Optimal Polyethyleneimine Molecular Weight and Arrangement for Modification of γ-Cyclodextrin Metal Organic Frameworks (γ-CD-MOFs) for Post-Combustion CO2 Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of γ-CD MOFs
2.3. PEI Impregnation
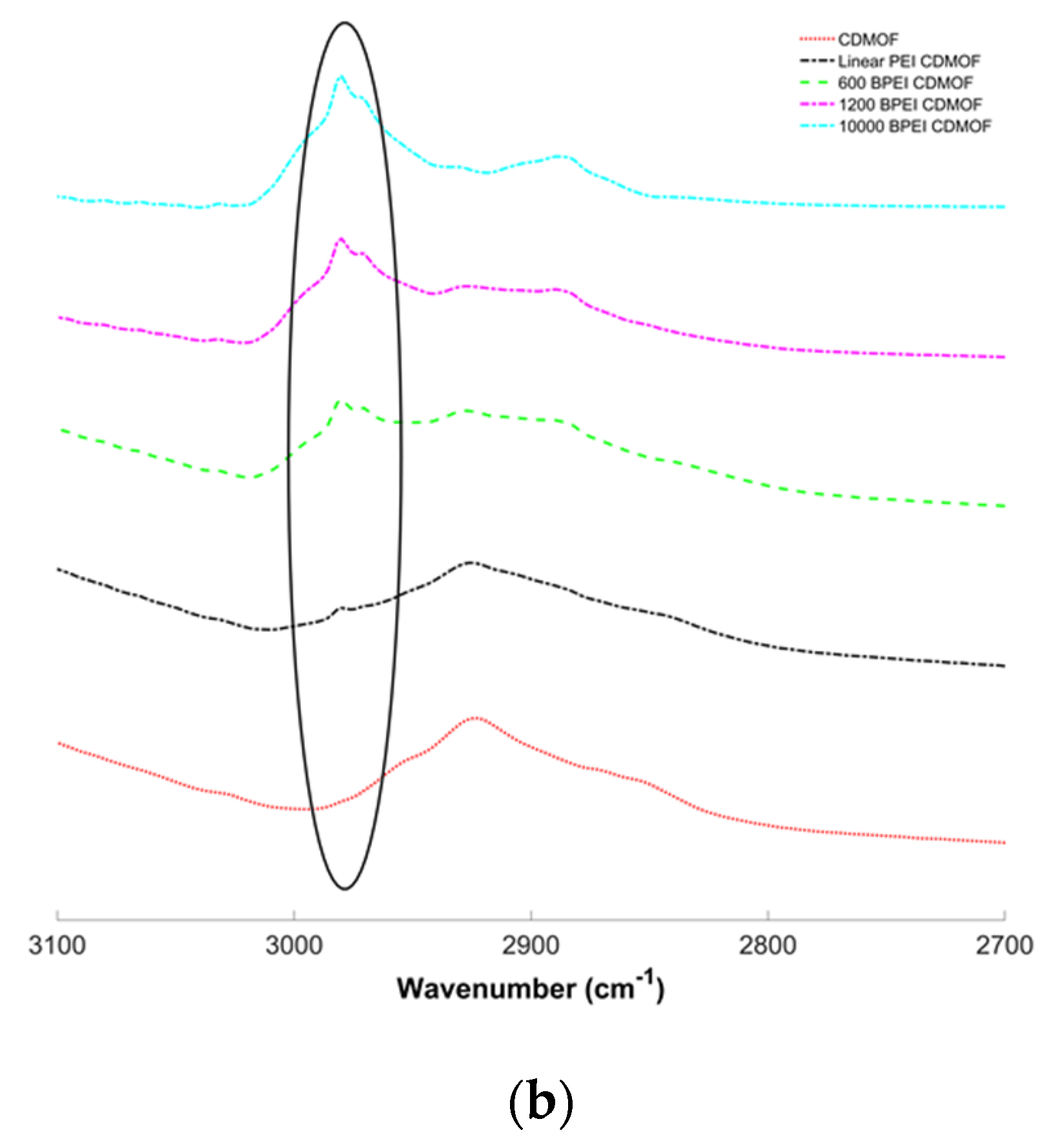
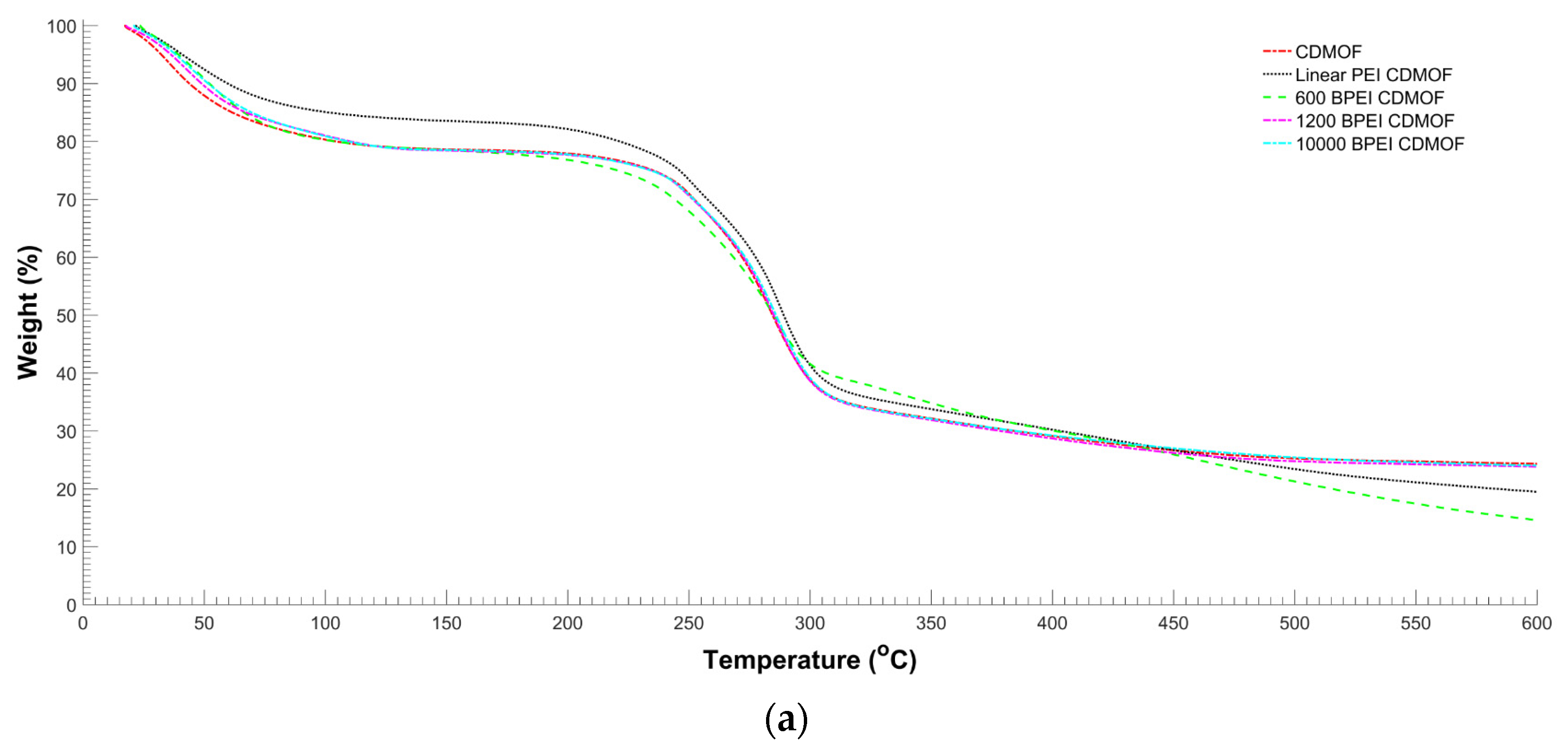
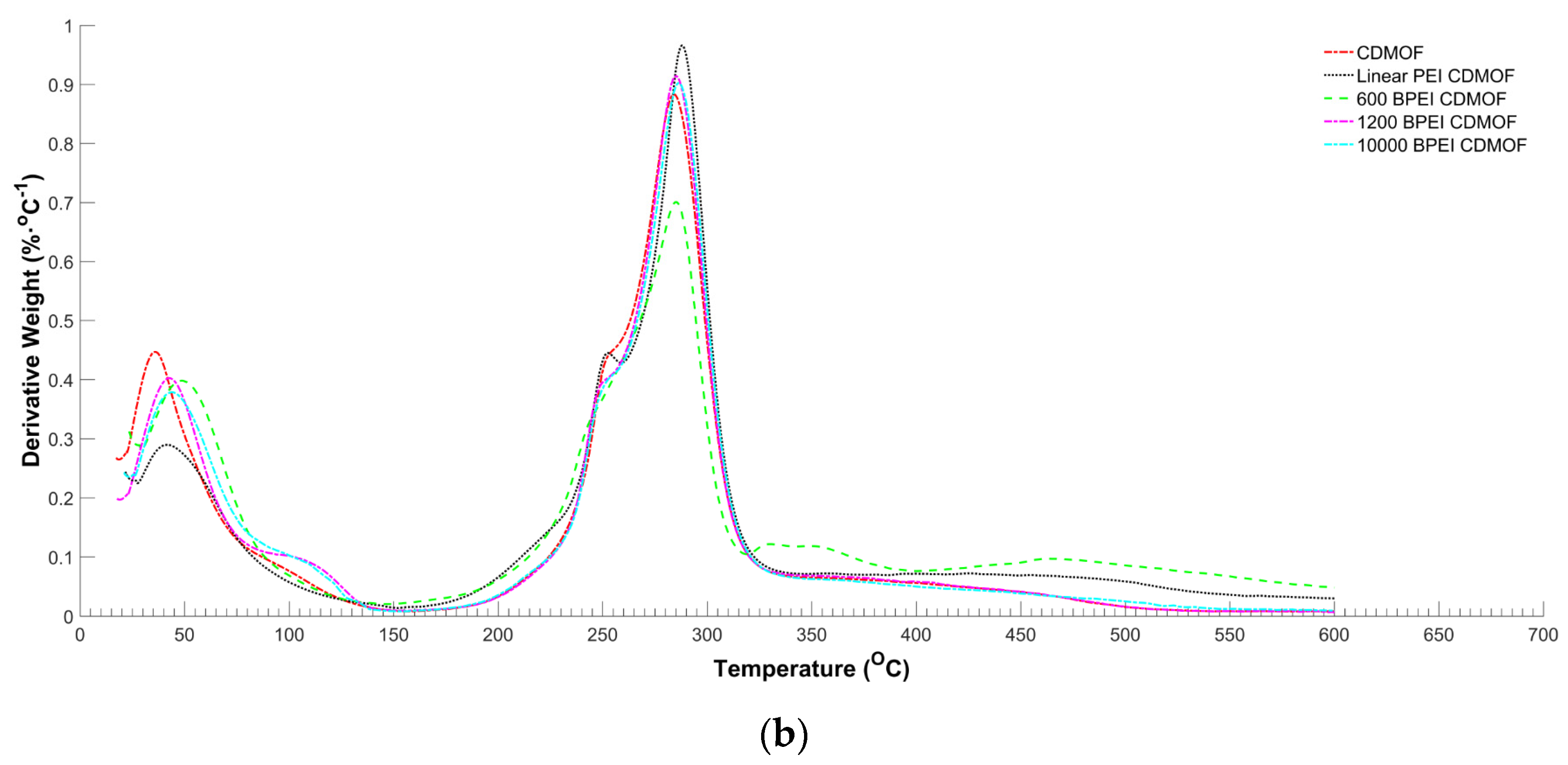
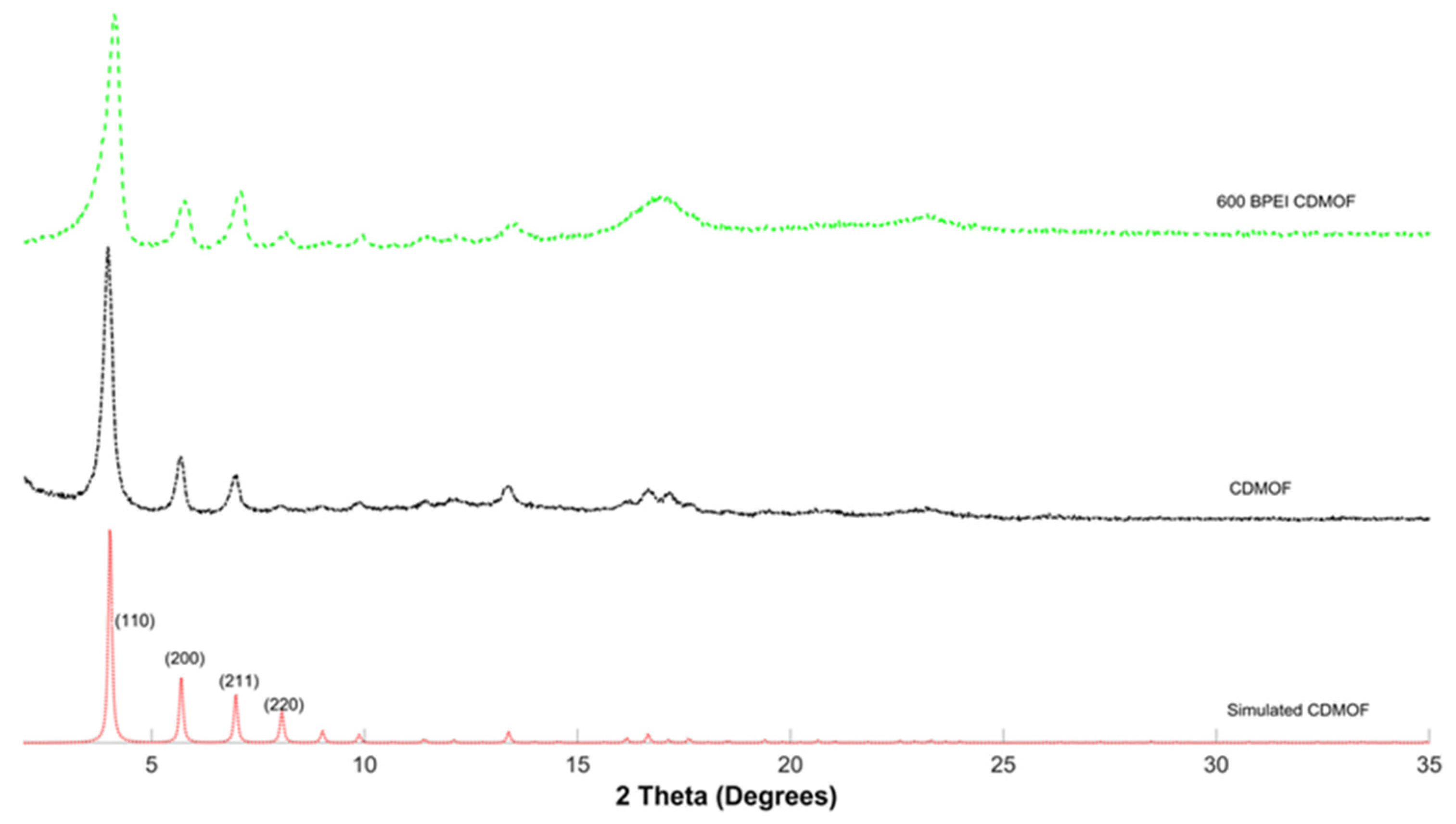
2.4. Characterization of γ-MOF
2.5. Experimental CO2 Sorption Quantification
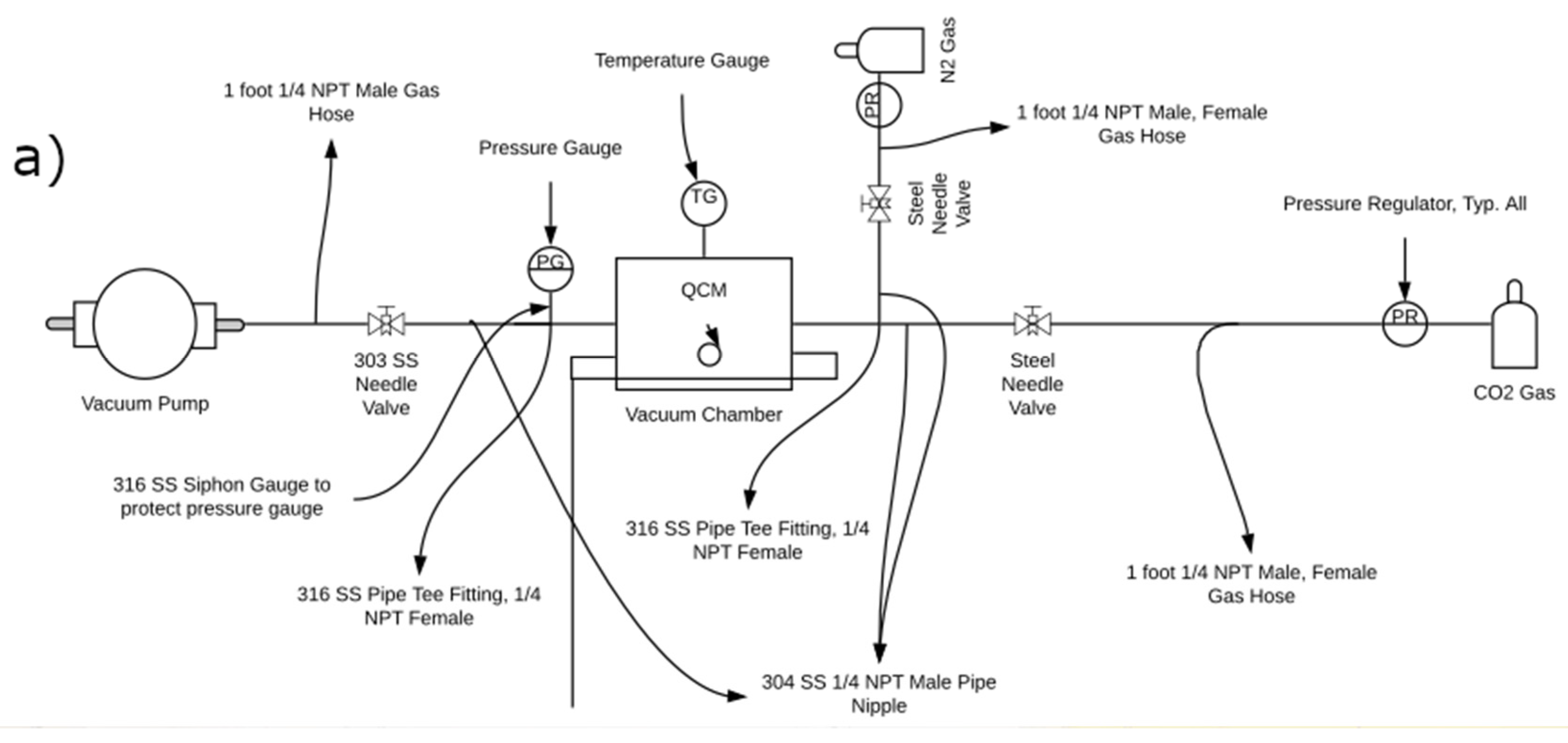
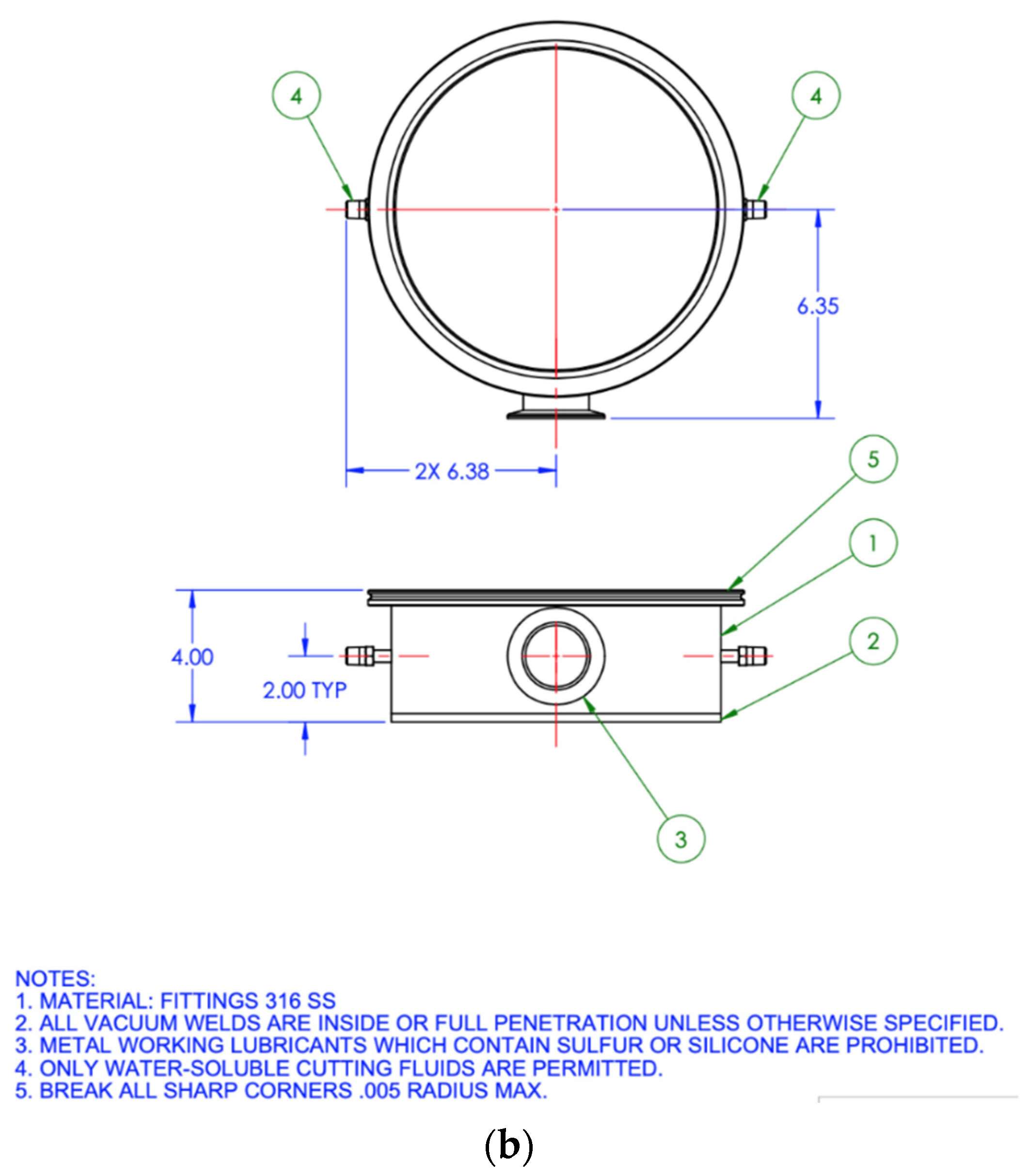
2.5.1. CO2 Sorption Testing Apparatus
2.5.2. CO2 Sorption Measurements
2.6. Molecular Docking Simulation
3. Results and Discussion
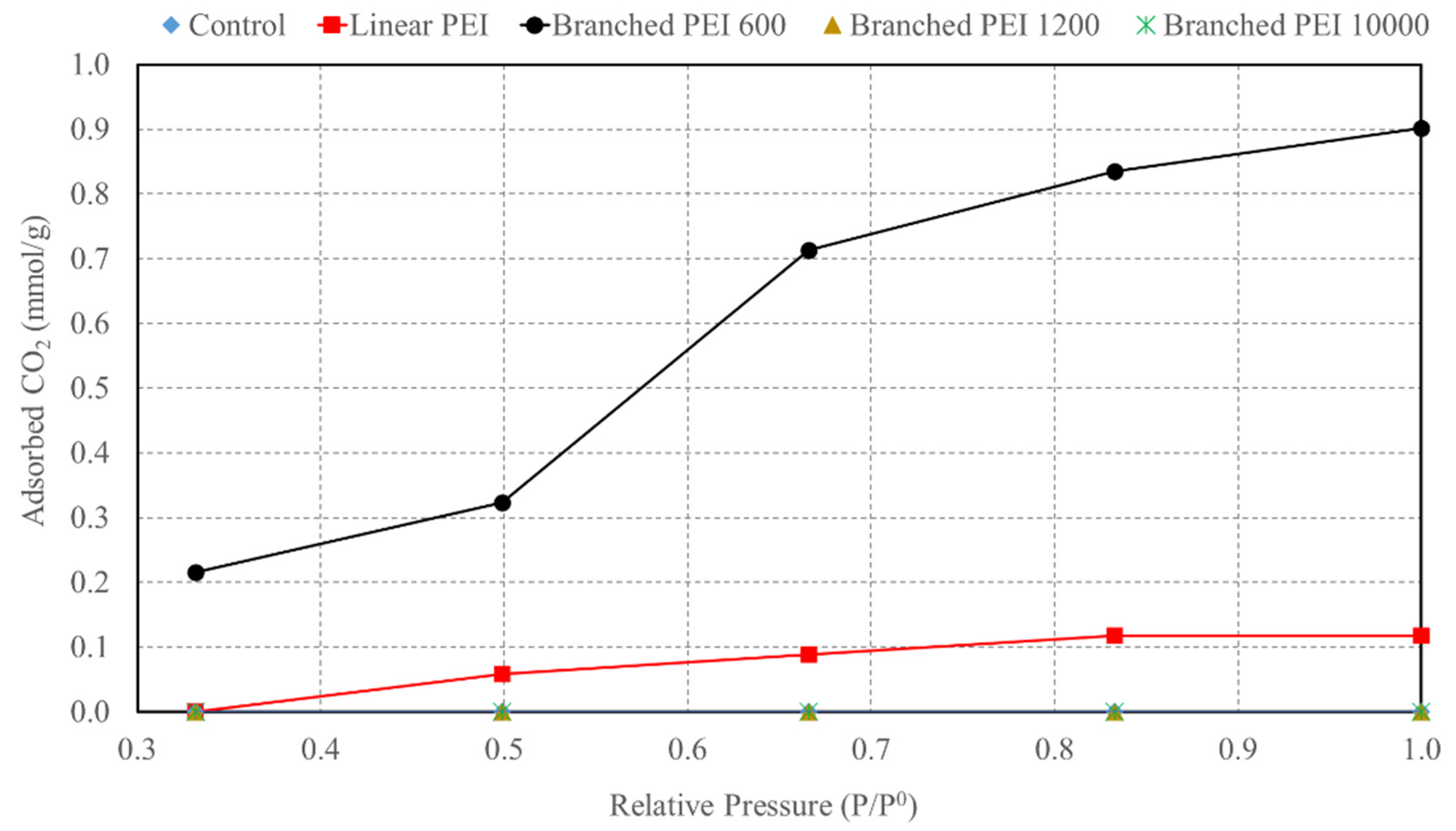
3.1. Impact of PEI Impregnation on γ-CD MOFs CO2 Sorption
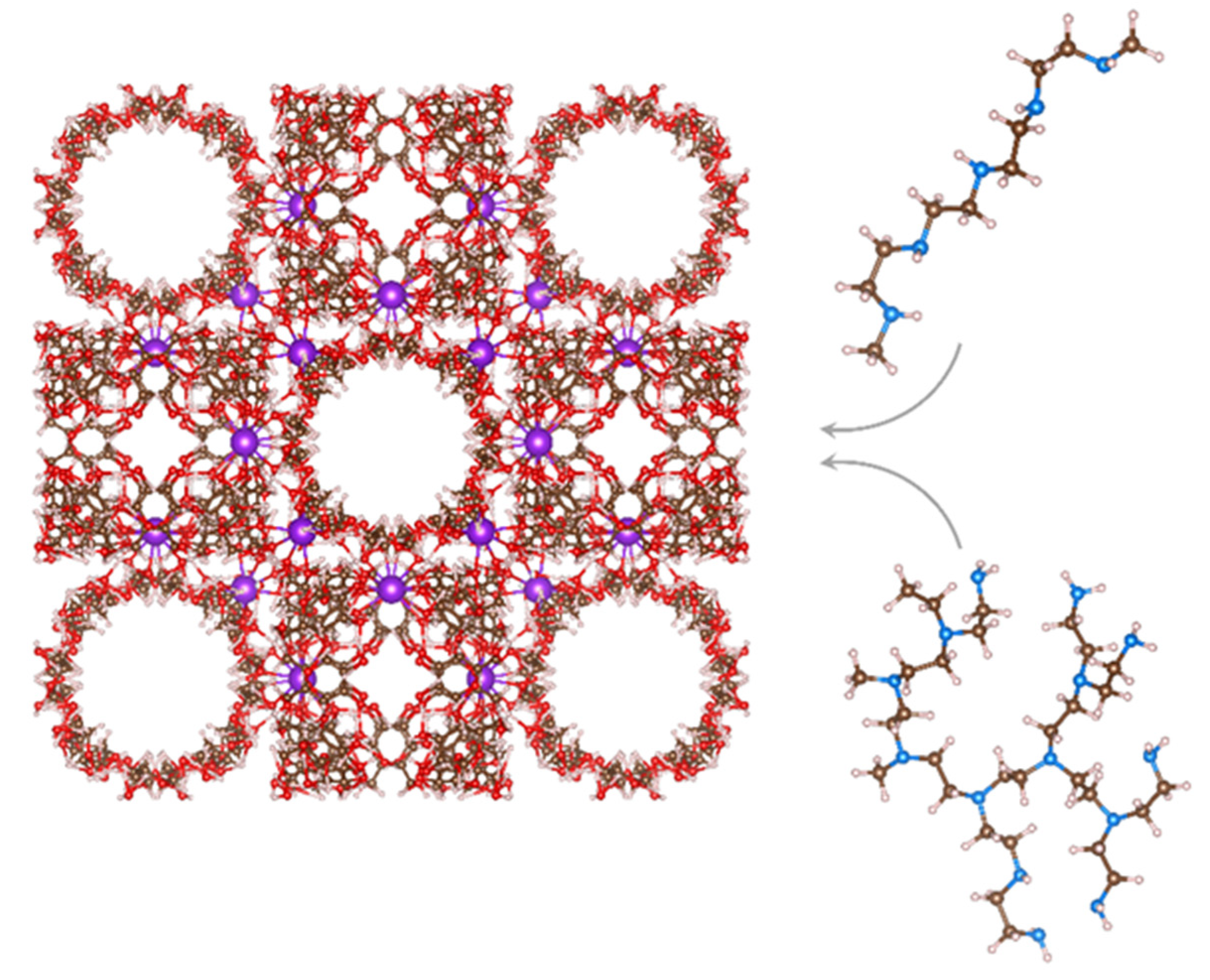
3.2. Molecular Docking Simulations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kjellström, E.; Nikulin, G.; Strandberg, G.; Christensen, O.B.; Jacob, D.; Keuler, K.; Geert Lenderink, G.; Van Meijgaard, E.; Schär, C.; Somot, S.; et al. A Review on Contemporary Metal–Organic Framework Materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Guillén Bolaños, T.; Bindi, M.; Brown, S.; Camilloni, I.A.; Diedhiou, A.; Djalante, R.; Ebi, K.; et al. The human imperative of stabilizing global climate change at 1.5 C. Science 2019, 365, eaaw6974. [Google Scholar] [CrossRef]
- Parry, M.; Arnell, N.; McMichael, T.; Nicholls, R.; Martens, P.; Kovats, S.; Livermore, M.; Rosenzweig, C.; Iglesias, A.; Fischer, G. Millions at risk: Defining critical climate change threats and targets. Glob. Environ. Change 2001, 11, 181–183. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F.; Parker, L.M.; Comeau, S.; Gattuso, J.P.; O’Connor, W.A.; Martin, S.; Pörtner, H.-O.; Ross, P.M. Impacts of ocean acidification on marine shelled molluscs. Mar. Biol. 2013, 160, 2207–2245. [Google Scholar] [CrossRef]
- Qazi, A.; Hussain, F.; Rahim, N.A.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards sustainable energy: A systematic review of renewable energy sources, technologies, and public opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon capture from flue gas and the atmosphere: A perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Hockstad, L.; Hanel, L. Inventory of US Greenhouse Gas Emissions and Sinks; (No. cdiac: EPA-EMISSIONS); US Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2018. [CrossRef]
- Zhang, X.; Singh, B.; He, X.; Gundersen, T.; Deng, L.; Zhang, S. Post-combustion carbon capture technologies: Energetic analysis and life cycle assessment. Int. J. Greenh. Gas Control. 2014, 27, 289–298. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013—A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Khraisheh, M.; Almomani, F.; Walker, G. Solid Sorbents as a Retrofit technology for CO2 Removal from natural Gas Under High pressure and temperature conditions. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Velasco, E.; Li, J. Metal-organic frameworks (MOFs) as effective sensors and scavengers for toxic environmental pollutants. Natl. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Su, X.; Bromberg, L.; Martis, V.; Simeon, F.; Huq, A.; Hatton, T.A. Postsynthetic functionalization of Mg-MOF-74 with tetraethylenepentamine: Structural characterization and enhanced CO2 adsorption. ACS Appl. Mater. Interfaces 2017, 9, 11299–11306. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, J.; Zhang, J.; Wang, X. Water stability and competition effects toward CO2 adsorption on metal organic frameworks. Sep. Purif. Rev. 2015, 44, 19–27. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.H. Green synthesis of metal–organic frameworks: A state-of-the-art review of potential environmental and medical applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Ho, T.M.; Howes, T.; Bhandari, B.R. Encapsulation of CO2 into amorphous and crystalline α-cyclodextrin powders and the characterization of the complexes formed. Food Chem. 2015, 187, 407–415. [Google Scholar] [CrossRef][Green Version]
- Guo, T.X.; Bedane, A.H.; Pan, Y.; Xiao, H.; Eić, M. Characteristics of carbon dioxide gas adsorption on β-cyclodextrin derivative. Mater. Lett. 2017, 189, 114–117. [Google Scholar] [CrossRef]
- Kathuria, A.; Pauwels, A.K.; Buntinx, M.; Shin, J.; Harding, T. Inclusion of ethanol in a nano-porous, bio-based metal organic framework. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 91–98. [Google Scholar] [CrossRef]
- Shen, X.; Du, H.; Mullins, R.H.; Kommalapati, R.R. Polyethylenimine applications in carbon dioxide capture and separation: From theoretical study to experimental work. Energy Technol. 2017, 5, 822–833. [Google Scholar] [CrossRef]
- Xian, S.; Xu, F.; Ma, C.; Wu, Y.; Xia, Q.; Wang, H.; Li, Z. Vapor-enhanced CO2 adsorption mechanism of composite PEI@ ZIF-8 modified by polyethyleneimine for CO2/N2 separation. Chem. Eng. J. 2015, 280, 363–369. [Google Scholar] [CrossRef]
- Le, M.U.T.; Lee, S.Y.; Park, S.J. Preparation and characterization of PEI-loaded MCM-41 for CO2 capture. Int. J. Hydrogen Energy 2014, 39, 12340–12346. [Google Scholar]
- Yan, T.K.; Nagai, A.; Michida, W.; Kusakabe, K.; binti Yusup, S. Crystal growth of cyclodextrin-based metal-organic framework for carbon dioxide capture and separation. Procedia Eng. 2016, 148, 30–34. [Google Scholar] [CrossRef]
- Venkatasubramanian, A.; Navaei, M.; Bagnall, K.R.; McCarley, K.C.; Nair, S.; Hesketh, P.J. Gas Adsorption characteristics of metal–organic frameworks via quartz crystal microbalance techniques. J. Phys. Chem. C 2012, 116, 15313–15321. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Auto dock 4 and auto dock tools 4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2010, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Thompson, M.A. Molecular Docking Using ArgusLab, an Efficient Shape-Based Search Algorithm and the a Score Scoring Function. In Proceedings of the American Chemical Society—228th National Meeting, Philadelphia, PA, USA, 22–26 August 2004. [Google Scholar]
- Xin, Q.; Ouyang, J.; Liu, T.; Li, Z.; Li, Z.; Liu, Y.; Wang, S.; Wu, H.; Jiang, Z.; Cao, X. Enhanced interfacial interaction and CO2 separation performance of mixed matrix membrane by incorporating polyethylenimine-decorated metal–Organic frameworks. ACS Appl. Mater. Interfaces 2015, 7, 1065–1077. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S.; Loong, L.H. Kinetic studies on carbon dioxide capture using activated carbon. Chem. Eng. Trans. 2013, 35, 361–366. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Liu, K.; Zhou, Z.; Jiang, L.; Shen, Y.; Zhao, D. Biocompatible cyclodextrin-based metal-organic frameworks for long-term sustained release of fragrances. Ind. Eng. Chem. Res. 2019, 58, 19767–19777. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Guo, T.; Zhang, G.; Qin, W.; Zhang, L.; Wang, C.; Zhu, W.; Yang, M.; Hu, X.; et al. Drug nanoclusters formed in confined nano-cages of CD-MOF: Dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm. Sin. B 2019, 9, 97–106. [Google Scholar] [CrossRef]
- Kathuria, A.; Harding, T.; Auras, R.; Kivy, M. Encapsulation of hexanal in biobased cyclodextrin metal organic framework for extended release. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 121–130. [Google Scholar] [CrossRef]
- Chen, L.; Watanabe, T.; Kanoh, H.; Hata, K.; Ohba, T. Cooperative CO2 adsorption promotes high CO2 adsorption density over wide optimal nanopore range. Adsorpt. Sci. Technol. 2018, 36, 625–639. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, C.; Lee, D.; El Badawy, A.; Kivy, M.B.; Kathuria, A. Optimal Polyethyleneimine Molecular Weight and Arrangement for Modification of γ-Cyclodextrin Metal Organic Frameworks (γ-CD-MOFs) for Post-Combustion CO2 Capture. Crystals 2022, 12, 1445. https://doi.org/10.3390/cryst12101445
Watson C, Lee D, El Badawy A, Kivy MB, Kathuria A. Optimal Polyethyleneimine Molecular Weight and Arrangement for Modification of γ-Cyclodextrin Metal Organic Frameworks (γ-CD-MOFs) for Post-Combustion CO2 Capture. Crystals. 2022; 12(10):1445. https://doi.org/10.3390/cryst12101445
Chicago/Turabian StyleWatson, Corinne, Dustin Lee, Amro El Badawy, Mohsen B. Kivy, and Ajay Kathuria. 2022. "Optimal Polyethyleneimine Molecular Weight and Arrangement for Modification of γ-Cyclodextrin Metal Organic Frameworks (γ-CD-MOFs) for Post-Combustion CO2 Capture" Crystals 12, no. 10: 1445. https://doi.org/10.3390/cryst12101445
APA StyleWatson, C., Lee, D., El Badawy, A., Kivy, M. B., & Kathuria, A. (2022). Optimal Polyethyleneimine Molecular Weight and Arrangement for Modification of γ-Cyclodextrin Metal Organic Frameworks (γ-CD-MOFs) for Post-Combustion CO2 Capture. Crystals, 12(10), 1445. https://doi.org/10.3390/cryst12101445








