Influence of Austenitisation Time and Temperature on Grain Size and Martensite Start of 51CrV4 Spring Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
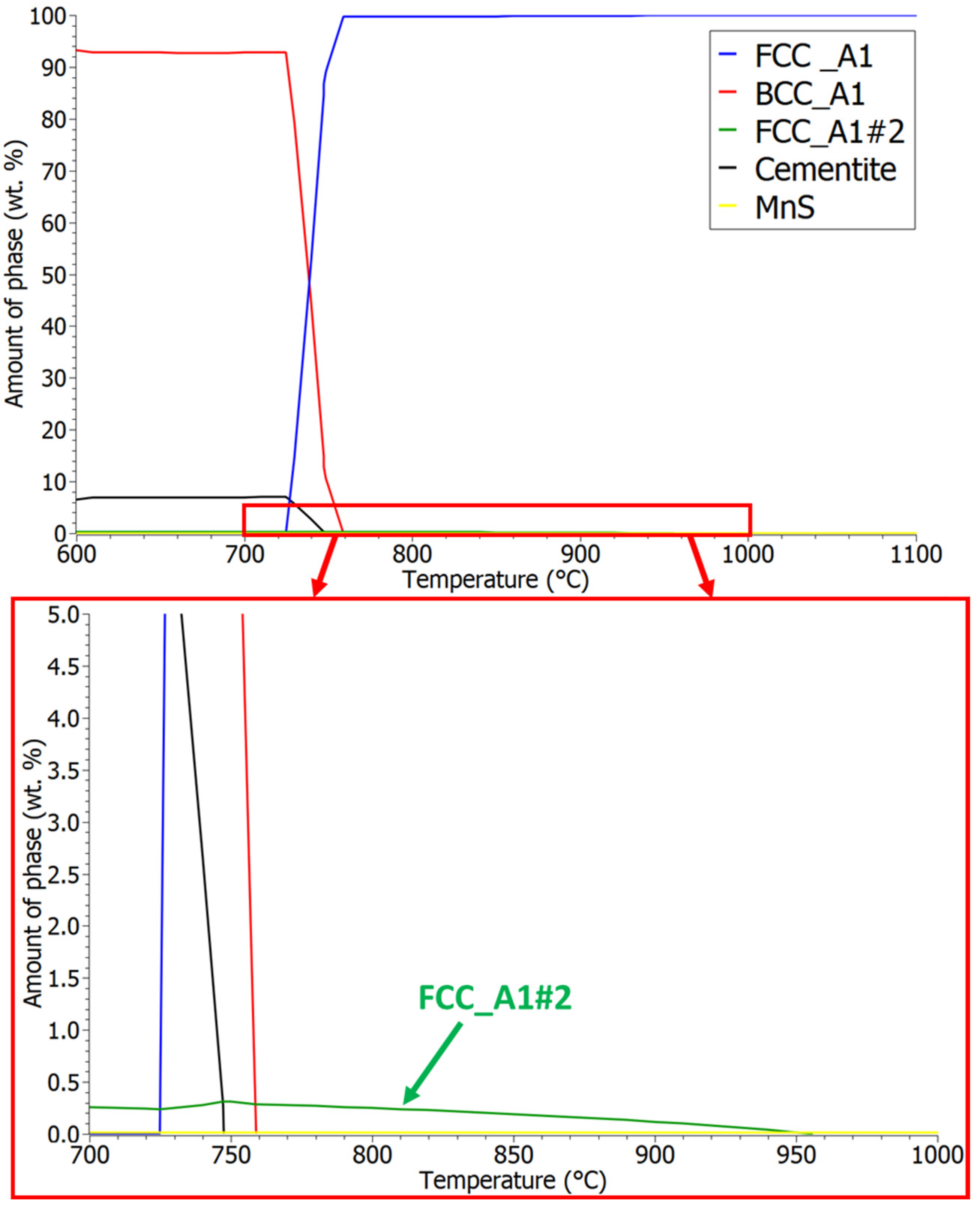
3.1. Thermodynamic Analysis
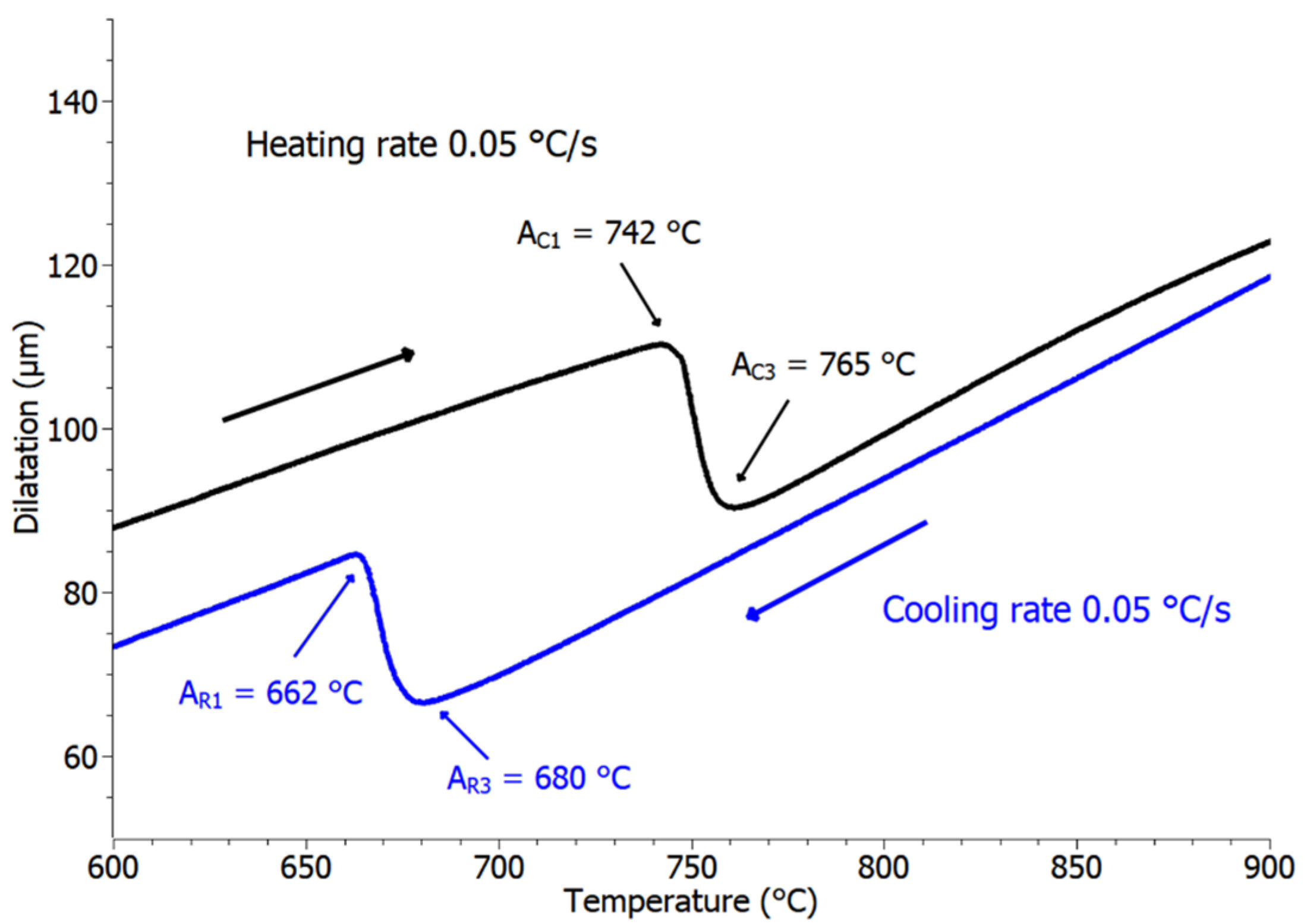
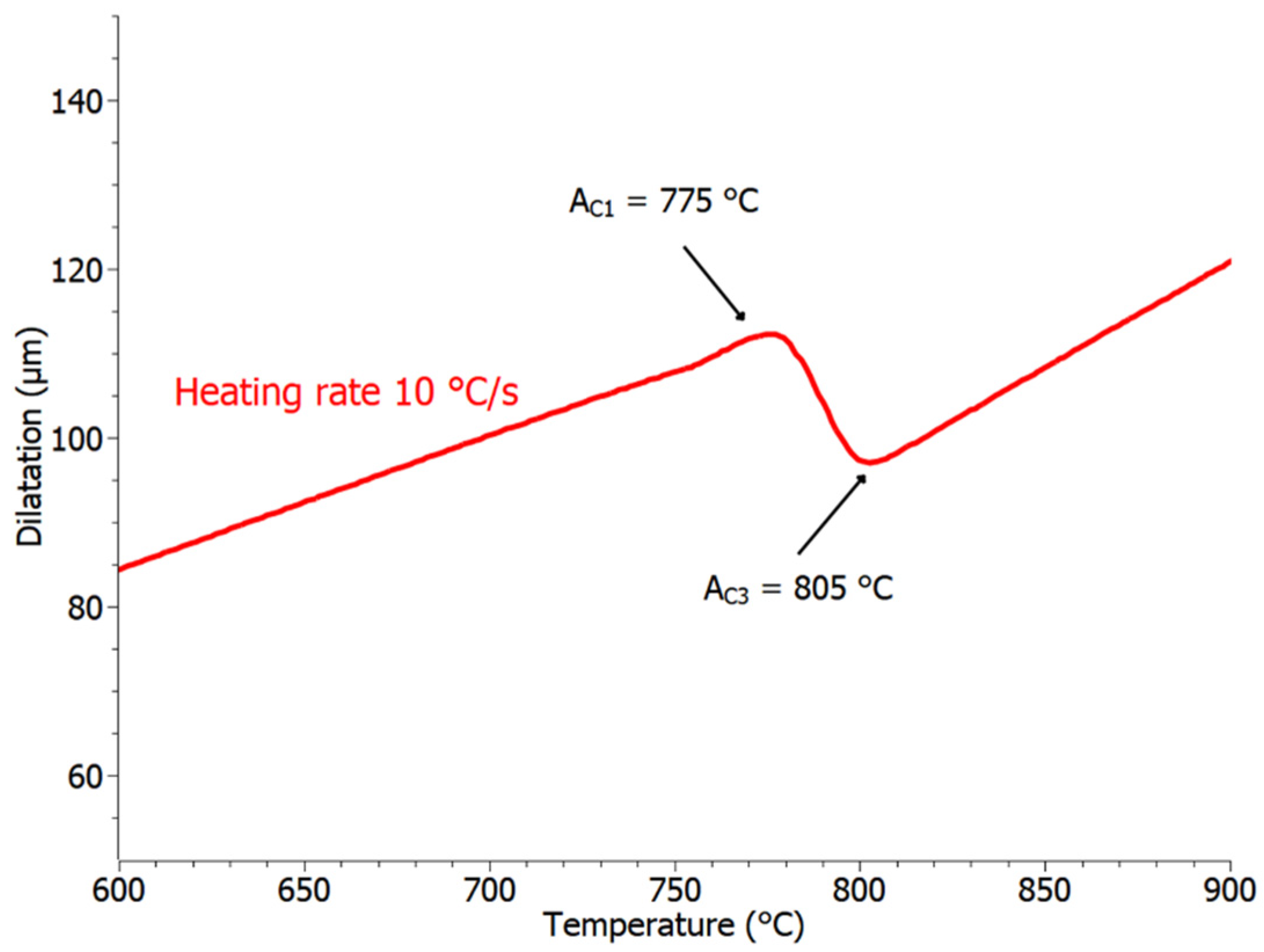
3.2. Transformation Temperatures
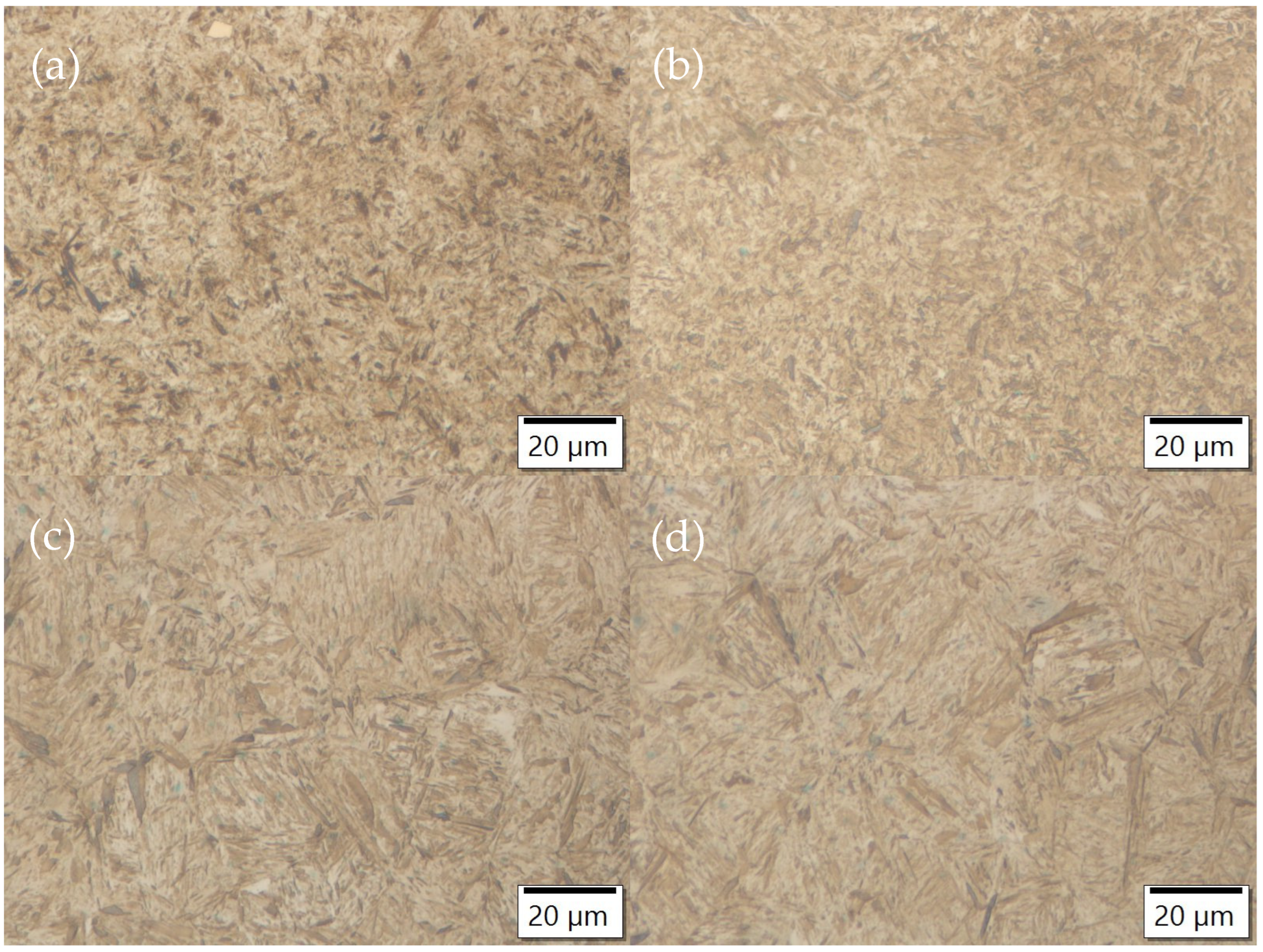
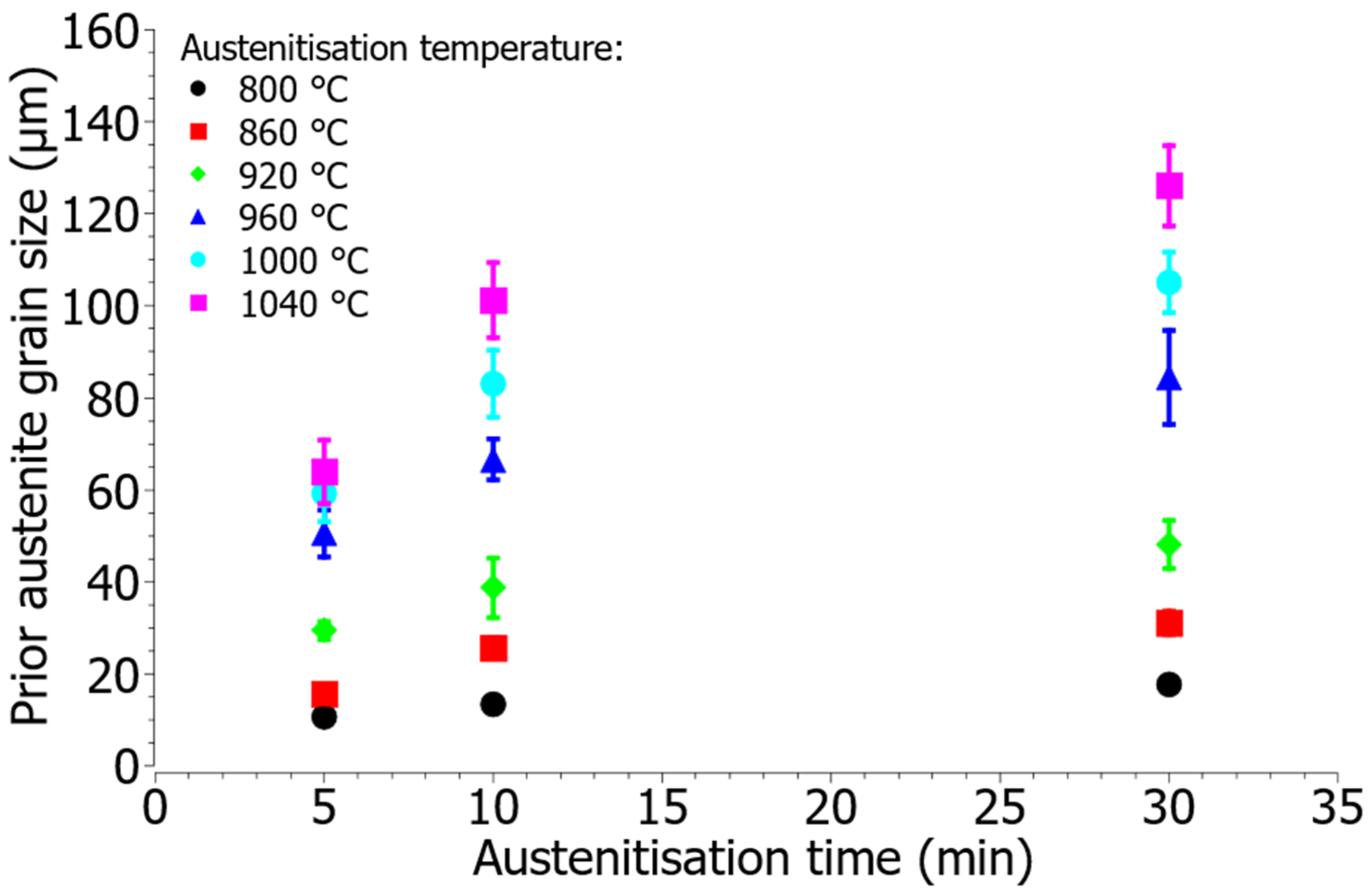
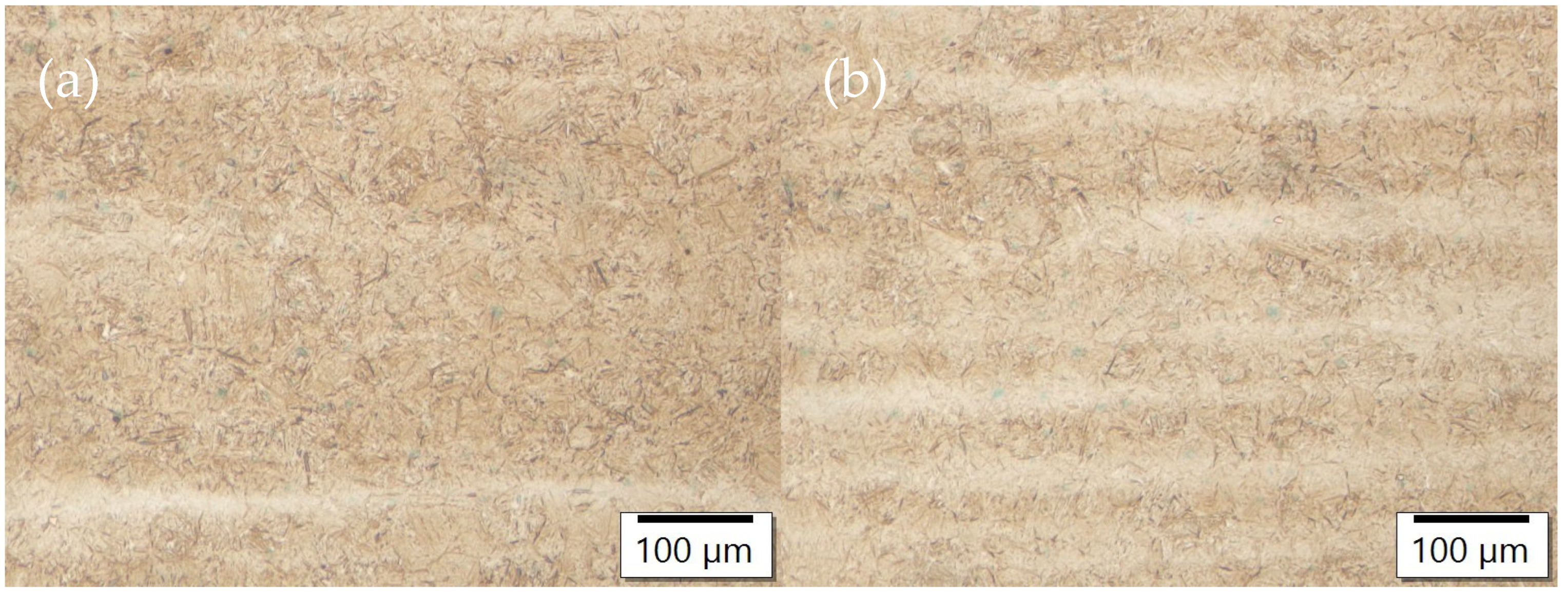
3.3. Optical Microscopy
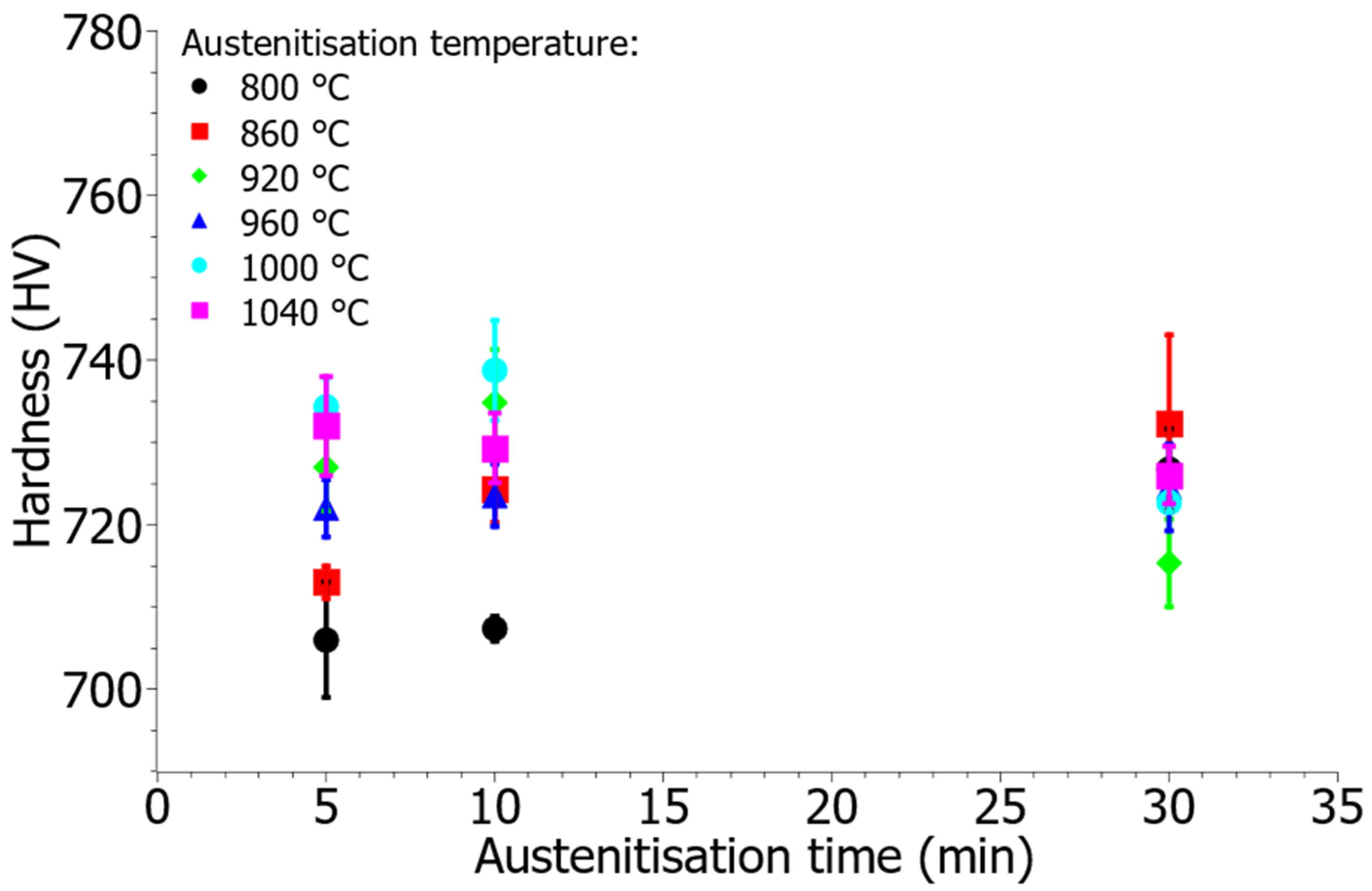
3.4. Hardness
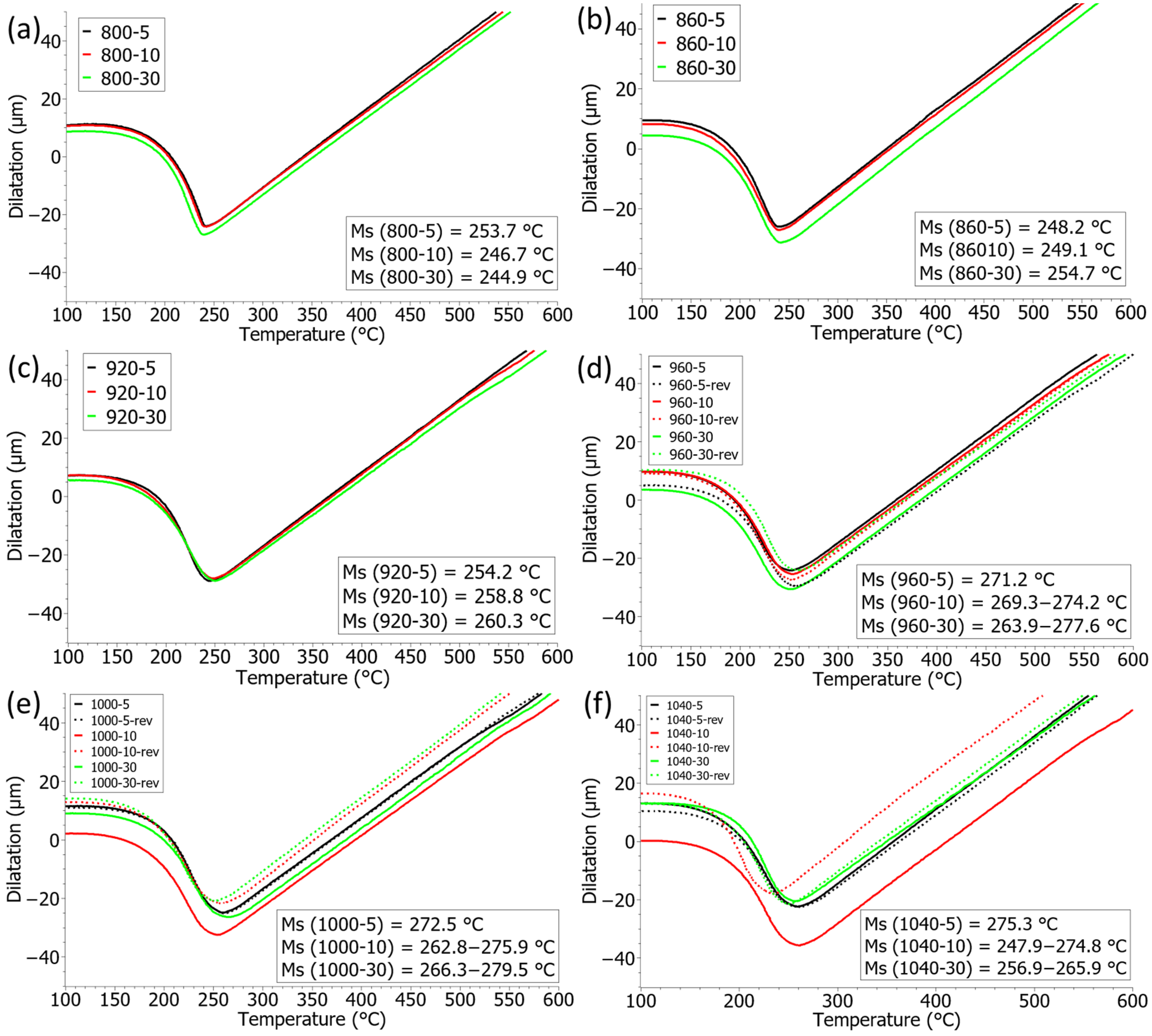
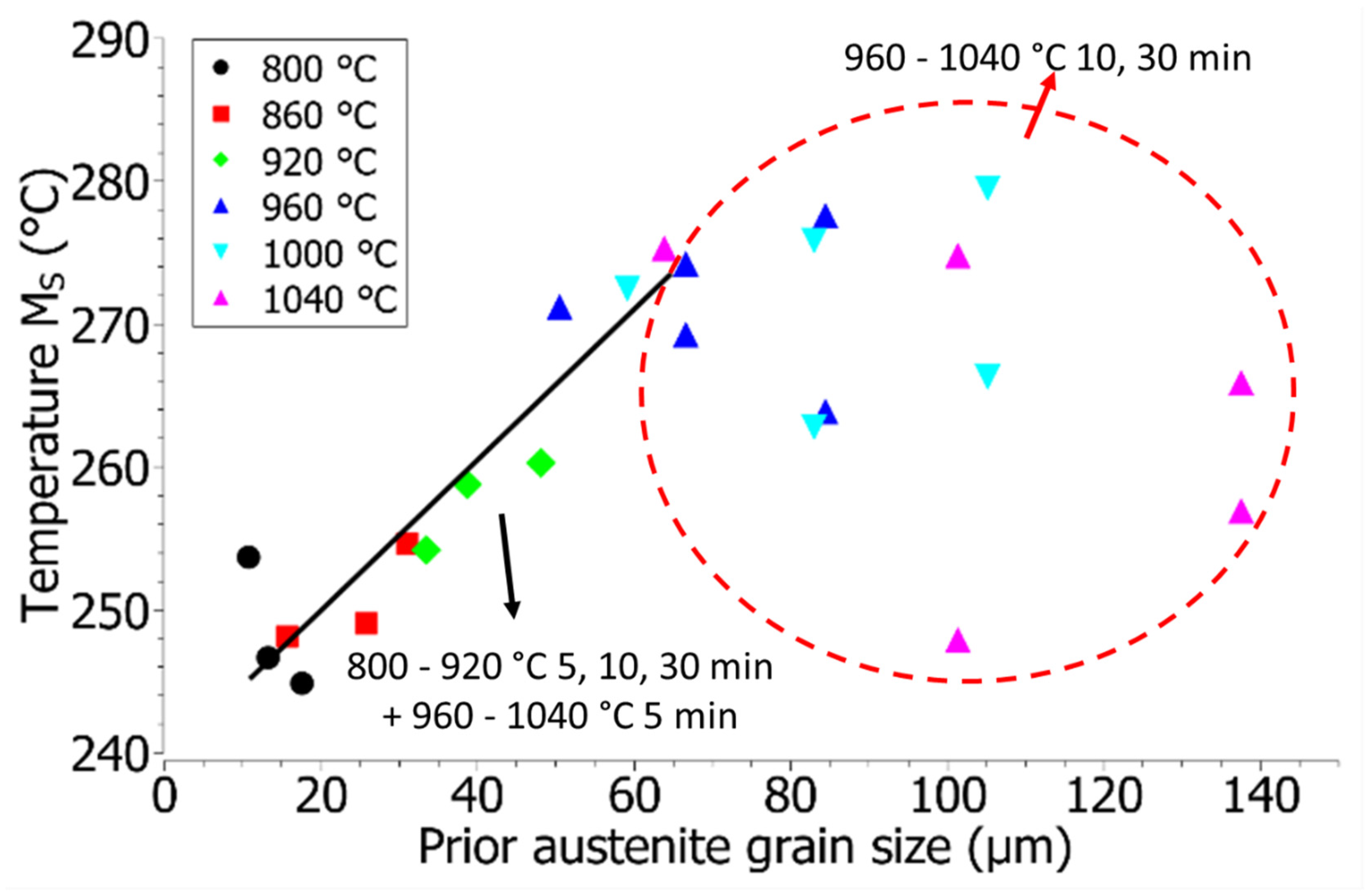
3.5. Dilatometry and MS Temperature
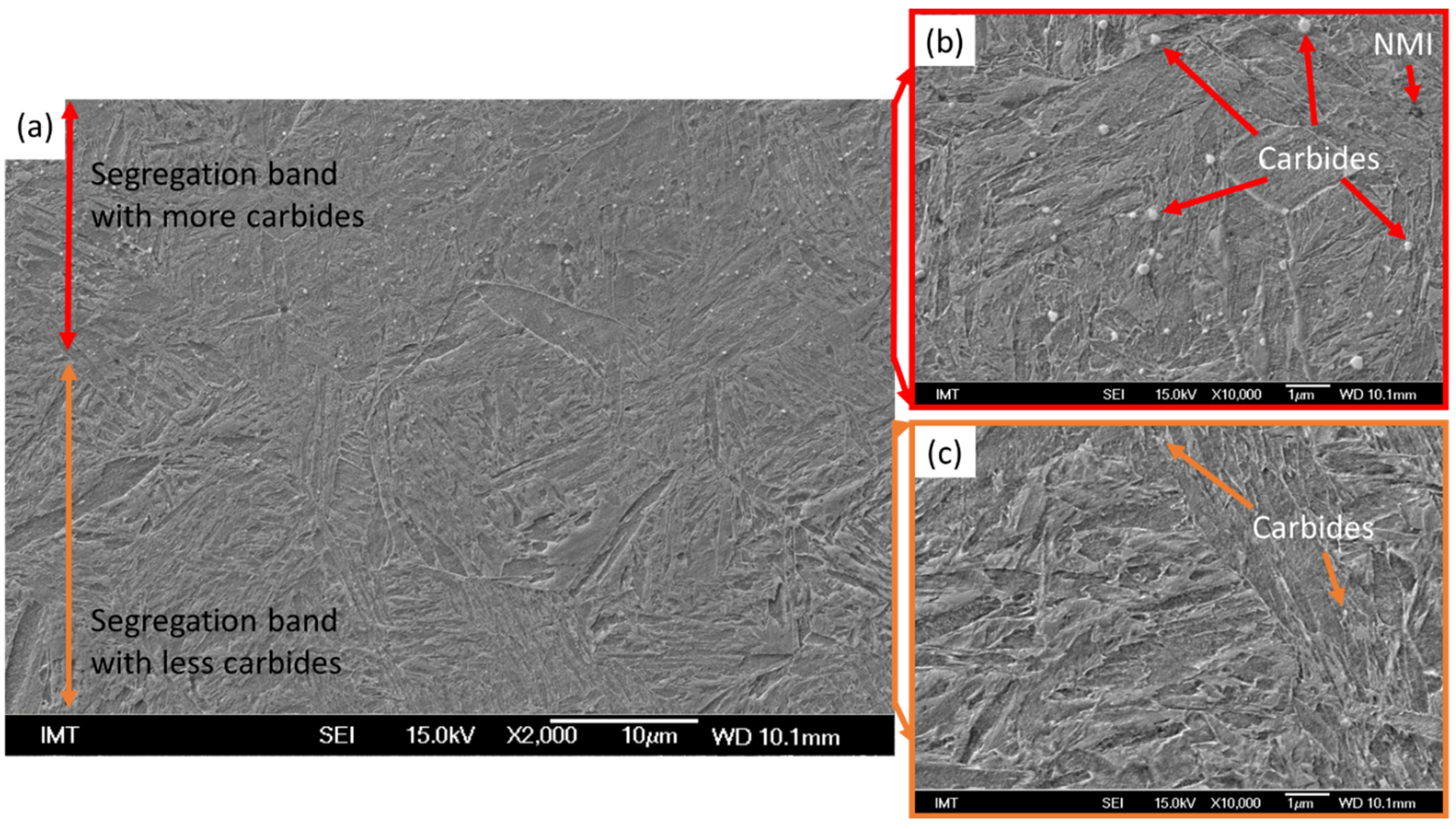
3.6. Scanning Electron Microscopy
4. Conclusions
- Prior austenite grains grow with increasing austenitisation temperature and times, with increasing temperature grain growth is more intense.
- The inhomogeneity of the microstructure can be observed in the quenched samples, which is the result of segregations during solidification. Due to the production route of the material, segregation bands with different distribution are present in the samples.
- At an austenitisation temperature of 800 °C, a partial transformation into austenite takes place; with a longer holding time at the temperature, a larger proportion of the transformation takes place, which affects the lowering of the martensite start temperature (MS).
- In the case of complete austenitic transformation, the MS temperature increases with the growth of crystal grains, and above 960 °C the MS temperature is also affected by the distribution of alloying elements. Due to the more intensive diffusion of alloying elements and the different degree of distribution of segregations, there are greater deviations in the MS temperature.
- Thermodynamic calculations using the Thermo-Calc program showed that the vanadium carbides in the investigated steel are stable up to 956 °C; using SEM, the vanadium carbides were analysed even at austenitisation temperatures of 1040 °C. The reason for the presence of carbides at such a high austenitisation temperature is local inhomogeneities and the limited time of carbide dissolution.
- There is an increased concentration of alloying elements in the segregation bands; the non-metallic inclusions and a higher concentration of carbides were characterised in the segregated areas.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Jiang, Z.; Li, Y.; Sun, M.; Wang, Q.; Chen, K.; Li, H. State of the Art in the Control of Inclusions in Spring Steel for Automobile—A Review. ISIJ Int. 2020, 60, 617–627. [Google Scholar] [CrossRef]
- Totten, G.E. (Ed.) Steel Heat Treatment Handbook: Metallurgy and Technologies; Taylor & Francis: Portland, OR, USA, 2006. [Google Scholar]
- Ishii, T.; Takahashi, K. Prediction of Fatigue Limit of Spring Steel Considering Surface Defect Size and Stress Ratio. Metals 2021, 11, 483. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, D.; Li, Y.; Wang, X.; Ren, X.; Wang, E. Effect of Quenching Conditions on the Microstructure and Mechanical Properties of 51CrV4 Spring Steel. Metals 2018, 8, 1056. [Google Scholar] [CrossRef]
- Xia, B.; Wang, B.; Zhang, P.; Ren, C.; Duan, Q.; Li, X.; Zhang, Z. Improving the High-Cycle Fatigue Life of a High-Strength Spring Steel for Automobiles by Suitable Shot Peening and Heat Treatment. Int. J. Fatigue 2022, 161, 106891. [Google Scholar] [CrossRef]
- James, M.N.; Hattingh, D.G.; Matthews, L. Embrittlement Failure of 51CrV4 Leaf Springs. Eng. Fail. Anal. 2022, 139, 106517. [Google Scholar] [CrossRef]
- Campbell, F.C. Elements of Metallurgy and Engineering Alloys; ASM International: Materials Park, OH, USA, 2008. [Google Scholar]
- Verhoeven, J.D. Steel Metallurgy for the Non-Metallurgist; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Liu, F.; Chen, K.; Kang, C.; Jiang, Z.; Ding, S. Effects of V–Nb Microalloying on the Microstructure and Properties of Spring Steel under Different Quenching-Tempering Times. J. Mater. Res. Technol. 2022, 19, 779–793. [Google Scholar] [CrossRef]
- Dai, Q.; Li, K.; Men, K.; Fang, Z.; Chen, W.; Yang, T.; Feng, C.; Wu, J.-M.; Misra, R.D.K. Effect of Vanadium on the Microstructure and Mechanical Properties of 2100mpa Ultrahigh Strength and High Plasticity Spring Steel Processed by a Novel Online Rapid-Induction Heat Treatment. Met. Mater. Int. 2022, 28, 4043687. [Google Scholar] [CrossRef]
- Dewangan, S. Effect of Heat Treatment into Tensile Strength, Hardness and Microstructural Attributes of TIG Welded Ti-6Al-4V Titanium Alloy. Aust. J. Mech. Eng. 2022, 1–10. [Google Scholar] [CrossRef]
- Burja, J.; Šuler, B.; Češnjaj, M.; Nagode, A. Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel. Metals 2021, 11, 392. [Google Scholar] [CrossRef]
- Celin, R.; Kafexhiu, F.; Klančnik, G.; Burja, J. Properties of the Simulated Coarse-Grained Microstructure of Quenched and Tempered High-Strength Steel. Mater. Technol. 2021, 55, 115–120. [Google Scholar] [CrossRef]
- Dewangan, S.; Chattopadhyaya, S. Analysing Effect of Quenching and Tempering into Mechanical Properties and Microstructure of 304-SS Welded Plates. Acta Metall. Slovaca 2022, 28, 140–146. [Google Scholar] [CrossRef]
- Speer, J.G.; Gaster, R.J. Austenitizing in steels. In Steel Heat Treating Fundamentals and Processes; Dossett, J., Totten, G.E., Eds.; ASM International: Materials Park, OH, USA, 2013; Volume 4A, pp. 309–316. [Google Scholar]
- Savran, V. Austenite Formation in C-Mn Steel. Ph.D. Thesis, Delft University of Technology, Delft, The Nederlanden, 2009. [Google Scholar]
- Pous-Romero, H.; Lonardelli, I.; Cogswell, D.; Bhadeshia, H.K.D.H. Austenite Grain Growth in a Nuclear Pressure Vessel Steel. Mater. Sci. Eng. A 2013, 567, 72–79. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.K. Effect of Austenite Grain Size on Martensitic Transformation of a Low Alloy Steel. Mater. Sci. Forum 2005, 475–479, 3169–3172. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.K. Prediction of Austenite Grain Growth during Austenitization of Low Alloy Steels. Mater. Des. 2008, 29, 1840–1844. [Google Scholar] [CrossRef]
- Jafarian, H.R.; Sabzi, M.; Mousavi Anijdan, S.H.; Eivani, A.R.; Park, N. The Influence of Austenitization Temperature on Microstructural Developments, Mechanical Properties, Fracture Mode and Wear Mechanism of Hadfield High Manganese Steel. J. Mater. Res. Technol. 2021, 10, 819–831. [Google Scholar] [CrossRef]
- Dong, D.; Chen, F.; Cui, Z. Modeling of Austenite Grain Growth During Austenitization in a Low Alloy Steel. J. Mater. Eng. Perform. 2016, 25, 152–164. [Google Scholar] [CrossRef]
- Chamanfar, A.; Chentouf, S.M.; Jahazi, M.; Lapierre-Boire, L.P. Austenite Grain Growth and Hot Deformation Behavior in a Medium Carbon Low Alloy Steel. J. Mater. Res. Technol. 2020, 9, 12102–12114. [Google Scholar] [CrossRef]
- Foder, J.; Burja, J.; Klančnik, G. Grain Size Evolution and Mechanical Properties of Nb, V–Nb, and Ti–Nb Boron Type S1100QL Steels. Metals 2021, 11, 492. [Google Scholar] [CrossRef]
- Maropoulos, S.; Karagiannis, S.; Ridley, N. The Effect of Austenitising Temperature on Prior Austenite Grain Size in a Low-Alloy Steel. Mater. Sci. Eng. A 2008, 483–484, 735–739. [Google Scholar] [CrossRef]
- Hong, S.C.; Lim, S.H.; Hong, H.S.; Lee, K.J.; Shin, D.H.; Lee, K.S. Effects of Nb on Strain Induced Ferrite Transformation in C-Mn Steel. Mater. Sci. Eng. A 2003, 355, 241–248. [Google Scholar] [CrossRef]
- Adamczyk, J.; Kalinowska-Ozgowicz, E.; Ozgowicz, W.; Wusatowski, R. Interaction of Carbonitrides V(C,N) Undissolved in Austenite on the Structure and Mechanical Properties of Microalloyed V-N Steels. J. Mater. Process. Technol. 1995, 53, 23–32. [Google Scholar] [CrossRef]
- Matsuo, S.; Ando, T.; Grant, N.J. Grain Refinement and Stabilization in Spray-Formed AISI 1020 Steel. Mater. Sci. Eng. A 2000, 288, 34–41. [Google Scholar] [CrossRef]
- Devra, V.K.; Maity, J. Solute Drag Effect on Austenite Grain Growth in Hypoeutectoid Steel. Philos. Mag. Lett. 2020, 100, 245–259. [Google Scholar] [CrossRef]
- Lambers, H.G.; Tschumak, S.; Maier, H.J.; Canadinc, D. Role of Austenitization and Pre-Deformation on the Kinetics of the Isothermal Bainitic Transformation. Metall. Mater. Trans. A Phys. 2009, 40, 1355–1366. [Google Scholar] [CrossRef]
- Goulas, C.; Mecozzi, M.G.; Sietsma, J. Bainite Formation in Medium-Carbon Low-Silicon Spring Steels Accounting for Chemical Segregation. Metall. Mater. Trans. A 2016, 47, 3077–3087. [Google Scholar] [CrossRef]
- Lambers, H.; Tschumak, S.; Maier, H.J.; Canadinc, D. On the Bainitic and Martensitic Phase Transformation Behavior and the Mechanical Properties of Low Alloy 51CrV4 Steel. Int. J. Struct. Chang. Solids 2011, 3, 15–27. [Google Scholar]
- Klančnik, G.; Krajnc, L.; Nagode, A.; Burja, J. Isothermal Quenching of As-Cast Medium Carbon, High-Silicon AR Steel. Materials 2022, 15, 5595. [Google Scholar] [CrossRef]
- Zhi, C.; Yuan, G.; Xian-Guo, Y.; Hong, G.; Yao, H.; Hai-Dong, Z.; Min-Na, Z. Enhanced Axial Tension-Tension Fatigue Resistance of a 51CrV4 Spring Steel by Cryogenic Treatment. Rev. Chim. 2021, 72, 138–151. [Google Scholar] [CrossRef]
- Sij Group. SIQUAL 8159 Steel. Available online: https://steelselector.sij.si/steels/VCV150.html (accessed on 26 September 2022).
- Kawulok, R.; Schindler, I.; Kawulok, P.; Rusz, S.; Opěla, P.; Mališ, M.; Vašek, Z.; Subíková, M.; Váňová, P. Effect of Plastic Deformation on CCT Diagram of Spring Steel 51CrV4. In Proceedings of the METAL 2015—24th International Conference on Metallurgy and Materials, Brno, Czech Republic, 3–5 June 2015; pp. 345–350. [Google Scholar]
- Gaggiotti, M.; Albini, L.; di Nunzio, P.E.; di Schino, A.; Stornelli, G.; Tiracorrendo, G. Ultrafast Heating Heat Treatment Effect on the Microstructure and Properties of Steels. Metals 2022, 12, 1313. [Google Scholar] [CrossRef]
- Moravec, J.; Nováková, I.; Vondráček, J. Influence of Heating Rate on the Transformation Temperature Change in Selected Steel Types. Manuf. Technol. 2020, 20, 217–222. [Google Scholar] [CrossRef]
- Yang, H.S.; Bhadeshia, H.K.D.H. Austenite Grain Size and the Martensite-Start Temperature. Scr. Mater. 2009, 60, 493–495. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Cr | V | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.52 | 0.26 | 0.76 | 0.013 | 0.005 | 1.00 | 0.18 | 0.09 | 0.21 | bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajželj, A.; Burja, J. Influence of Austenitisation Time and Temperature on Grain Size and Martensite Start of 51CrV4 Spring Steel. Crystals 2022, 12, 1449. https://doi.org/10.3390/cryst12101449
Bajželj A, Burja J. Influence of Austenitisation Time and Temperature on Grain Size and Martensite Start of 51CrV4 Spring Steel. Crystals. 2022; 12(10):1449. https://doi.org/10.3390/cryst12101449
Chicago/Turabian StyleBajželj, Anže, and Jaka Burja. 2022. "Influence of Austenitisation Time and Temperature on Grain Size and Martensite Start of 51CrV4 Spring Steel" Crystals 12, no. 10: 1449. https://doi.org/10.3390/cryst12101449
APA StyleBajželj, A., & Burja, J. (2022). Influence of Austenitisation Time and Temperature on Grain Size and Martensite Start of 51CrV4 Spring Steel. Crystals, 12(10), 1449. https://doi.org/10.3390/cryst12101449










