Assessment of the Physical Properties of an Experimental Adhesive Dentin Bonding Agent with Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filler Nanoparticles and Their Inclusion in Dentin Adhesives
2.2. Morphological and Mapping Analysis of Filler CNPs
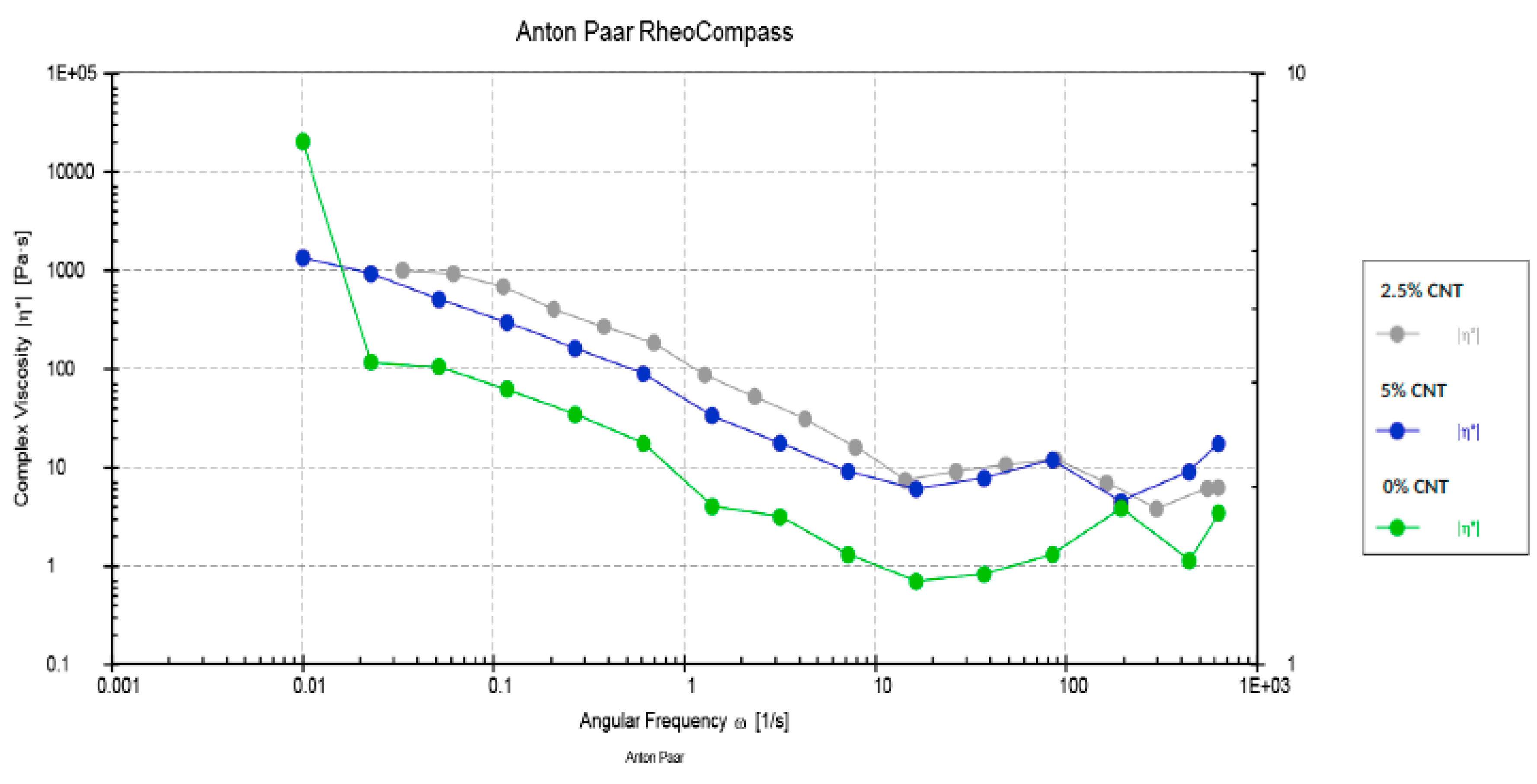
2.3. Rheological Assessment
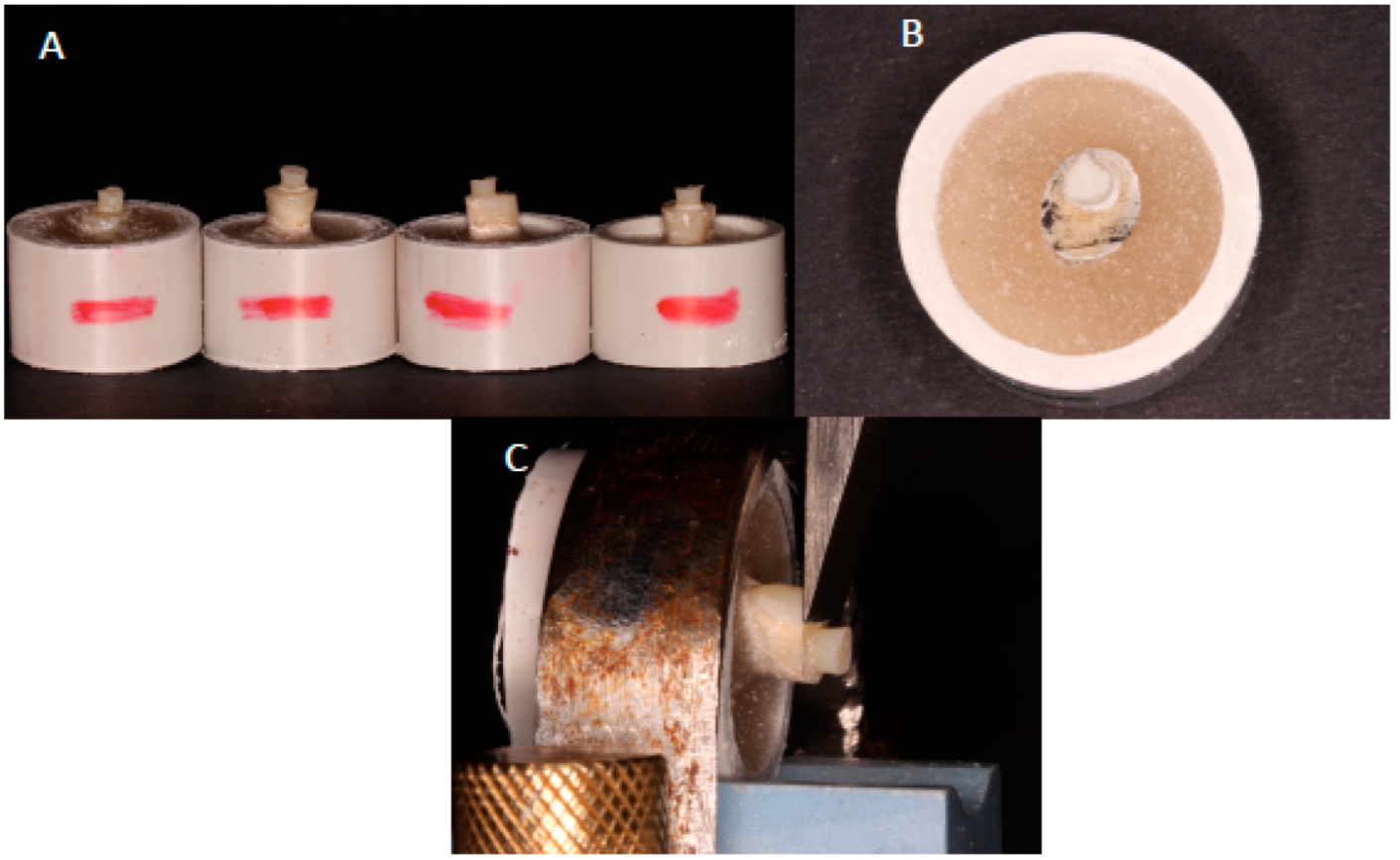
2.4. Cutting of Tooth Samples and Adhesive Bonding Protocol
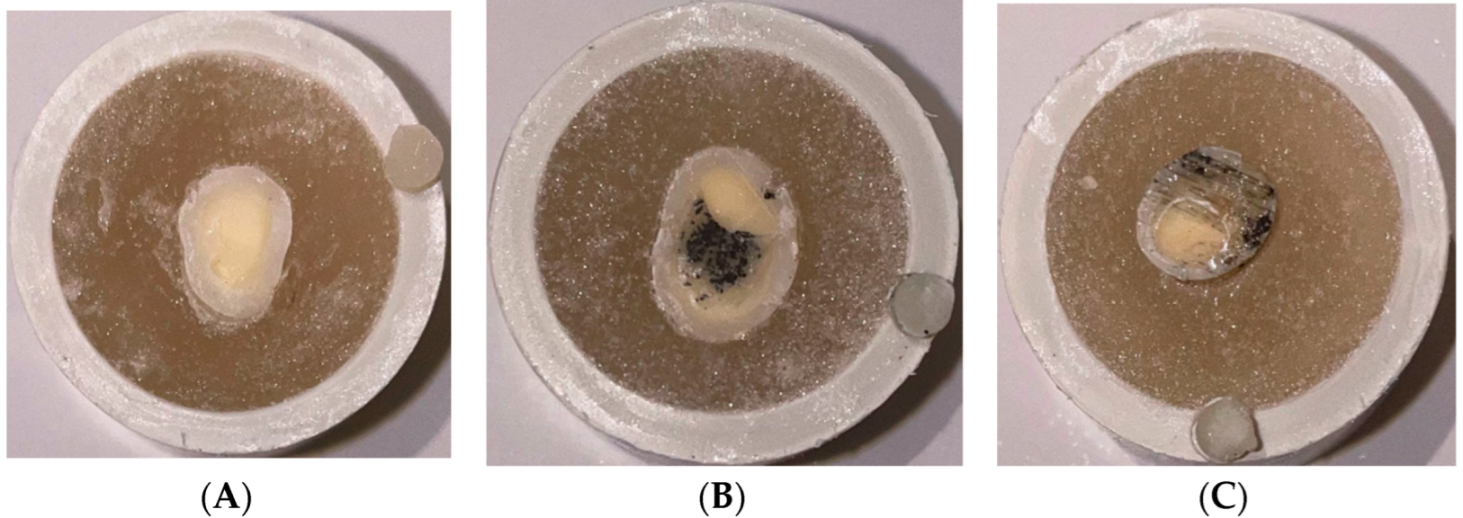
2.5. SBS Testing and Analysis of the Failure Modes
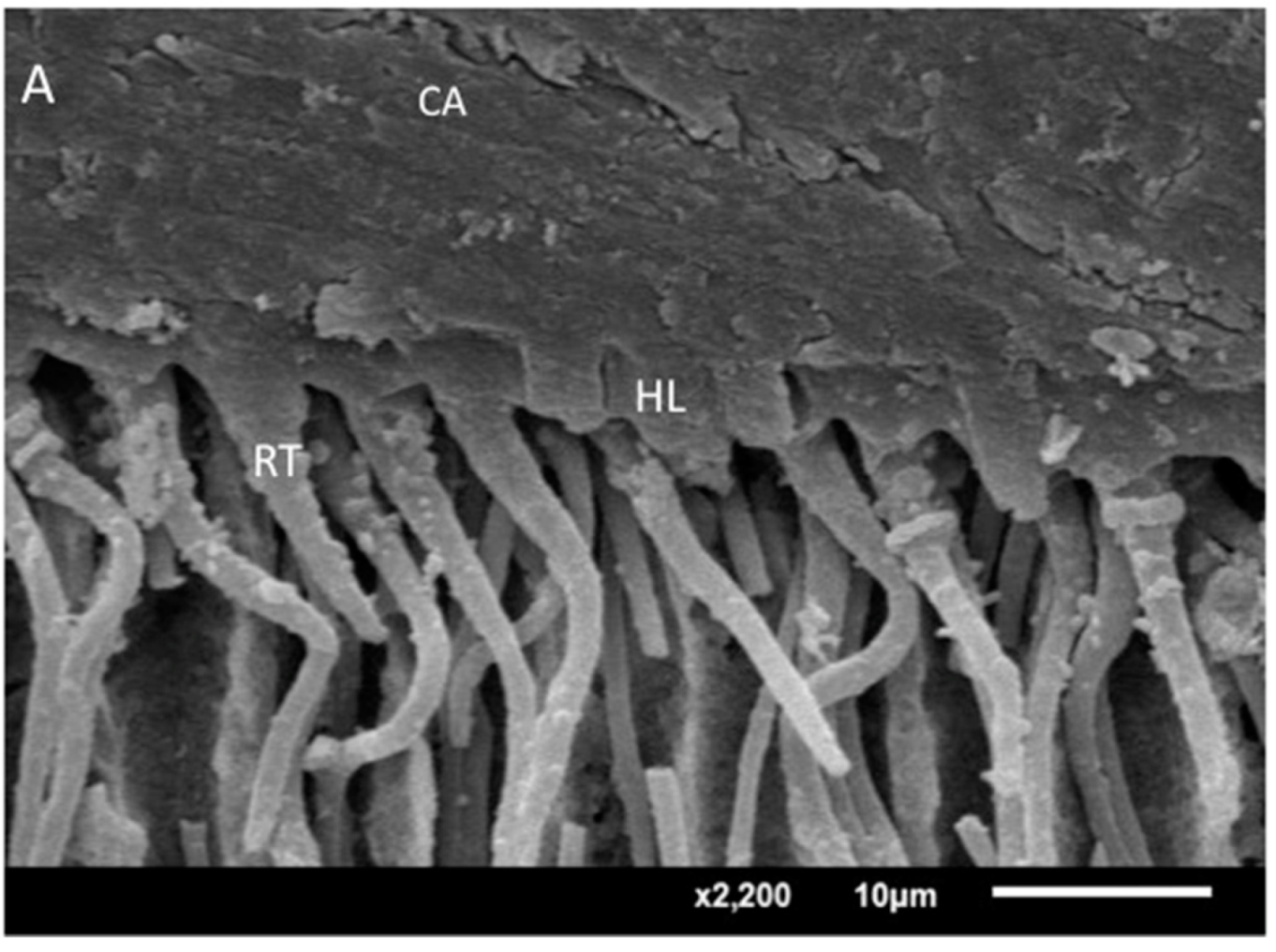
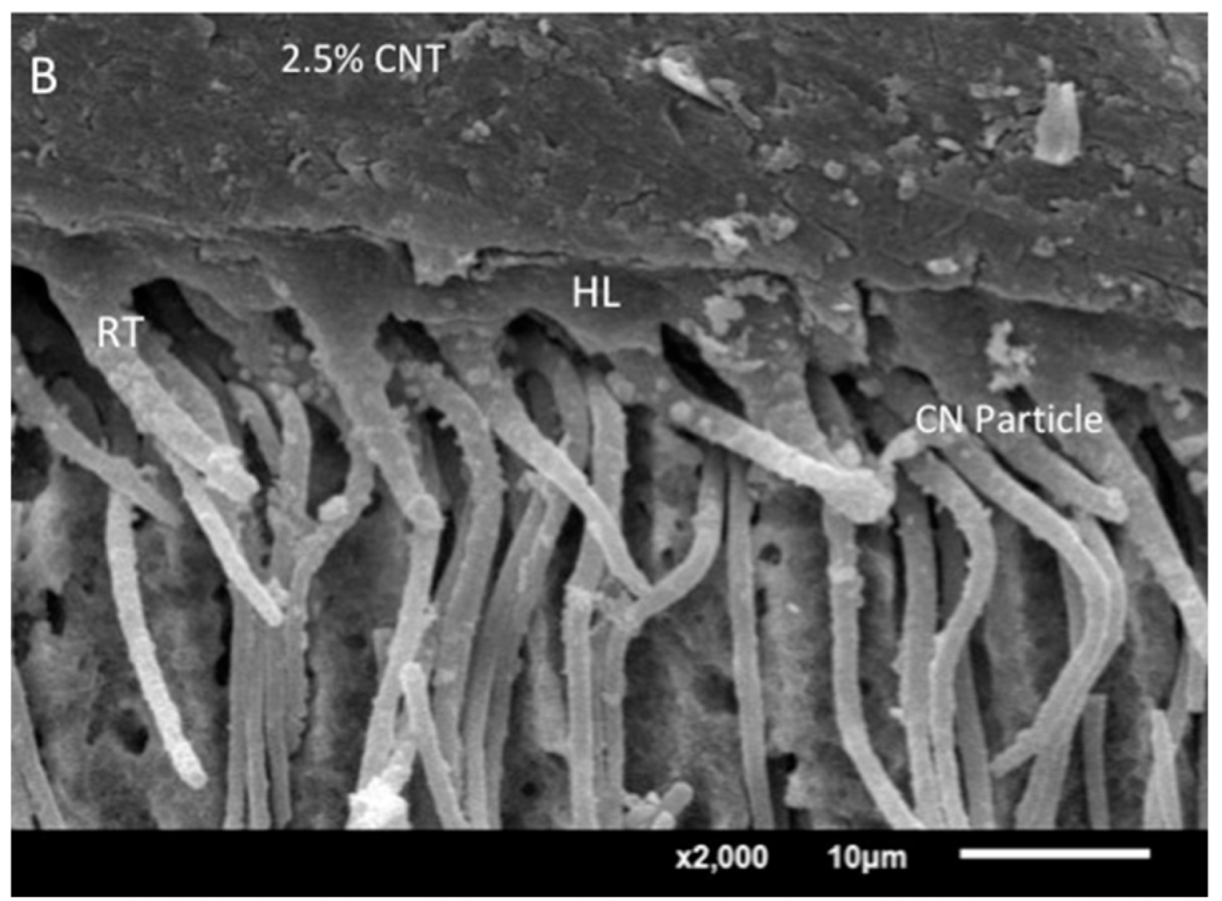
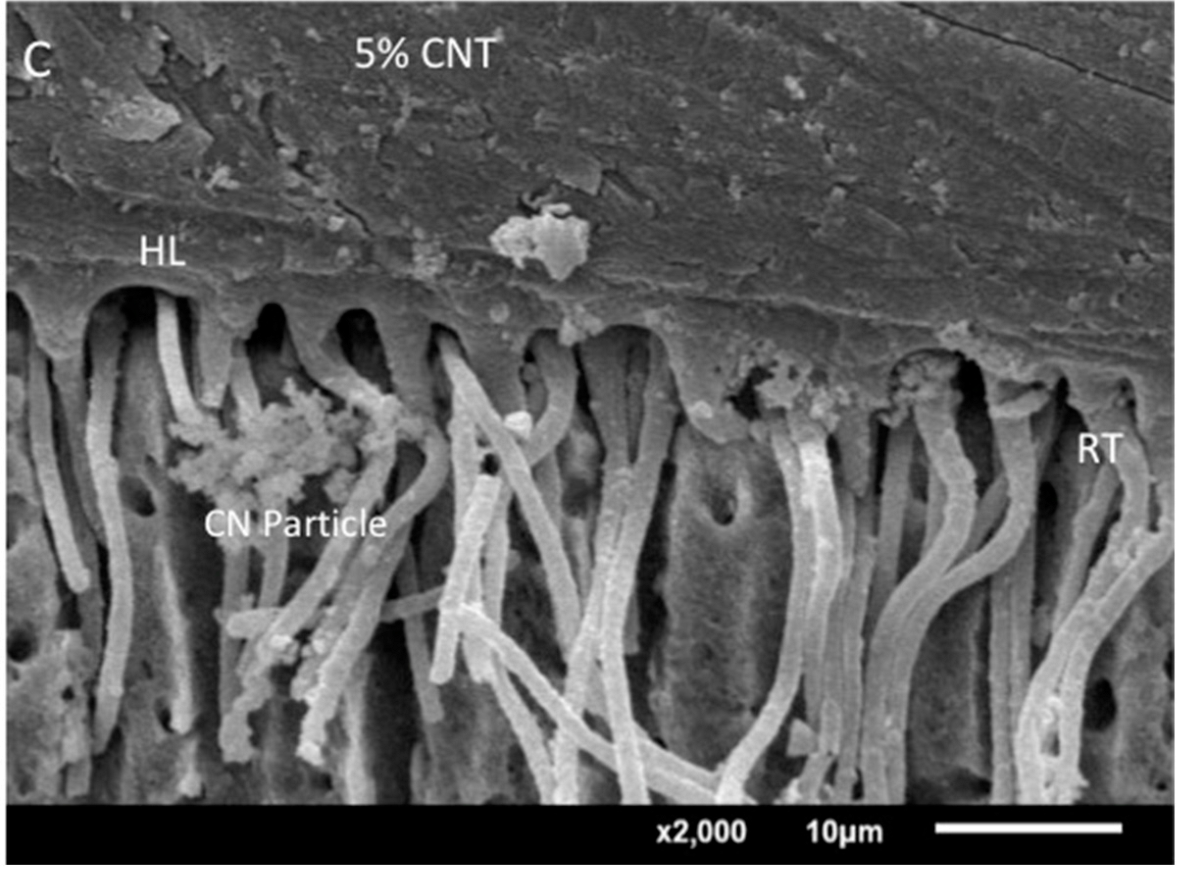
2.6. Analysis of the Adhesive−Dentin Interface
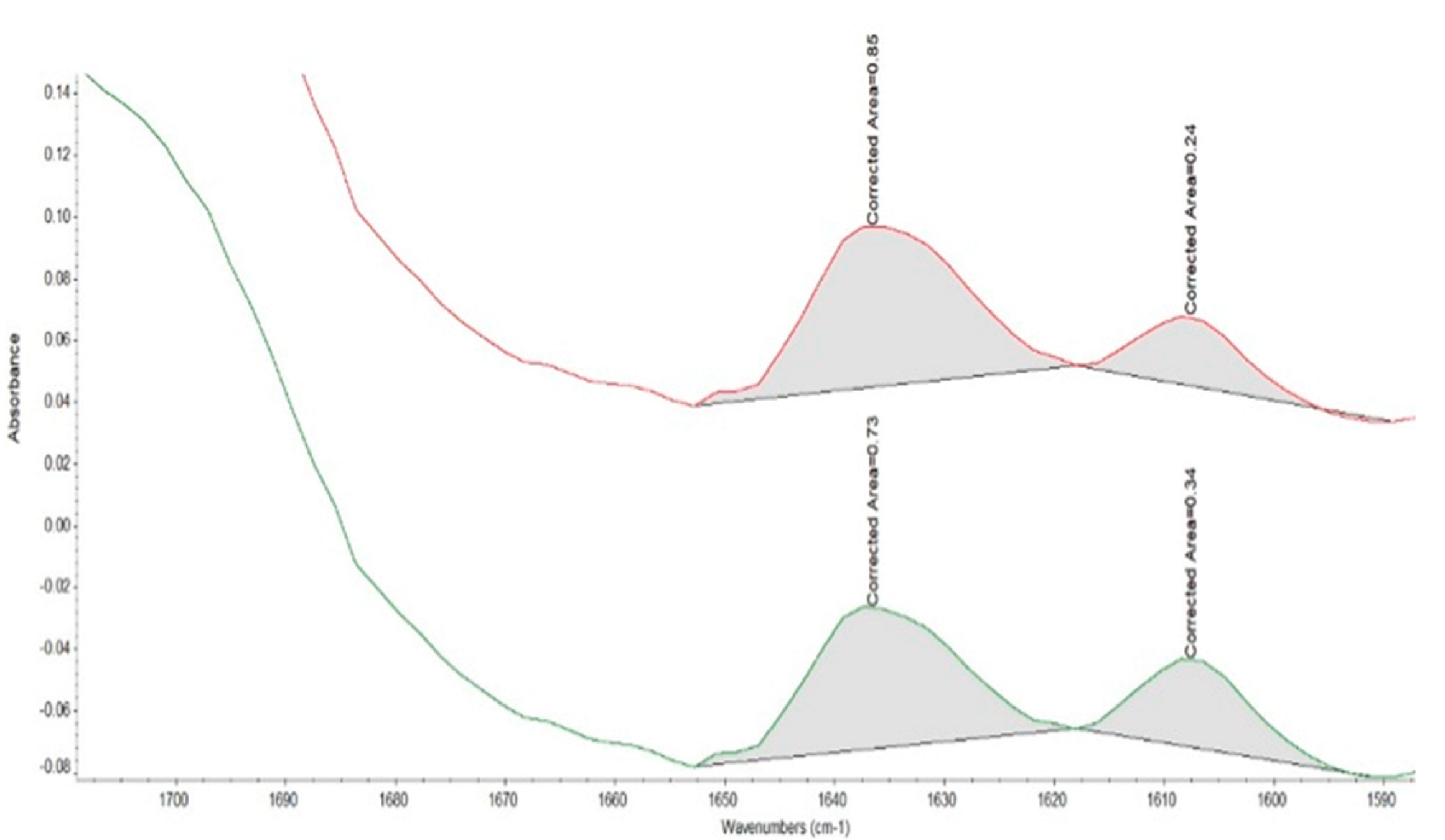
2.7. FTIR Spectroscopy for the Calculation of Degree of Conversion (DC) Values
2.8. Statistical Analysis
3. Results
3.1. Morphological and Mapping Analysis of Filler CNPs
3.2. Rheological Assessment
3.3. SBS Testing and FMA
3.4. Analysis of the Adhesive–Dentin Interface
3.5. FTIR and DC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Hua, F.; Yan, J.; Yang, H.; Huang, C. Effects of Plant Extracts on Dentin Bonding Strength: A Systematic Review and Meta-Analysis. Front. Bioeng. Biotechnol. 2022, 10, 836042. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Taneja, S.; Kumari, M.; Bansal, S. Effect of saliva and blood contamination on the shear bond strength of fifth-, seventh-, and eighth-generation bonding agents: An in vitro study. J. Conserv. Dent. 2017, 20, 157–160. [Google Scholar] [CrossRef]
- Hass, V.; Luque-Martinez, I.; Munoz, M.A.; Reyes, M.F.; Abuna, G.; Sinhoreti, M.A.; Liu, A.Y.; Loguercio, A.D.; Wang, Y.; Reis, A. The effect of proanthocyanidin-containing 10% phosphoric acid on bonding properties and MMP inhibition. Dent. Mater. 2016, 32, 468–475. [Google Scholar] [CrossRef]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives-A Review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [Green Version]
- Ghahramani, Y.; Javanmardi, N. Graphene oxide quantum dots and their applications via stem cells: A mini-review. Adv. Appl. NanoBio-Technol. 2021, 2, 54–56. [Google Scholar]
- Vijay, R.; Mendhi, J.; Prasad, K.; Xiao, Y.; MacLeod, J.; Ostrikov, K.K.; Zhou, Y. Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients. Nanomaterials 2021, 11, 2977. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Perez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- De Vasconcellos, L.M.R.; do Prado, R.F.; Sartori, E.M.; Mendonca, D.B.S.; Mendonca, G.; Marciano, F.R.; Lobo, A.O. In vitro osteogenesis process induced by hybrid nanohydroxyapatite/graphene nanoribbons composites. J. Mater. Sci. Mater. Med. 2019, 30, 81. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rojas, M.A.; Vega-Cantu, Y.I.; Cordell, G.A.; Rodriguez-Garcia, A. Dental Applications of Carbon Nanotubes. Molecules 2021, 26, 4423. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Yousefi, K.; Hashemi, S.A.; Afsa, M.; Bahran, I.S.; Gholami, A.; Ghahramani, Y.; Alizadeh, A.; Chiang, W.H. Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering. Nanomaterials 2021, 11, 2800. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.L.J.; Xu, S. Progress in Research on Carbon Nanotubes Reinforced Cementitious Composites. Adv. Mater. Sci. Eng. 2015, 2015, 307435. [Google Scholar] [CrossRef] [Green Version]
- Kechagioglou, P.; Andriotis, E.; Papagerakis, P.; Papagerakis, S. Multiwalled Carbon Nanotubes for Dental Applications. Methods Mol. Biol. 2019, 1922, 121–128. [Google Scholar]
- Khan, A.S.; Hussain, A.N.; Sidra, L.; Sarfraz, Z.; Khalid, H.; Khan, M.; Manzoor, F.; Shahzadi, L.; Yar, M.; Rehman, I.U. Fabrication and in vivo evaluation of hydroxyapatite/carbon nanotube electrospun fibers for biomedical/dental application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 387–396. [Google Scholar] [CrossRef]
- Akasaka, T.; Nakata, K.; Uo, M.; Watari, F. Modification of the dentin surface by using carbon nanotubes. Biomed. Mater. Eng. 2009, 19, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Suo, L.; Li, Z.; Luo, F.; Chen, J.; Jia, L.; Wang, T.; Pei, X.; Wan, Q. Effect of dentin surface modification using carbon nanotubes on dental bonding and antibacterial ability. Dent. Mater. J. 2018, 37, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, S.; Alateeq, A.; Alshaya, A.H.; Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation. Polymers 2021, 13, 3794. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamaguchi, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of metal conditioners on polymerization behavior of bonding agents. J. Oral Sci. 2005, 47, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles-An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion. A scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, micro-Raman, and bond strength study. Microsc. Res. Tech. 2021, 84, 2082–2094. [Google Scholar] [PubMed]
- Dyatlova, O.A.; Gomis-Bresco, J.; Malic, E.; Telg, H.; Maultzsch, J.; Zhong, G.; Geng, J.; Woggon, U. Dielectric screening effects on transition energies in aligned carbon nanotubes. Phys. Rev. B 2012, 85, 245449. [Google Scholar] [CrossRef]
- Williams, J.; Broughton, W.; Koukoulas, T.; Rahatekar, S.S. Plasma treatment as a method for functionalising and improving dispersion of carbon nanotubes in epoxy resins. J. Mater. Sci. 2012, 48, 1005–1013. [Google Scholar] [CrossRef]
- Osikoya, A.O.; Wankasi, D.; Vala, R.M.K.; Dikio, C.W.; Afolabi, A.O.; Ayawei, N.; Dikio, E.D. Synthesis, characterization and sorption studies of nitrogen–doped carbon nanotubes. Digest. J. Nanomat. Biostruct. 2015, 10, 125–134. [Google Scholar]
- Yue, N.; Wang, L.; He, X.; Liu, H.; Zhang, W. Optimizing the SEM specimen preparation method for accurate microanalysis of carbon nanotube/nanocluster hybrids. J. Microsc. 2021, 282, 267–273. [Google Scholar] [CrossRef]
- Lee, J.H.; Um, C.M.; Lee, I.B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Sirisha, K.; Rambabu, T.; Ravishankar, Y.; Ravikumar, P. Validity of bond strength tests: A critical review-Part II. J. Conserv. Dent. 2014, 17, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Al-Aali, K.A.; Alhamdan, M.M.; Abduljabbar, T. Influence of Concentration Levels of beta-Tricalcium Phosphate on the Physical Properties of a Dental Adhesive. Nanomaterials 2022, 12, 125–134. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; AlRefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers 2021, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Sachdeva, P.; Goswami, M.; Singh, D. Comparative evaluation of shear bond strength and nanoleakage of conventional and self-adhering flowable composites to primary teeth dentin. Contemp. Clin. Dent. 2016, 7, 326–331. [Google Scholar]
- Terada, M.; Abe, S.; Akasaka, T.; Uo, M.; Kitagawa, Y.; Watari, F. Development of a multiwalled carbon nanotube coated collagen dish. Dent. Mater. J. 2009, 28, 82–88. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, T.R.; de Oliveira, M.; Arrais, C.A.; Ambrosano, G.M.; Rueggeberg, F.; Giannini, M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J. Prosthet. Dent. 2015, 113, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Utneja, S.; Talwar, S.; Nawal, R.R.; Sapra, S.; Mittal, M.; Rajain, A.; Verma, M. Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: An in vitro study. J. Conserv. Dent. 2018, 21, 681–690. [Google Scholar] [CrossRef] [PubMed]

| SBS (MPa) (Mean ± SD) | Failure Mode Analysis, FMA (%) | |||||
|---|---|---|---|---|---|---|
| Group (n = 10) | NTC | TC | p-Value * | Adhesive | Cohesive | Mixed |
| 0% CNP-adhesive | 19.71 ± 2.8 a,A | - | < 0.01 | 100 | 0 | 0 |
| - | 15.11 ± 3.8 b,A | 100 | 0 | 0 | ||
| 2.5 wt.% CNP- adhesive | 25.15 ± 3.0 a,B | - | 70 | 0 | 30 | |
| - | 22.43 ± 3.4 b,B | 90 | 0 | 10 | ||
| 5.0 wt.% CNP- adhesive | 24.25 ± 3.0 a,B | - | 90 | 0 | 10 | |
| - | 19.75 ± 2.7 b,B | 80 | 0 | 20 | ||
| Serial No. | Group Name | DC (%) |
|---|---|---|
| 1 | 0% CNP (CA) | 68.24 (4.88) A |
| 2 | 2.5 % CNP adhesive | 42.91 (5.43) B |
| 3 | 5% CNP adhesive | 34.32 (4.60) C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binhasan, M.; Al-Habeeb, K.M.; Almuqbil, A.S.; Alhaidary, T.A.; Alfawaz, Y.F.; Farooq, I.; Vohra, F.; Abduljabbar, T. Assessment of the Physical Properties of an Experimental Adhesive Dentin Bonding Agent with Carbon Nanoparticles. Crystals 2022, 12, 1441. https://doi.org/10.3390/cryst12101441
Binhasan M, Al-Habeeb KM, Almuqbil AS, Alhaidary TA, Alfawaz YF, Farooq I, Vohra F, Abduljabbar T. Assessment of the Physical Properties of an Experimental Adhesive Dentin Bonding Agent with Carbon Nanoparticles. Crystals. 2022; 12(10):1441. https://doi.org/10.3390/cryst12101441
Chicago/Turabian StyleBinhasan, Mashael, Khaled M Al-Habeeb, Abdullah S. Almuqbil, Tarik A. Alhaidary, Yasser F. Alfawaz, Imran Farooq, Fahim Vohra, and Tariq Abduljabbar. 2022. "Assessment of the Physical Properties of an Experimental Adhesive Dentin Bonding Agent with Carbon Nanoparticles" Crystals 12, no. 10: 1441. https://doi.org/10.3390/cryst12101441
APA StyleBinhasan, M., Al-Habeeb, K. M., Almuqbil, A. S., Alhaidary, T. A., Alfawaz, Y. F., Farooq, I., Vohra, F., & Abduljabbar, T. (2022). Assessment of the Physical Properties of an Experimental Adhesive Dentin Bonding Agent with Carbon Nanoparticles. Crystals, 12(10), 1441. https://doi.org/10.3390/cryst12101441







