Assessment of the Possibility of Galvanic Corrosion in Aluminum Microchannel Heat Exchangers
Abstract
:1. Introduction
2. Test Methodology
3. Research
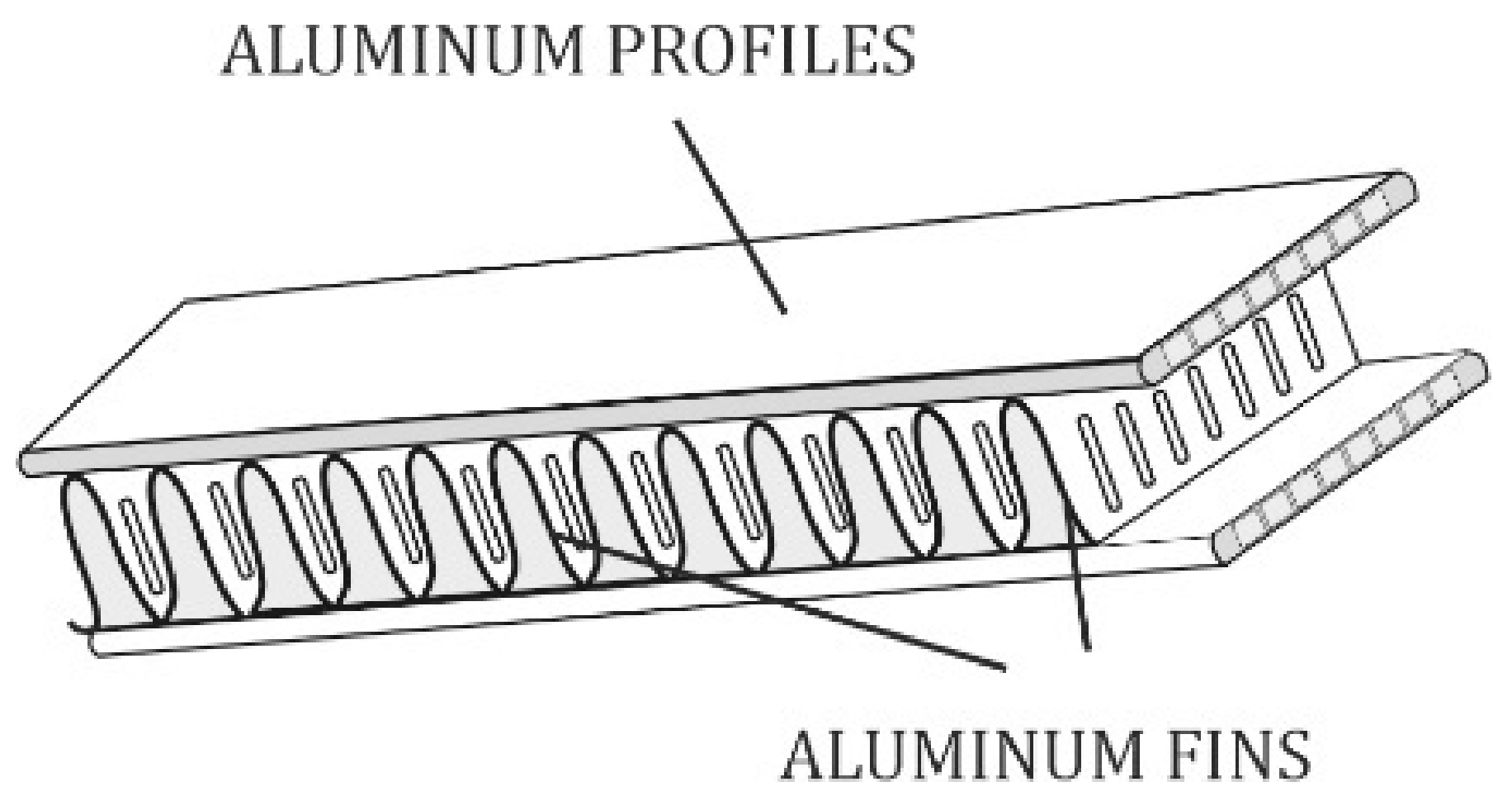
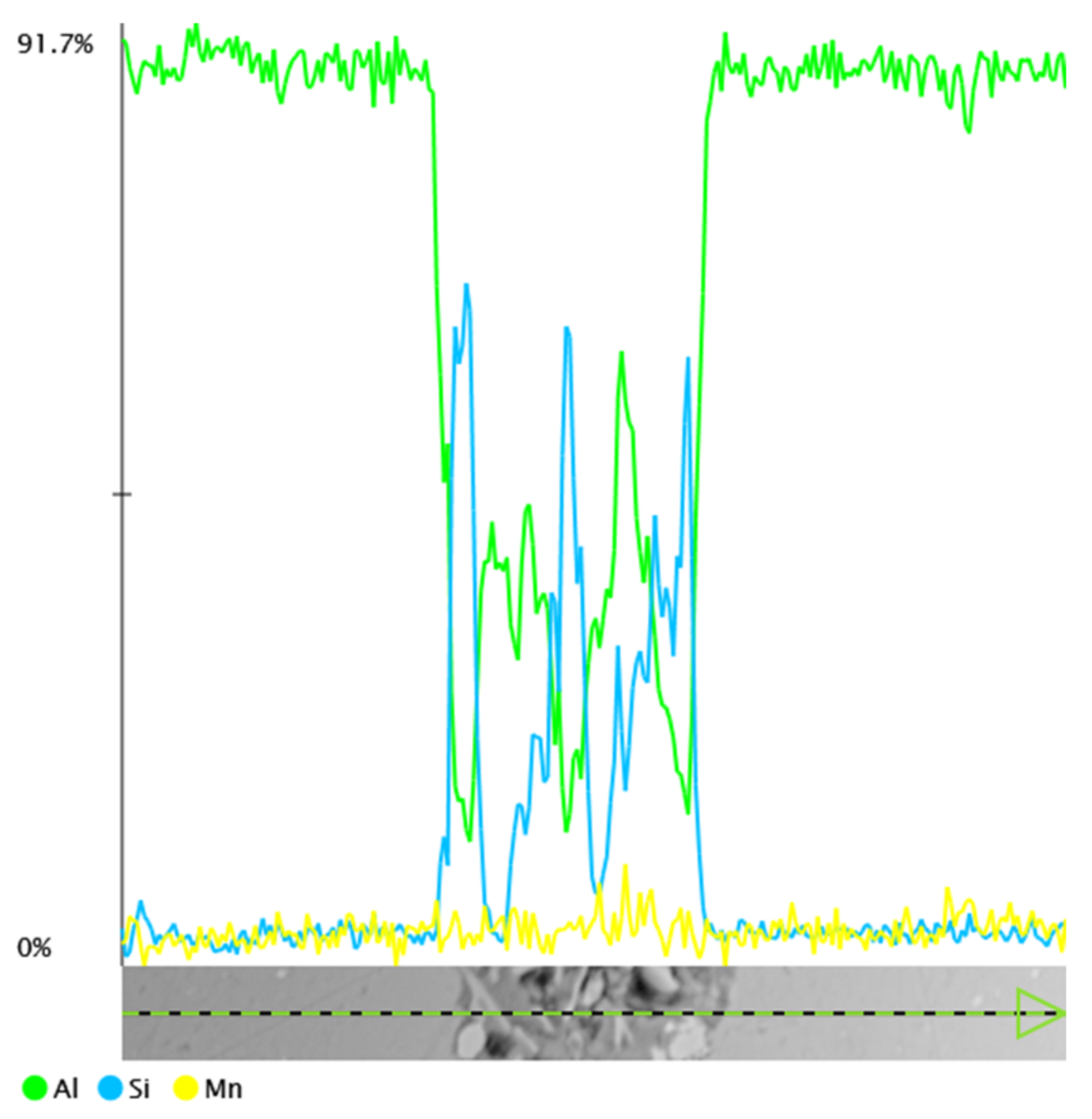
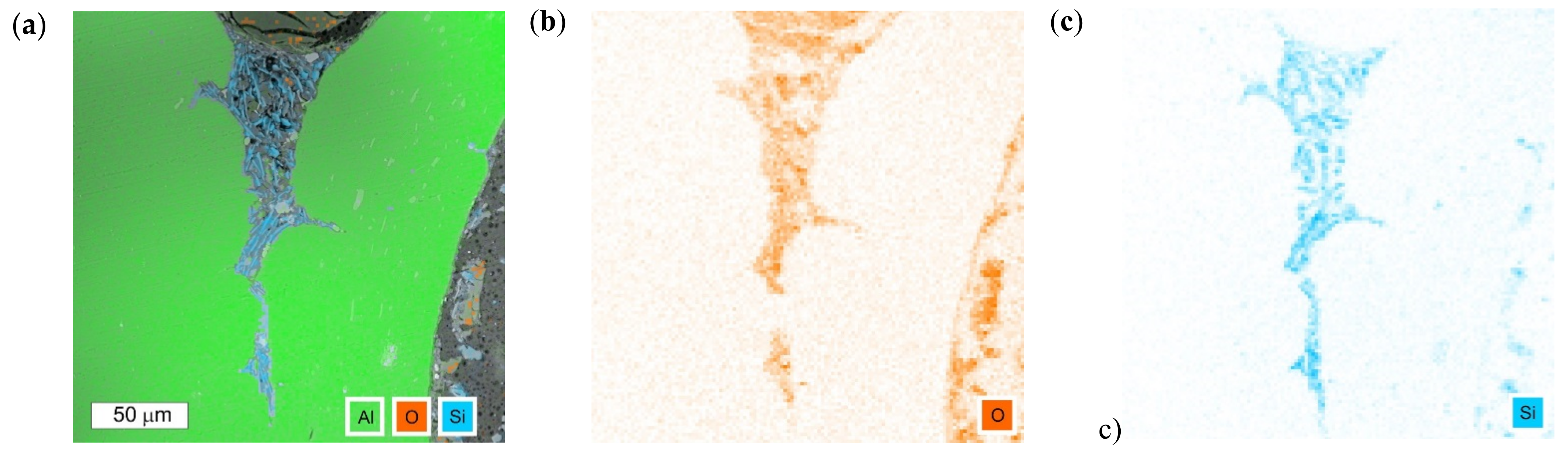
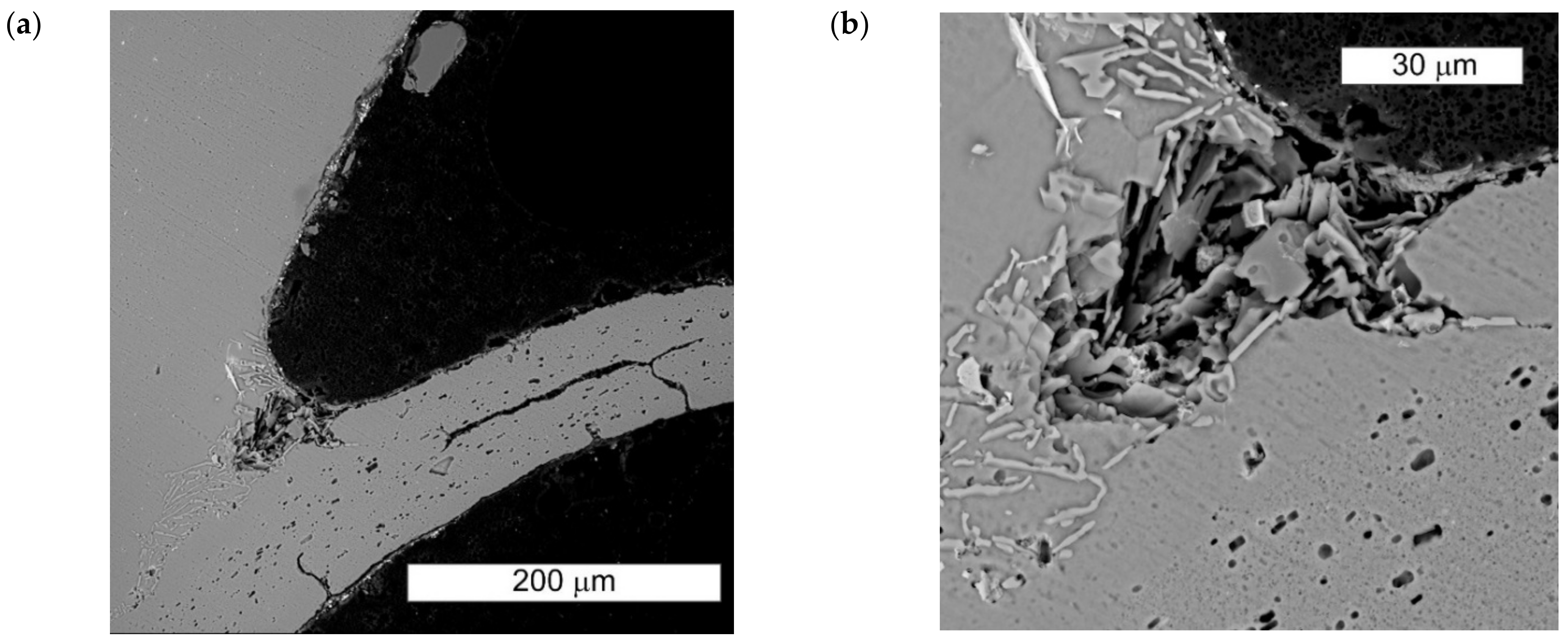
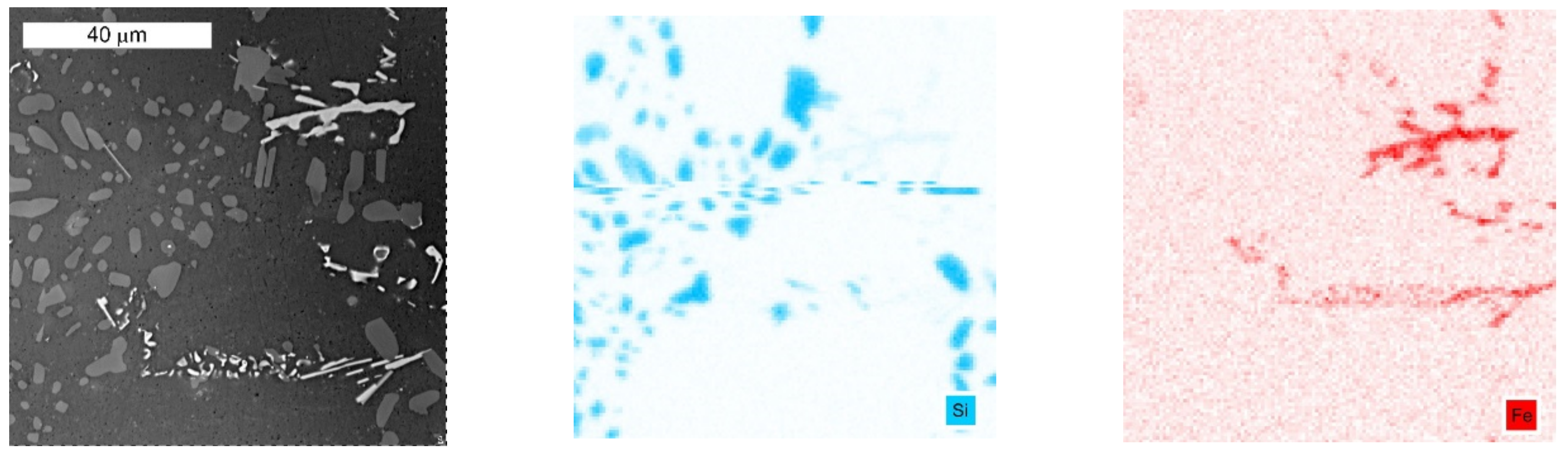
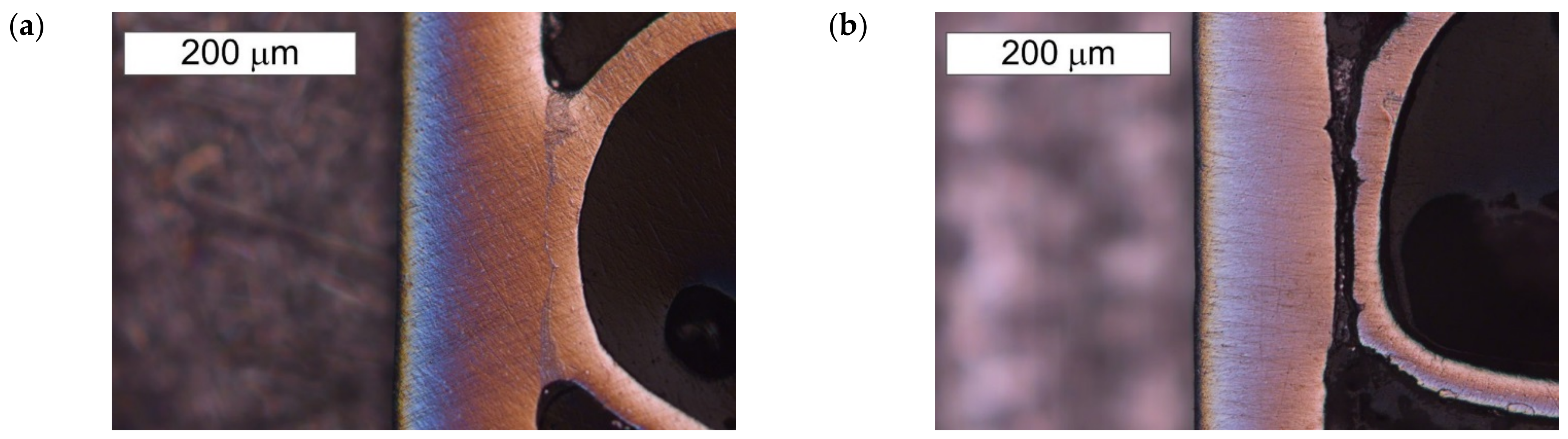
3.1. Microscopic Examination of Decommissioned Heat Exchangers
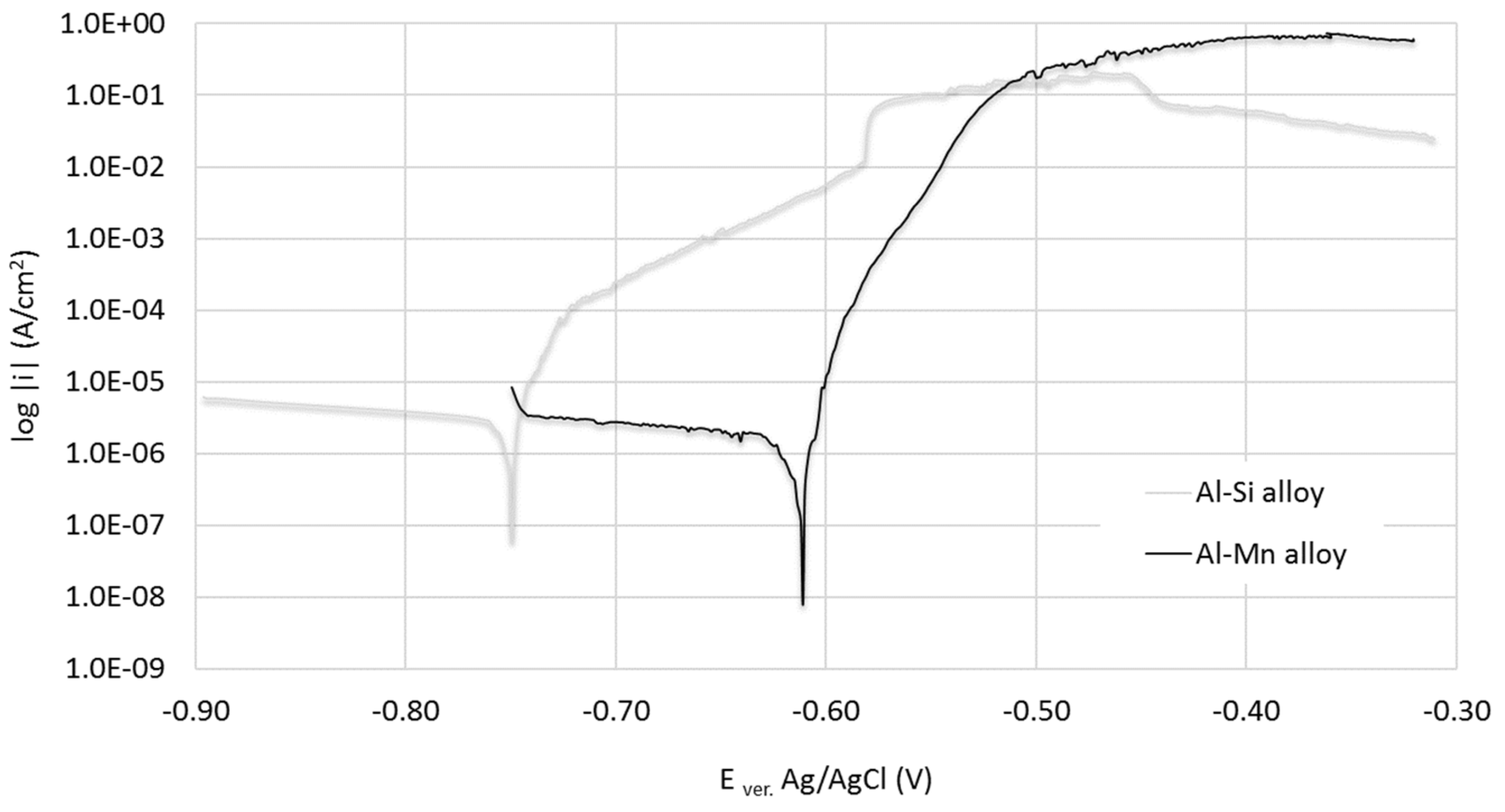
3.2. Electrochemical Test
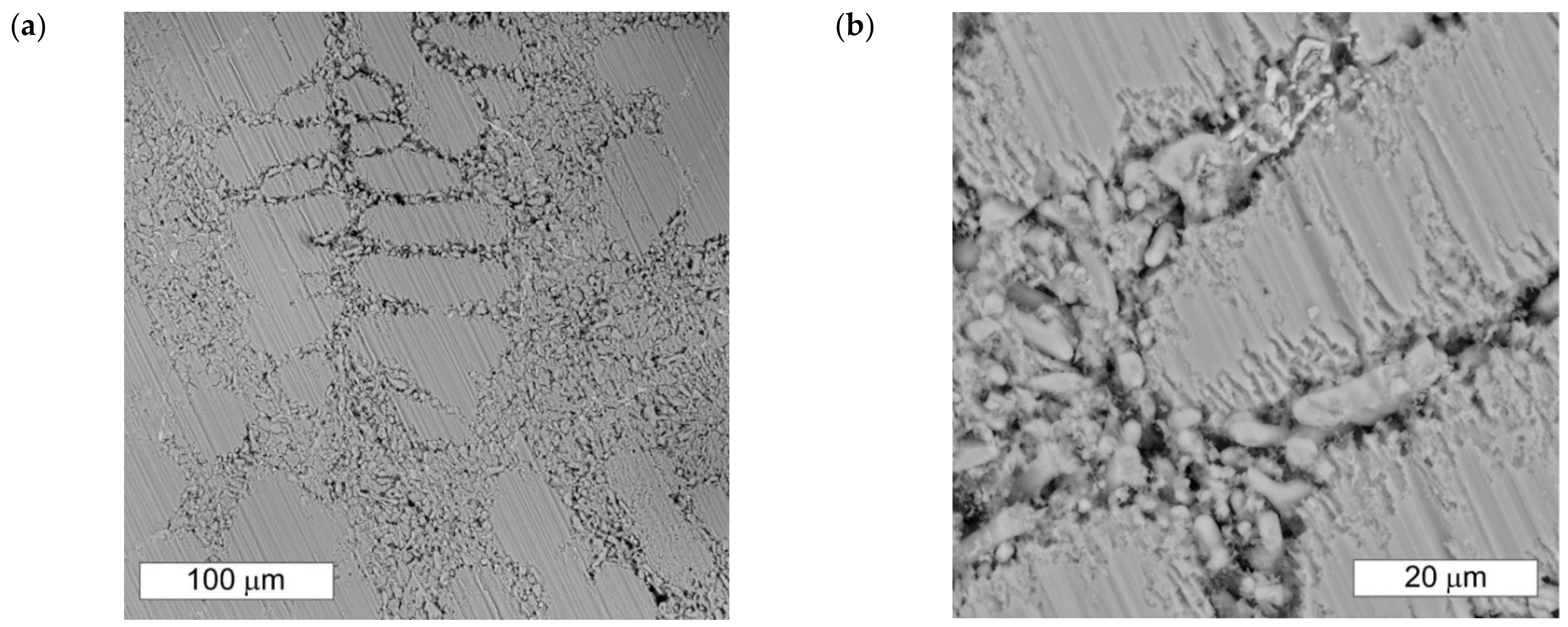
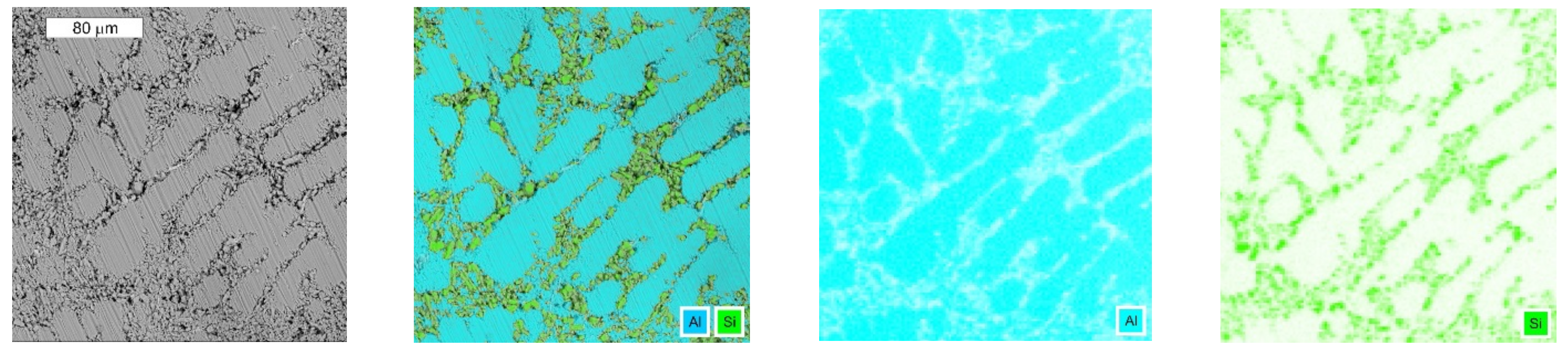
3.3. Microscopic Examination after the NSS Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lachowicz, M.M.; Lachowicz, M.B.; Gertruda, A. Role of microstructure in corrosion of microchannel heat exchangers. Inżynieria Mater. Mater. Eng. 2018, 39, 94–99. [Google Scholar] [CrossRef]
- Lachowicz, M. Electrochemical and Microstructural Aspects of the Development of Corrosion Damage to Parts of Machines and Devices; Wydawnictwo Naukowe Instytutu Technologii i Eksploatacji—Sieć Badawcza Łukasiewicz: Radom, Poland, 2020. (In Polish) [Google Scholar]
- Venegas, T.; Qu, M.; Nawaz, K.; Wang, L. Critical review and future prospects for desiccant coated heat exchangers: Materials, design, and manufacturing. Renew. Sustain. Energy Rev. 2021, 151, 111531. [Google Scholar] [CrossRef]
- Zhao, L.H.; Wang, R.Z.; Ge, T.S. Desiccant coated heat exchanger and its applications. Int. J. Refrig. 2021, 130, 217–232. [Google Scholar] [CrossRef]
- Vivekh, P.; Kumja, M.; Bui, D.T.; Chua, K.J. Recent developments in solid desiccant coated heat exchangers—A review. Appl. Energy 2018, 229, 778–803. [Google Scholar] [CrossRef]
- Bordo, K.; Gudla, V.C.; Peguet, L.; Afseth, A.; Ambat, R. Electrochemical profiling of multi-clad aluminium sheets used in automotive heat exchangers. Corros. Sci. 2018, 131, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Lacaze, J.; Tierce, S.; Lafont, M.C.; Thebault, Y.; Pébère, N.; Mankowski, G.; Blanc, C.; Robidou, H.; Vaumousse, D.; Daloz, D. Study of the microstructure resulting from brazed aluminium materials used in heat exchangers. Mater. Sci. Eng. A 2005, 413–414, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Iwao, S.; Edo, M.; Chiba, H. Mechanism of intergranular corrosion of brazed Al–Mn–Cu alloys with various Si content. J. Jpn. Inst. Light Met. 2017, 67, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Peta, K.; Siwak, P.; Grochalski, K. Research on mechanical properties of aluminum alloys used in automotive industry. Inżynieria Mater. Mater. Eng. 2017, 38, 114–118. [Google Scholar] [CrossRef]
- Zhao, H.; Woods, R. 10—Controlled atmosphere brazing of aluminum. In Woodhead Publishing Series in Welding and Other Joining Technologies, Advances in Brazing; Sekulić, D.P., Ed.; Woodhead Publishing: Thorston, UK, 2013; pp. 280–323e. [Google Scholar] [CrossRef]
- Guia-Tello, J.C.; Pech-Canul, M.I.; Trujillo, E.; Pech-Canul, M. Effect of brazing parameters on fillet size and microstructure of cladded fin-microchannel tube joints. Trans. Nonferrous Met. Soc. China 2020, 30, 3240–3253. [Google Scholar] [CrossRef]
- Shutov, I.V.; Kamaeva, L.V.; Krivilyov, M.D.; Yu, C.N.; Mesarovic, S.D.; Sekulic, D.P. Effect of processing parameters on microstructure in brazing of Al–Si alloys. J. Cryst. Growth 2020, 530, 125287. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Hu, W.; Zhou, D. Corrosion Process of Multilayer Aluminum Brazed Sheet by EIS and EN Techniques. Surf. Rev. Lett. 2018, 26, 1850224. [Google Scholar] [CrossRef]
- Tierce, S.; Pébère, N.; Blanc, C.; Mankowski, G.; Robidou, H.; Vaumousse, D.; Lacaze, J. Solidification and phase transformations in brazed aluminium alloys used in automotive heat exchangers. Int. J. Cast Met. Res. 2005, 18, 370–376. [Google Scholar] [CrossRef]
- Yuan, Z.; Tao, F.; Wen, J.; Tu, Y. The Dependence of Microstructural Evolution and Corrosion Resistance of a Sandwich Multi-Layers Brazing Sheets on the Homogenization Annealing. IEEE Access 2019, 7, 121388–121394. [Google Scholar] [CrossRef]
- Marshall, G.J.; Bolingbroke, R.K.; Gray, A. Microstructural Control in an Aluminum Core Alloy for Brazing Sheet Applications. Metall. Mater. Trans. A 1993, 24, 1935–1942. [Google Scholar] [CrossRef]
- Yuan, Z.; Tu, Y.; He, H.; Yuan, T.; Zhang, Q.; Peng, X. Influence of final annealing temperature on the microstructural evolution and corrosion resistance of a Sandwich multi-layered aluminum sheet. Mater. Res. Express 2019, 6, 026536. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, I.J.; Kim, J.G. Simulation Approach for Cathodic Protection Prediction of Aluminum Fin-Tube Heat Exchanger Using Boundary Element Method. Metals 2019, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Ul-Hamid, A.; Alhems, L.M.; Saeed, A. Review of common failures in heat exchangers—Part I: Mechanical and elevated temperature failures. Eng. Fail. Anal. 2020, 109, 104396. [Google Scholar] [CrossRef]
- Faes, W.; Lecompte, S.; Ahmed, Z.; Van Bael, J.; Salenbien, R.; Verbeken, K.; De Paepe, M. Corrosion and corrosion prevention in heat exchangers. Corros. Rev. 2019, 37, 131–155. [Google Scholar] [CrossRef]
- Lachowicz, M.M. A metallographic case study of formicary corrosion in heat exchanger copper tubes. Eng. Fail. Anal. 2020, 111, 104502. [Google Scholar] [CrossRef]
- Zeng, F.L.; Wei, Z.L.; Li, J.F.; Li, C.X.; Tan, X.; Zhang, Z.; Zheng, Z.Q. Corrosion mechanism associated with Mg2Si and Si particles in Al–Mg–Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, J.; Gu, X.; Qin, P.; Dai, N.; Li, X.; Kruth, J.P.; Zhang, L.C. Improved corrosion behavior of ultrafine-grained eutectic Al-12Si alloy produced by selective laser melting. Mater. Des. 2018, 146, 239–248. [Google Scholar] [CrossRef]
- Oya, Y.; Kojima, Y.; Hara, N. Influence of Silicon on Intergranular Corrosion for Aluminum Alloys. Mater. Trans. 2013, 54, 1200–1208. [Google Scholar] [CrossRef]



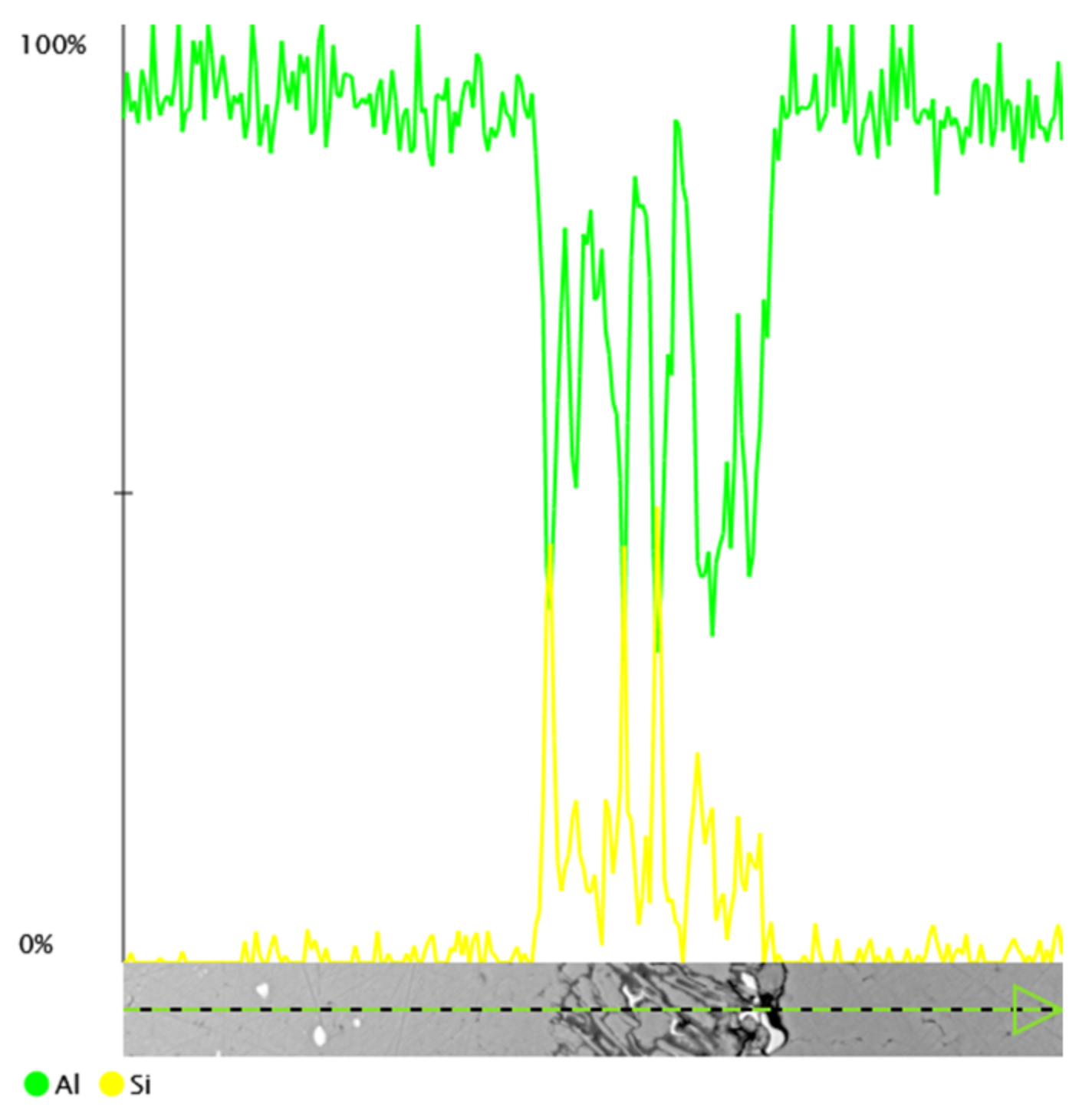
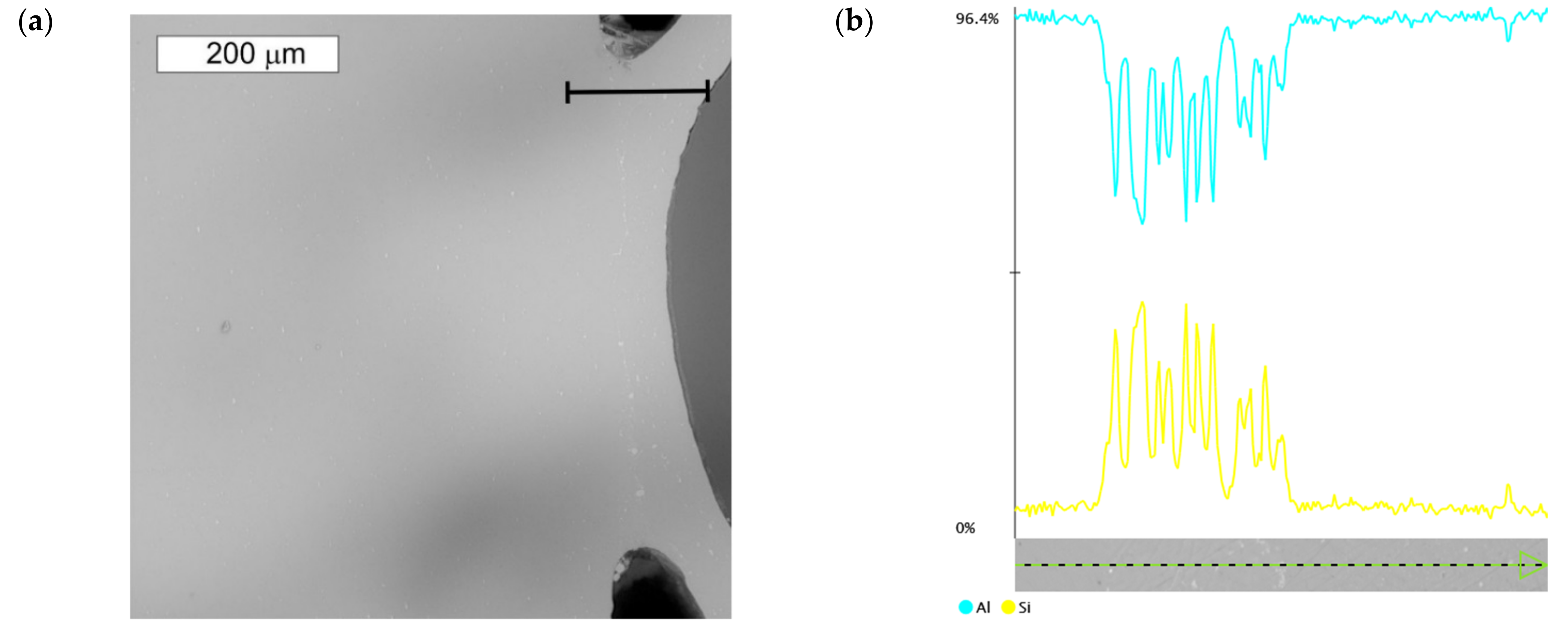
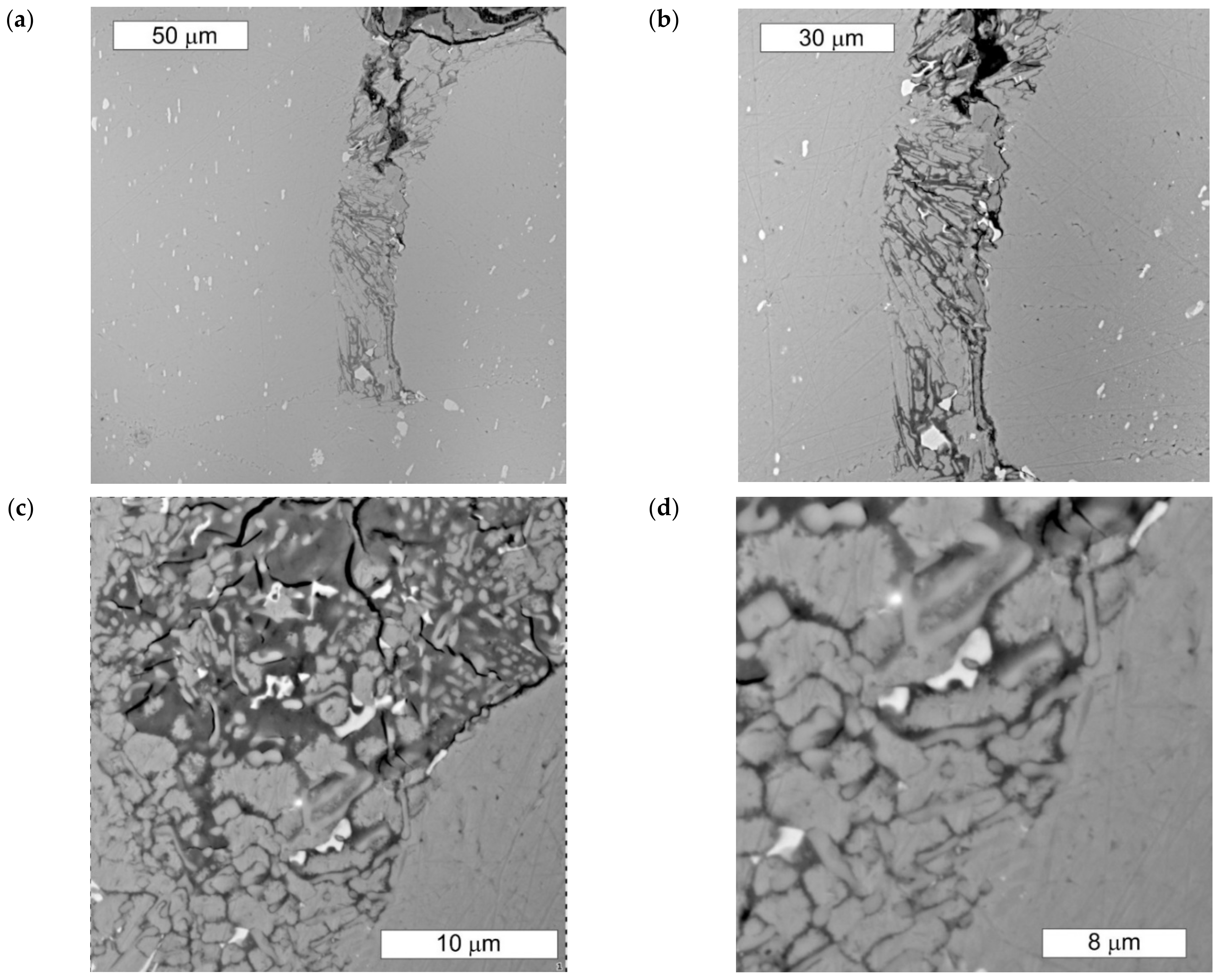


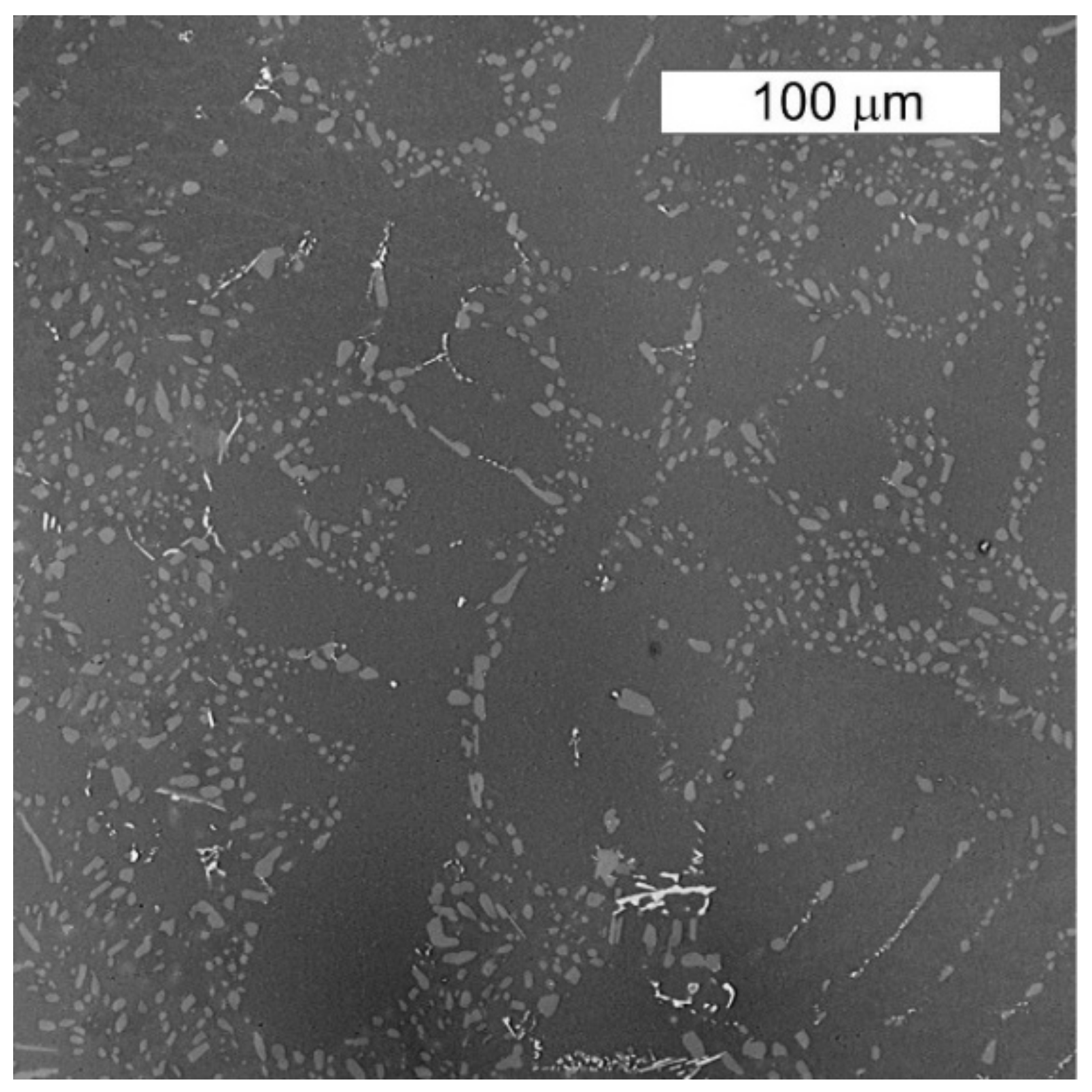
| ALLOY | Mn | Fe | Si | Cu | Zn | Mg | Cr | Al |
|---|---|---|---|---|---|---|---|---|
| AW 3003 | 1.0 ÷ 1.5 | max. 0.70 | max. 0.60 | 0.05 ÷ 0.20 | max. 0.10 | - | - | reszta |
| AW 3103 | 0.9 ÷ 1.5 | max. 0.70 | max. 0.50 | max. 0.10 | max. 0.20 | max. 0.30 | max. 0.10 | |
| AW 3203 | 1.0 ÷ 1.5 | max. 0.70 | max. 0.60 | max. 0.05 | max. 0.10 | - | - |
| ALLOY | Si | Fe | Mn | Cu | Zn | Mg | Al |
|---|---|---|---|---|---|---|---|
| AW 4343 | 6.8 ÷ 8.2 | max. 0.80 | max. 0.10 | max. 0.25 | max. 0.20 | - | reszta |
| AW 4145 | 9.3 ÷ 10.7 | max. 0.80 | max. 0.15 | 3.3 ÷ 4.7 | max. 0.20 | max. 0.15 | reszta |
| AW 4047 | 11.0 ÷ 13.0 | max. 0.80 | max. 0.15 | max. 0.30 | max. 0.20 | max. 0.10 | reszta |
| AW 4045 | 9.0 ÷ 11.0 | max. 0.80 | max. 0.05 | max. 0.30 | max. 0.10 | max. 0.05 | reszta |
| ALLOY | Si | Fe | Cu | Mn | Mg | Zn | Al |
|---|---|---|---|---|---|---|---|
| AC 44000 | 10.4 | 0.15 | 0.01 | 0.01 | 0.35 | 0.02 | reszta |
| AW 3003 | 0.38 | 0.52 | 0.10 | 1.23 | 0.01 | 0.03 | reszta |
| PARAMETER | E0 (I = 0) (mV) | Ecorr vs. Ag/AgCl (mV) | icorr (µA/cm2) |
|---|---|---|---|
| AC 44000 | −803 ± 6 | −750 ± 5 | 2.01 ± 0.4 |
| AW 3003 | −633 ± 5 | −610 ± 3 | 0.93 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, M.M.; Lachowicz, M.B.; Gertruda, A. Assessment of the Possibility of Galvanic Corrosion in Aluminum Microchannel Heat Exchangers. Crystals 2022, 12, 1439. https://doi.org/10.3390/cryst12101439
Lachowicz MM, Lachowicz MB, Gertruda A. Assessment of the Possibility of Galvanic Corrosion in Aluminum Microchannel Heat Exchangers. Crystals. 2022; 12(10):1439. https://doi.org/10.3390/cryst12101439
Chicago/Turabian StyleLachowicz, Marzena M., Maciej B. Lachowicz, and Adam Gertruda. 2022. "Assessment of the Possibility of Galvanic Corrosion in Aluminum Microchannel Heat Exchangers" Crystals 12, no. 10: 1439. https://doi.org/10.3390/cryst12101439





