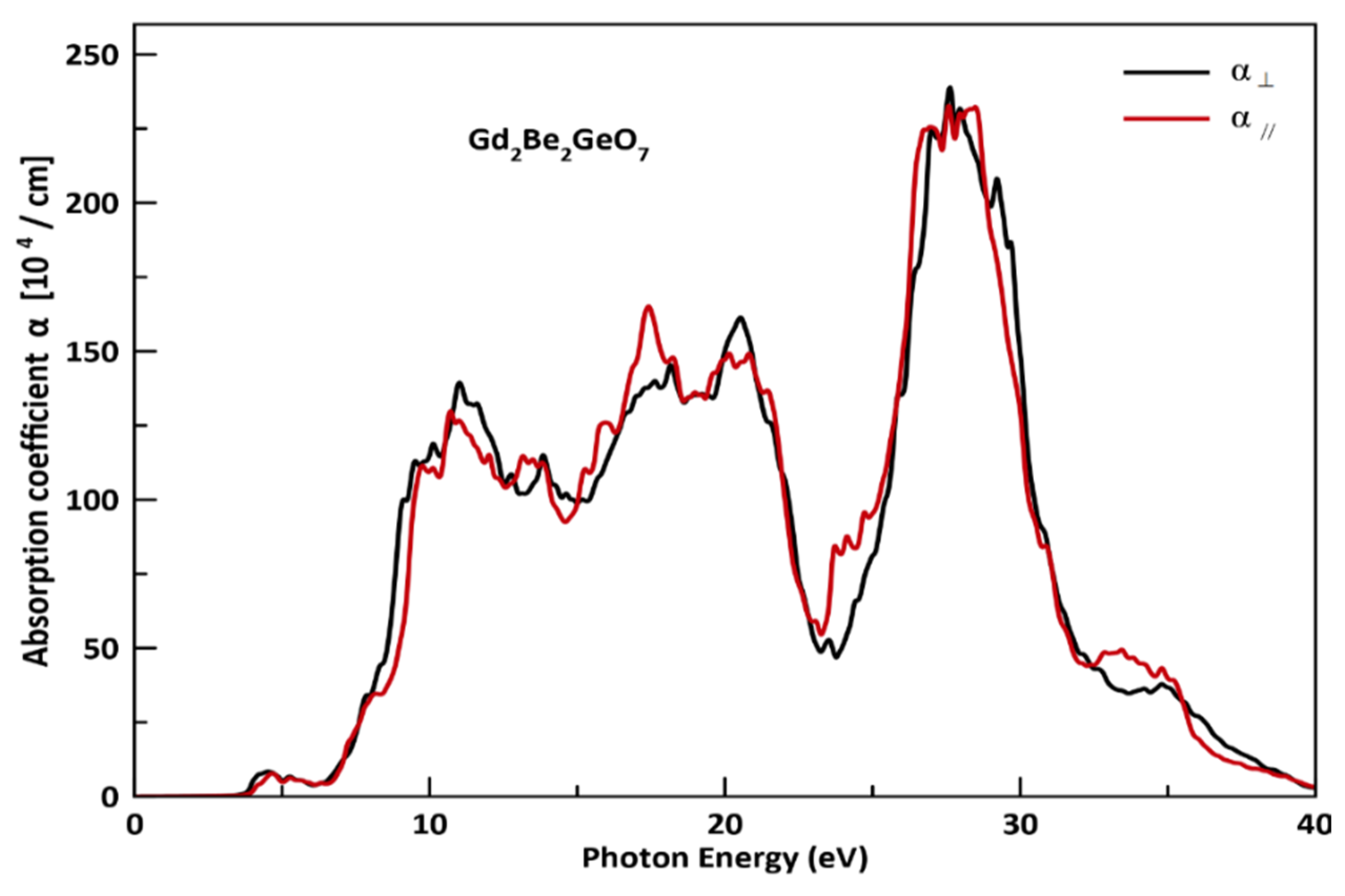
3.3. Optical Properties
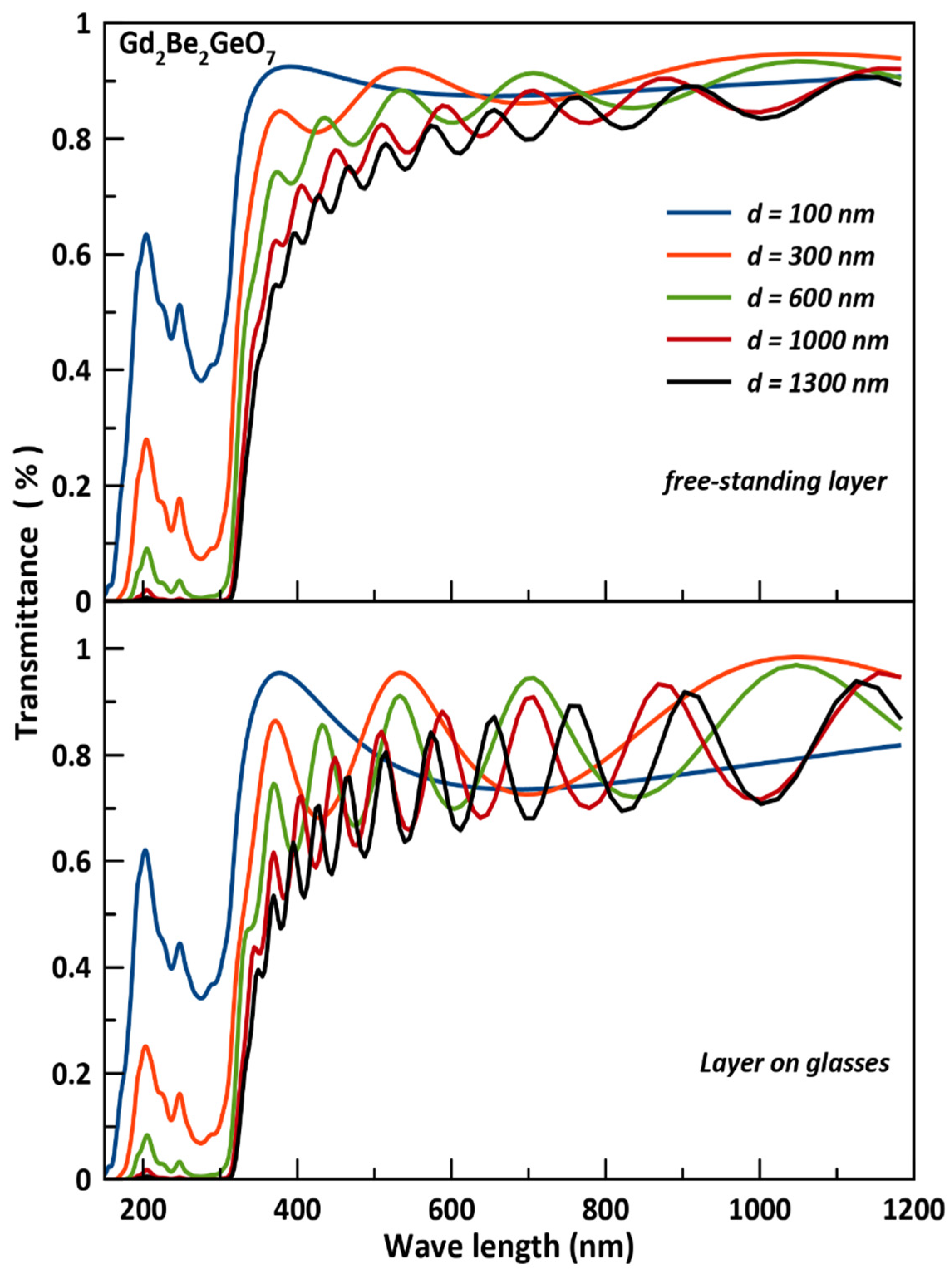
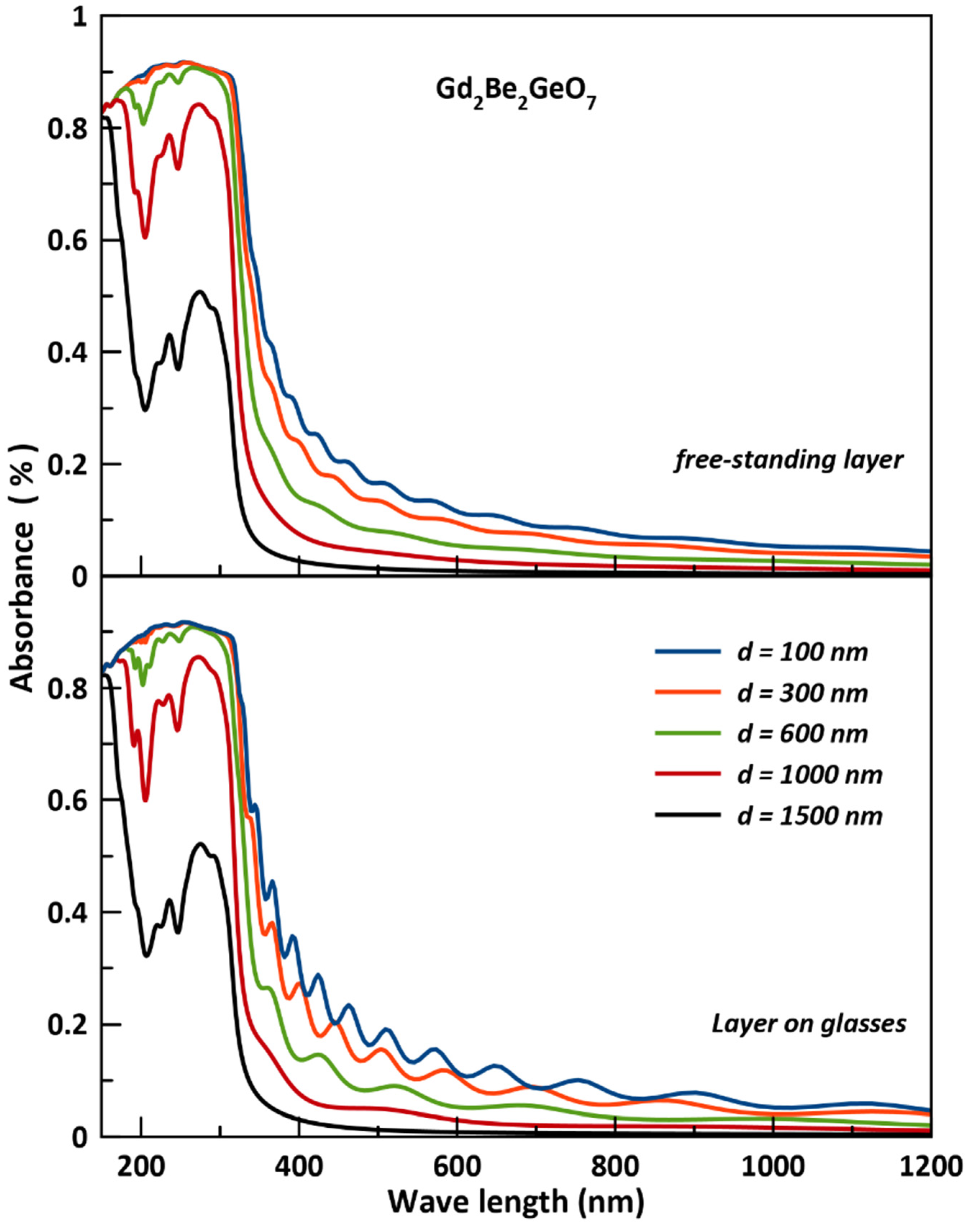
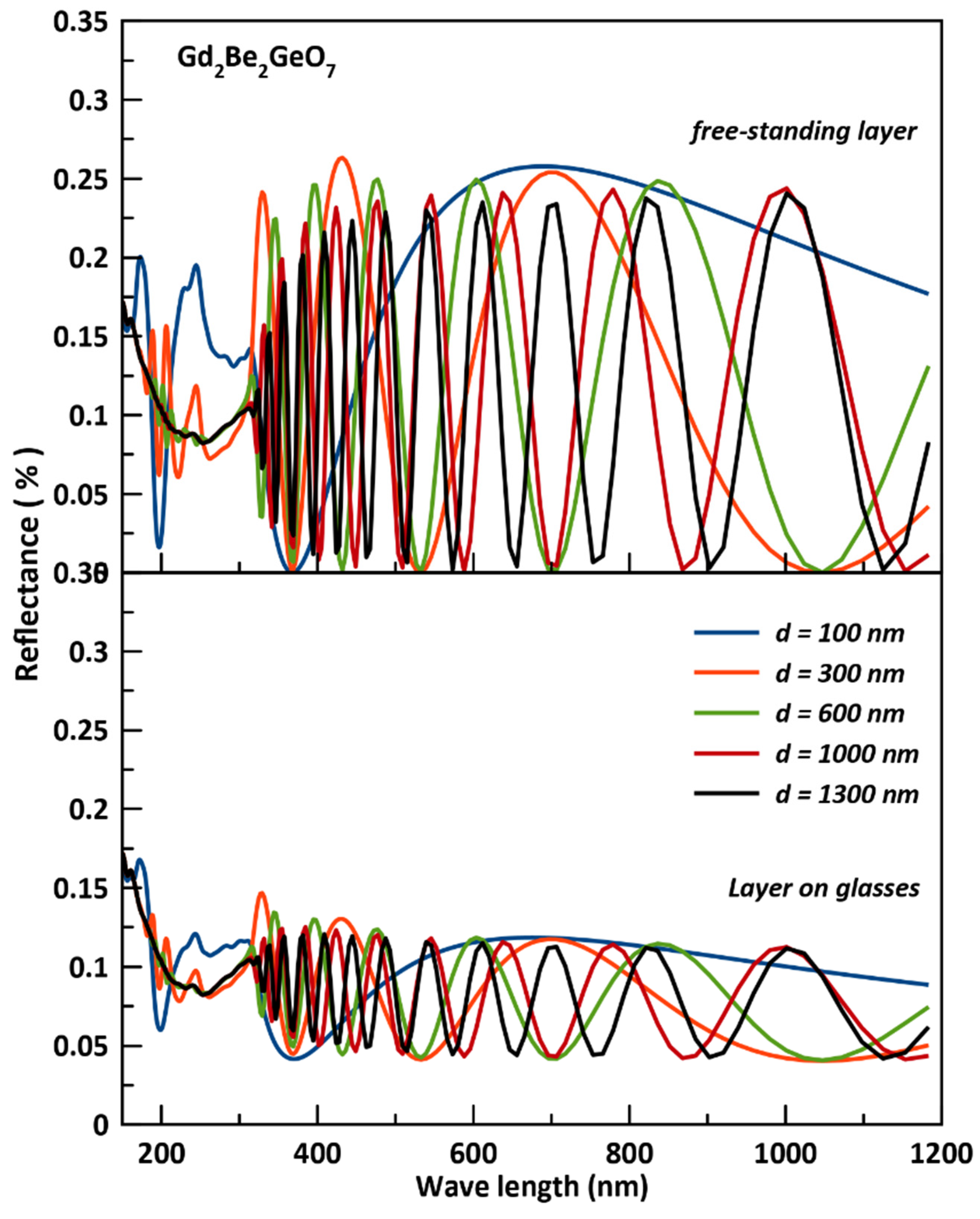
3.4. Optical Coating Characteristics
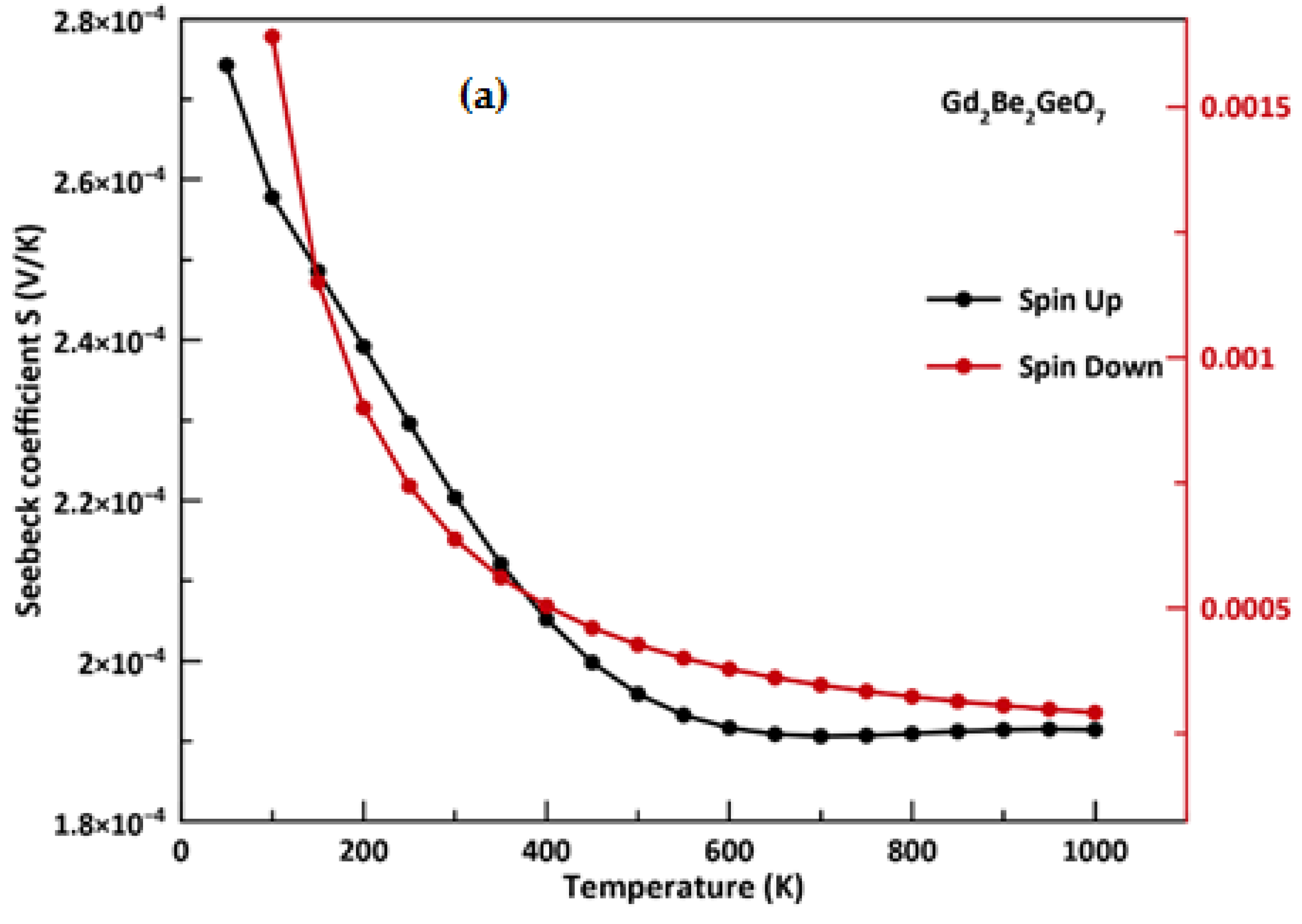
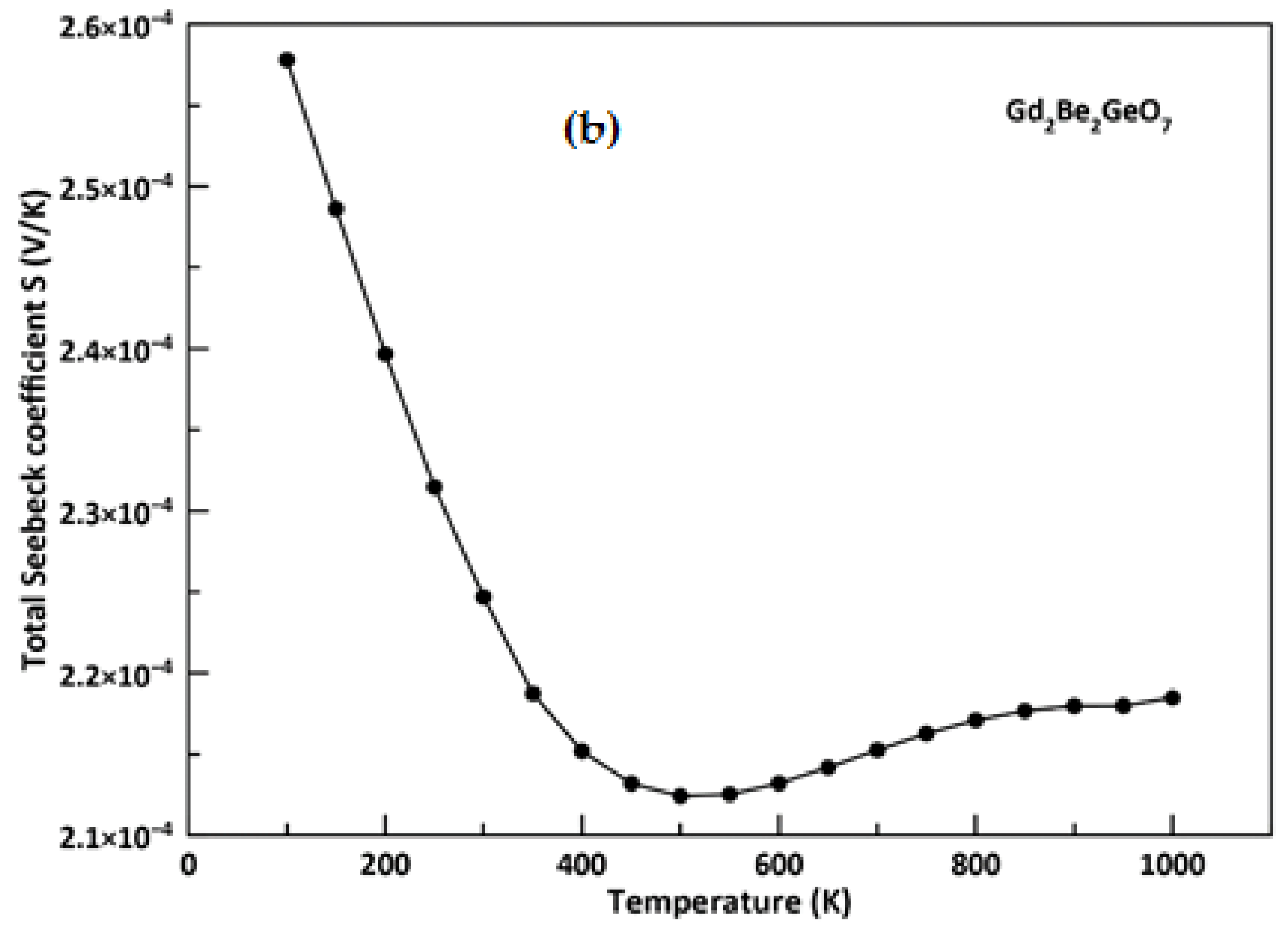
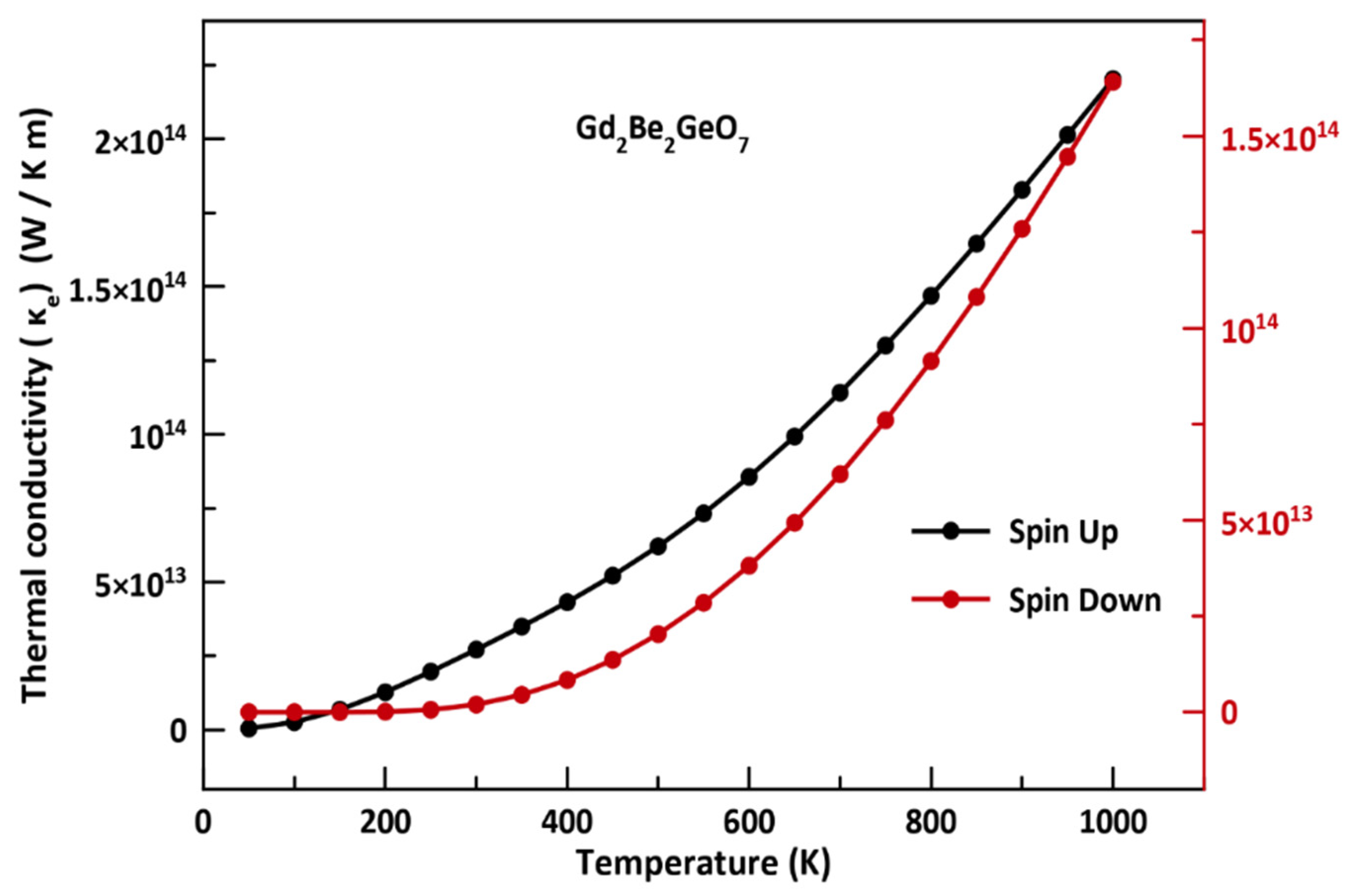
3.5. Thermoelectric Properties
4. Conclusions
- The calculated lattice constants agree well with the available experimental data.
- The studied Gd2Be2GeO7 as thin film could be a good transparent in the visible spectrum (400–700 nm) for very thin thickness of 100 nm which makes it a suitable candidate for optical coating design devices; furthermore, it can be more efficient for applications in the ultraviolet (UV) region of the spectrum, and it could be useful for the high frequency UV device applications regarding the high absorbance peaks found in the range of 200 nm to 300 nm.
- From spin-polarized thermoelectric calculations, the material under discussion presents adequate Seebeck coefficients around room temperature; furthermore, we found higher values of the figure of merit (around 0.99 and 0.70) over large temperature ranges which suggest that this material exhibits good thermoelectric performance and it could be a promising material for applications in thermoelectric generators.
- Finally, the electrical conductivity (σ/τ) as a function of temperature exhibits the behavior of a typical semiconductor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nurse, R.W.; Midgley, H.G. The system CaO-fe2O3-al2O 3-sio2. J. Iron Steel Inst. 1953, 174, 121. [Google Scholar]
- Burzo, E. Melilites and related silicateS. In Sorosilicates; Springer: Berlin/Heidelberg, Germany, 2009; Volume 14, pp. 29–60. [Google Scholar]
- Louisnathan, S.J. Refinement of the crystal structure of a natural gehlenite, Ca2Al(Al, Si)2O7. Can. Mineral. 1971, 10, 822–837. [Google Scholar]
- Kuang, X.; Green, M.A.; Niu, H.; Zajdel, P.; Dickinson, C.; Claridge, J.B.; Jantsky, L.; Rosseinsky, M.J. Interstitial oxide ion conductivity in the layered tetrahedral network melilite structure. Nat. Mater. 2008, 7, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Xia, Z.; Ma, C.; Molokeev, M.S.; Liu, Q.; Rickert, K.; Poeppelmeier, K.R. Chemical Unit Cosubstitution and Tuning of Photoluminescence in the Ca2(Al1–xMgx)(Al1–xSi1+x)O7:Eu2+ Phosphor. J. Am. Chem. Soc. 2015, 137, 12494–12497. [Google Scholar] [CrossRef]
- Louisnathan, S.J. Refinement of the crystal structure of hardystonite, Ca2ZnSi2O7. Z. Krist. Cryst. Mater. 1969, 130, 427. [Google Scholar] [CrossRef]
- Weiss, J.; Rosenstein, G. Addition of Ba2TiSi2O8 to manganese-doped barium titanate: Effect on oxygen diffusion and grain-boundary composition. J. Mater. Sci. 1988, 23, 3263–3271. [Google Scholar] [CrossRef]
- Peng, C.; Tsao, R.; Chou, Z. Gugiaite, Ca2BeSi2O7, a new beryllium mineral and its relation to the melilite group. Sci. Sin. 1962, 11, 977–988. [Google Scholar]
- Warren, B.E. The structure of melilite (Ca,Na)2(Mg,Al)1(Si,Al)2O7. Z. Kristallogr. 1930, 74, 131–138. [Google Scholar] [CrossRef]
- Ben Yahia, H.; Essehli, R.; Belharouak, I.; Gaudin, E. Crystal structure and magnetic properties of K2CoV2O7. Mater. Res. Bull. 2015, 71, 7–10. [Google Scholar] [CrossRef]
- Matsubara, S.; Miyawaki, R.; Kato, A.; Yokoyama, K.; Okamoto, A. Okayamalite, Ca2B2SiO7, a new mineral, boron analogue of gehlenite. Mineral. Mag. 1998, 62, 703–706. [Google Scholar] [CrossRef]
- Akasaka, M.; Ohashi, H. 57Fe mössbauer study of synthetic Fe3+-melilites. Phys. Chem. Miner. 1985, 12, 13–18. [Google Scholar] [CrossRef]
- Smith, J.V. Reexamination of the crystal structure of melilite. Amer. Miner. 1953, 38, 643–661. [Google Scholar]
- Smith, J.V. X-ray-emission microanalysis of rock-forming minerals. I. Experimental techniques. J. Geol. 1965, 73, 830–864. [Google Scholar] [CrossRef]
- Tyutyunnik, A.P.; Zubkov, V.G.; Surat, L.L.; Slobodin, B.V.; Svensson, G. Synthesis and crystal structure of the pyrovanadate Na2ZnV2O7. Powder Diffr. 2005, 20, 189–192. [Google Scholar] [CrossRef]
- Kloss, S.D.; Weidmann, N.; Niklaus, R.; Schnick, W. High-Pressure Synthesis of Melilite-type Rare-Earth Nitridophosphates RE2P3N7 and a Ba2Cu[Si2O7]-type Polymorph. Inorg. Chem. 2016, 55, 9400–9409. [Google Scholar] [CrossRef] [PubMed]
- Ochi, Y.; Morikawa, H.; Minato, I.; Marumo, F. Preparation and magnetic property of new rare earth compounds R2GeBe2O7 (R = La, Pr, Sm, Gd, Dy, Er) and Y2GeBe2O7. Mat. Res. Bull. 1982, 17, 911–916. [Google Scholar] [CrossRef]
- Ashtar, M.; Bai, Y.; Xu, L.; Wan, Z.; Wei, Z.; Liu, Y.; Marwat, M.A.; Tian, Z. Structure and Magnetic Properties of Melilite-Type Compounds RE2Be2GeO7 (RE = Pr, Nd, Gd–Yb) with Rare-Earth Ions on Shastry–Sutherland Lattice. Inorg. Chem. 2021, 60, 3626–3634. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.K. Linear methods in band theory. Phys. Rev. B. 1975, 12, 3060–3083. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.J. Plane Waves, Pseudopotentials and the LAPW Method; Kluwer Academic: Dodrecht, The Netherlands, 1994. [Google Scholar]
- Schwarz, K.; Blaha, P.; Madsen, G.K.; Kvasnicka, D.; Luitz, J. An Augmented Plane Waves + Local Orbital Program for calculating Crystal Properties; Institut Für Physikalische und Theoretische Chemie: Vienna, Austria, 2001. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D.; Johnson, E.R. A simple effective potential for exchange. J. Chem. Phys. 2006, 124, 221101. [Google Scholar] [CrossRef] [PubMed]
- Tran, F.; Blaha, P. Accurate Band Gaps of Semiconductors and Insulators with a Semilocal Exchange-Correlation Potential. Phys. Rev. Lett. 2009, 102, 226401. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.; Tran, F.; Blaha, P. Merits and limits of the modified Becke-Johnson exchange potential. Phys. Rev. B 2011, 83, 195134. [Google Scholar] [CrossRef] [Green Version]
- Koller, D.; Tran, F.; Blaha, P. Improving the modified Becke-Johnson exchange potential. Phys. Rev. B 2012, 85, 155109. [Google Scholar] [CrossRef] [Green Version]
- Madsen, G.K.; Singh, D.J. BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244. [Google Scholar] [CrossRef] [Green Version]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809. [Google Scholar] [CrossRef]
- Available online: https://materialsproject.org/materials/mp-650715?chemsys=Gd-Be-Ge-O (accessed on 17 August 2022).
- Ambrosch-Draxl, C.; Sofo, J.O. Linear optical properties of solids within the full-potential linearized augmented planewave method. Comput. Phys. Commun. 2006, 175, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Amin, B.; Ahmad, I.; Maqbool, M.; Goumri-Said, S.; Ahmad, R. Ab initio study of the bandgap engineering of Al1−xGaxN for optoelectronic applications. J. Appl. Phys. 2011, 109, 023109. [Google Scholar] [CrossRef] [Green Version]
- Bassani, F.; Parravicini, G.P.; Ballinger, R.A.; Birman, J.L. Electronic States and Optical Transitions in Solids; Pergamon Press: Oxford, UK, 1973. [Google Scholar]
- Kramers, H.A. Collected Science Papers; North Holland: Amsterdam, The Netherlands, 1956. [Google Scholar]
- Kronig, R.D.L. On the Theory of Dispersion of X-Rays. J. Opt. Soc. Am. 1926, 12, 547. [Google Scholar] [CrossRef]
- Macleod, H.A. Thin Film Optical Filters, 2nd ed.; Adam Hilger: Bristol, UK, 1986. [Google Scholar]
- Born, M.; Wolf, E. Principles of Optics, 3rd ed.; Pergamon: Oxford, UK, 1965. [Google Scholar]
- Derkaoui, Z.; Kebbab, Z.; Miloua, R.; Benramdane, N. Theoretical study of optical characteristics of multilayer coatings ZnO/CdS/CdTe using first-principles calculations. Solid State Commun. 2009, 149, 1231–1235. [Google Scholar] [CrossRef]
- Miloua, R.; Kebbab, Z.; Chiker, F.; Sahraoui, K.; Khadraoui, M.; Benramdane, N. Determination of layer thickness and optical constants of thin films by using a modified pattern search method. Opt. Lett. 2012, 37, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Miloua, R.; Kebbab, Z.; Chiker, F.; Khadraoui, M.; Sahraoui, K.; Bouzidi, A.; Medles, M.; Mathieu, C.; Benramdane, N. Peak, multi-peak and broadband absorption in graphene-based one-dimensional photonic crystal. Opt. Commun. 2014, 330, 135–139. [Google Scholar] [CrossRef]
- Boukabrine, F.; Chiker, F.; Miloua, R.; Kebbab, Z.; Khenata, R.; Prakash, D.; Bin Omran, S.; Verma, K. Combined theoretical studies of the optical characteristics of II-IV-V2 semiconductor thin films. Opt. Mater. 2016, 54, 200–206. [Google Scholar] [CrossRef]
- Lafort, A.; Kebaili, H.; Goumri-Said, S.; Deparis, O.; Cloots, R.; De Coninck, J.; Voué, M.; Mirabella, F.; Maseri, F.; Lucas, S. Optical properties of thermochromic VO2 thin films on stainless steel: Experimental and theoretical studies. Thin Solid Film. 2011, 519, 3283–3287. [Google Scholar] [CrossRef]
- Azam, S.; Khan, S.A.; Minar, J.; Goumri-Said, S. Exploring the electronic structure and optical properties of new inorganic luminescent materials Ba(Si,Al)5(O,N)8 compounds for light-emitting diodes devices. Curr. Appl. Phys. 2015, 15, 1160–1167. [Google Scholar] [CrossRef]
- Liu, M.-L.; Chen, I.-W.; Huang, F.-Q.; Chen, L.-D. Improved Thermoelectric Properties of Cu-Doped Quaternary Chalcogenides of Cu2CdSnSe4. Adv. Mater. 2009, 21, 3808–3812. [Google Scholar] [CrossRef]
- Fan, F.-J.; Yu, B.; Wang, Y.-X.; Zhu, Y.-L.; Liu, X.-J.; Yu, S.-H.; Ren, Z. Colloidal synthesis of Cu2CdSnSe4 nanocrystals and hot-pressing to enhance the thermoelectric figure-of-merit. J. Am. Chem. Soc. 2011, 133, 15910–15913. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goumri-Said, S. Probing Optoelectronic and Thermoelectric Properties of Lead-Free Perovskite SnTiO3: HSE06 and Boltzmann Transport Calculations. Crystals 2022, 12, 1317. [Google Scholar] [CrossRef]
- Goumri-Said, S.; Alrebdi, T.A.; Deligoz, E.; Ozisik, H.; Kanoun, M.B. Revisiting the Electronic Structures and Phonon Properties of Thermoelectric Antimonide-Tellurides: Spin–Orbit Coupling Induced Gap Opening in ZrSbTe and HfSbTe. Crystals 2021, 11, 917. [Google Scholar] [CrossRef]
- Ding, G.; Gao, G.; Yao, K. High-efficient thermoelectric materials: The case of orthorhombic IV-VI compounds. Sci. Rep. 2015, 5, 9567. [Google Scholar] [CrossRef]
- Khan, W.; Azam, S.; Kanoun, M.B.; Goumri-Said, S. Optoelectronic structure and related transport properties of BiCuSeO-based oxychalcogenides: First principle calculations. Solid State Sci. 2016, 58, 86–93. [Google Scholar] [CrossRef]
- Azam, S.; Goumri-Said, S.; Khan, S.A.; Kanoun, M.B. Electronic, optical and thermoelectric properties of new metal-rich homological selenides with palladium–indium: Density functional theory and Boltzmann transport model. J. Phys. Chem. Solids 2020, 138, 109229. [Google Scholar] [CrossRef]

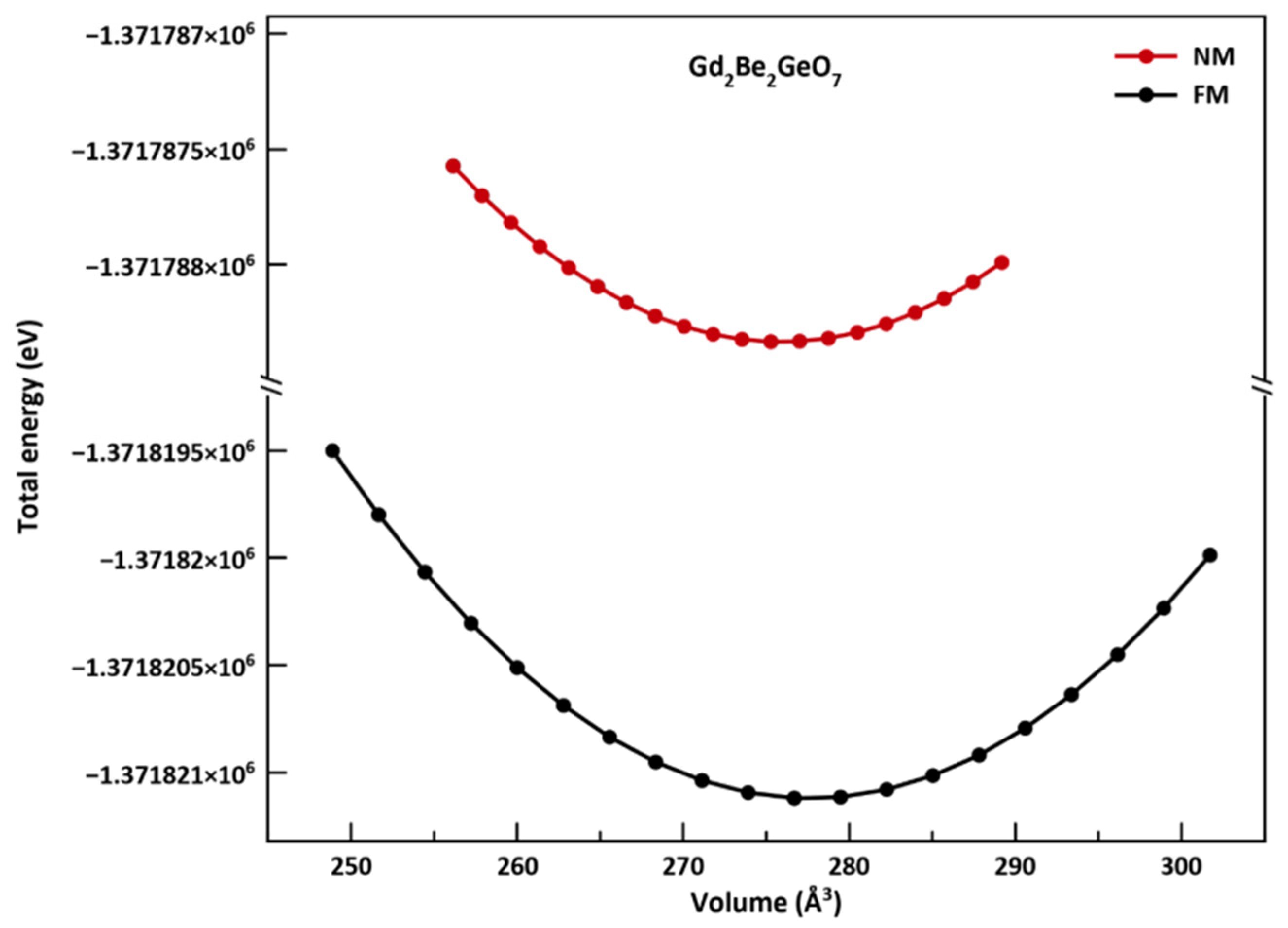




| Gd2Be2GeO7 | a () | c () | V () | B0 (GPa) | B’ | E0 (×106 eV) | Reference | |
| 7.516 | 4.878 | 275.654 | 160.95 | 3.880 | −1.371788 | Present work | NM | |
| 7.525 | 4.883 | 276.567 | 165.91 | 4.274 | −1.371821 | Present work | FM | |
| 7.480 | 4.842 | 270.910 | - | - | - | [18] | ||
| 7.494 | 4.842 | 271.894 | - | - | - | [19] |
| k-Points | Majority Spin (Spin-up) | Minority Spin (Spin-down) | ||
|---|---|---|---|---|
| HVB | LCB | HVB | LCB | |
| Γ | −0.199 | 6.327 | −0.183 | 3.792 |
| X | −0.132 | 7.154 | −0.246 | 3.792 |
| M | −0.087 | 7.283 | −0.212 | 3.796 |
| Z | 0.007 | 6.862 | −0.139 | 3.788 |
| R | −0.044 | 7.025 | −0.191 | 3.79 |
| A | −0.08 | 7.026 | −0.164 | 3.803 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiker, F.; Baki, N.; Khachai, Y.A.; Khachai, H.; Miloua, R.; Khenata, R.; Bin-Omran, S.; Bouhemadou, A.; Kanoun, M.B.; Goumri-Said, S. Spin-Polarized Study of the Structural, Optoelectronic, and Thermoelectric Properties of the Melilite-Type Gd2Be2GeO7 Compound. Crystals 2022, 12, 1397. https://doi.org/10.3390/cryst12101397
Chiker F, Baki N, Khachai YA, Khachai H, Miloua R, Khenata R, Bin-Omran S, Bouhemadou A, Kanoun MB, Goumri-Said S. Spin-Polarized Study of the Structural, Optoelectronic, and Thermoelectric Properties of the Melilite-Type Gd2Be2GeO7 Compound. Crystals. 2022; 12(10):1397. https://doi.org/10.3390/cryst12101397
Chicago/Turabian StyleChiker, Fafa, Najet Baki, Yasser Abderrahim Khachai, Houari Khachai, Redouane Miloua, Rabah Khenata, Saad Bin-Omran, Abdelmadjid Bouhemadou, Mohammed Benali Kanoun, and Souraya Goumri-Said. 2022. "Spin-Polarized Study of the Structural, Optoelectronic, and Thermoelectric Properties of the Melilite-Type Gd2Be2GeO7 Compound" Crystals 12, no. 10: 1397. https://doi.org/10.3390/cryst12101397





