Effect of Al Content on the Microstructure and Tensile Properties of Zr-Co-Al Alloy Prepared by Rapid Solidification
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
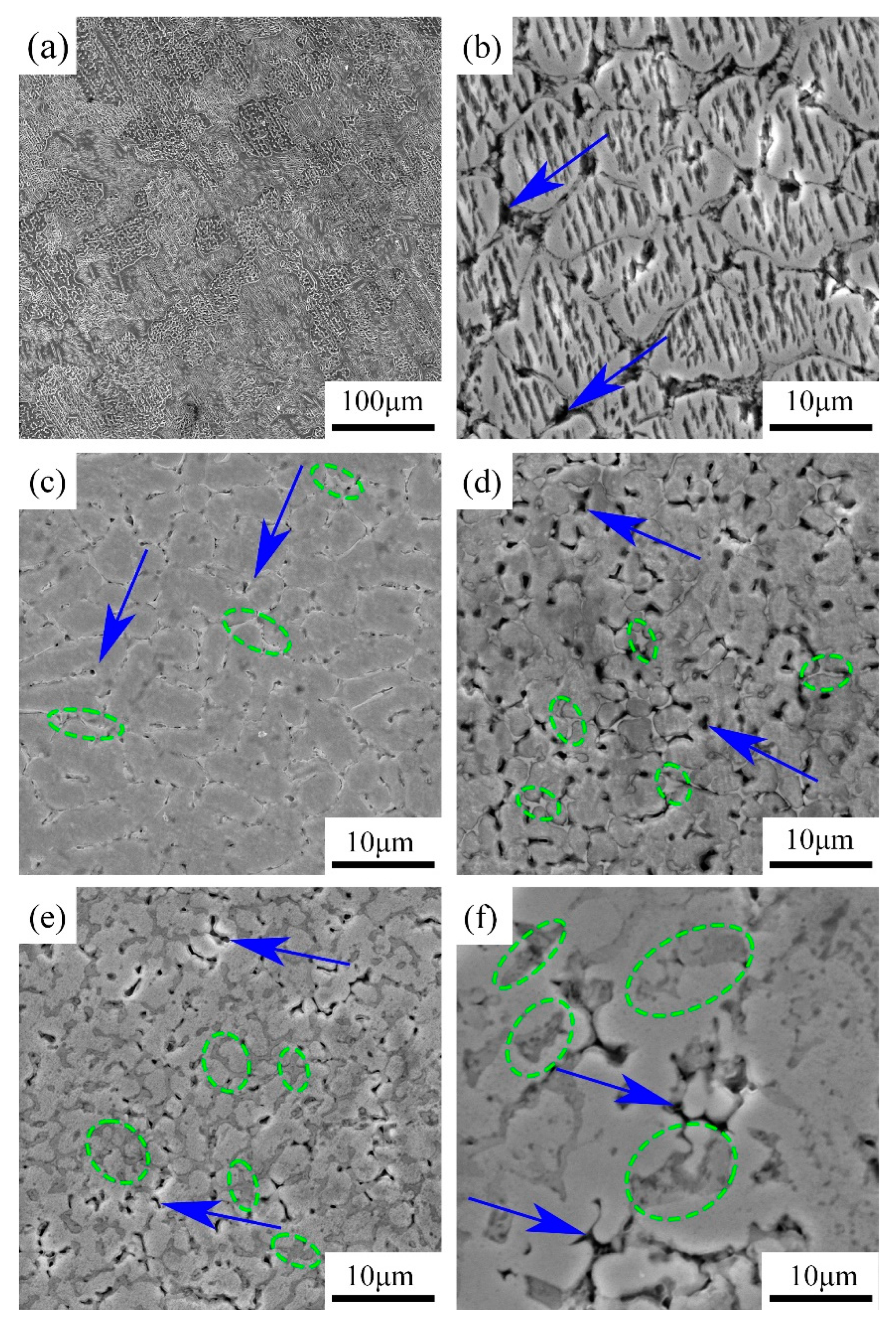
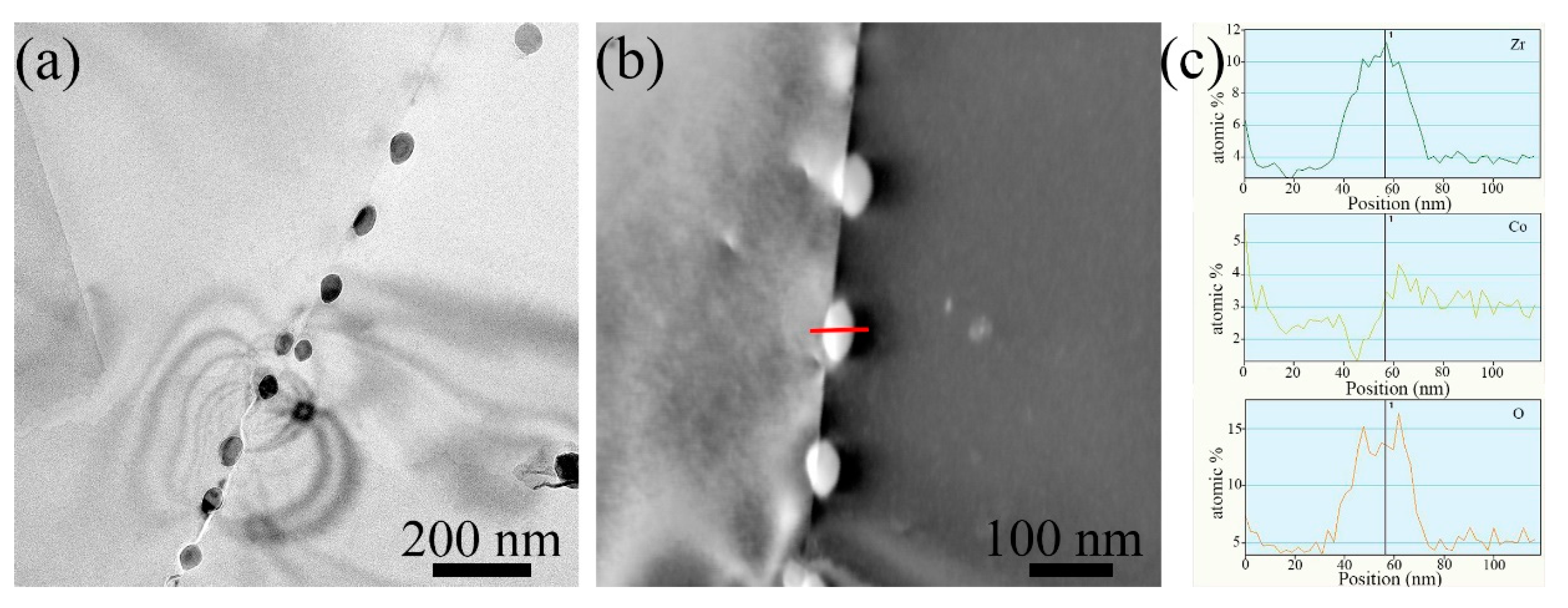
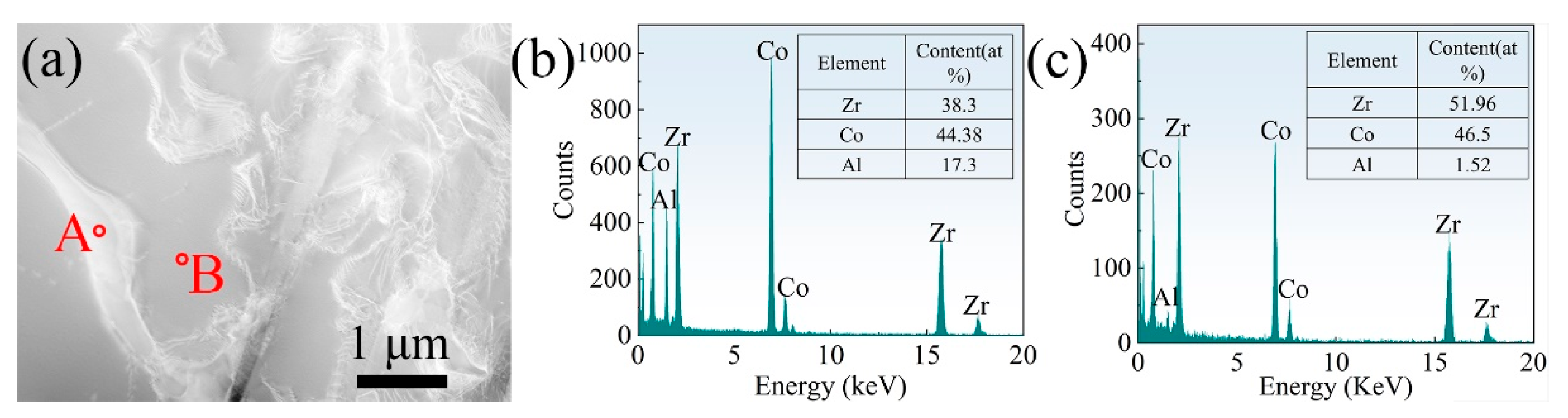
3.1. Microstructure
3.2. Hardness
3.3. Tensile Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hua, N.; Pang, S.; Li, Y.; Wang, J.; Li, R.; Georgarakis, K.; Yavari, A.R.; Vaughan, G.; Zhang, T. Ni- and Cu-free Zr–Al–Co–Ag bulk metallic glasses with superior glass-forming ability. J. Mater. Res. 2011, 26, 539–546. [Google Scholar] [CrossRef]
- Wada, T.; Qin, F.; Wang, X.; Yoshimura, M.; Inoue, A.; Sugiyama, N.; Ito, R.; Matsushita, N. Formation and bioactivation of Zr-Al-Co bulk metallic glasses. J. Mater. Res. 2011, 24, 2941–2948. [Google Scholar] [CrossRef]
- Tan, J.; Pan, F.S.; Zhang, Y.; Sun, B.A.; He, J.; Zheng, N.; Stoica, M.; Kühn, U.; Eckert, J. Formation of Zr–Co–Al bulk metallic glasses with high strength and large plasticity. Intermetallics 2012, 31, 282–286. [Google Scholar] [CrossRef]
- Matsuda, M.; Nishimoto, T.; Matsunaga, K.; Morizono, Y.; Tsurekawa, S.; Nishida, M. Deformation structure in ductile B2-type Zr–Co–Ni alloys with martensitic transformation. J. Mater. Sci. 2011, 46, 4221–4227. [Google Scholar] [CrossRef]
- Matsuda, M.; Nishimoto, T.; Morizono, Y.; Tsurekawa, S.; Nishida, M. Enhancement of ductility in B2-type Zr–Co–Pd alloys with martensitic transformation. Intermetallics 2011, 19, 894–899. [Google Scholar] [CrossRef]
- Matsuda, M.; Iwamoto, Y.; Morizono, Y.; Tsurekawa, S.; Takashima, K.; Nishida, M. Enhancement of ductility in B2-type Zr–Co–Ni alloys with deformation-induced martensite and microcrack formation. Intermetallics 2013, 36, 45–50. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, C.; Jiang, J.-Z. Bulk metallic glass composites containing B2 phase. Prog. Mater. Sci. 2021, 121, 100799. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, H.; Wu, Y.; Song, W.; Chen, R.; Tan, C.; Xie, G.; Cao, D.; Wang, H.; Liu, X.; et al. Enhancing dynamic mechanical properties of bulk metallic glass composites via deformation-induced martensitic transformation. Scr. Mater. 2020, 186, 346–351. [Google Scholar] [CrossRef]
- Lu, W.F.; Li, C.J.; Sarac, B.; Şopu, D.; Yi, J.H.; Tan, J.; Stoica, M.; Eckert, J. Structural, elastic and electronic properties of CoZr in B2 and B33 structures under high pressure. J. Alloys Compd. 2017, 705, 445–455. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.F.; Li, C.J.; Yuan, Q.; Wu, Z.X.; Tan, J.; Gao, P.; You, X.; Yi, J.H. Effect of Al content on the mechanical properties and toughening mechanism of Zr-Co-Al alloys prepared by rapid solidification. Mater. Sci. Eng. A 2022, 831, 142237. [Google Scholar] [CrossRef]
- Jansson, K.; Nygren, M.; Östlund, Å. Crystallization behaviour of amorphous Zr1-xCox alloys with 0.20 ≤ x ≤ 0.41. Mater. Res. Bull. 1984, 19, 1091–1104. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.-Z. Formation of the B2–ZrCo phase and micro-hardness evolution in Zr–Co–Al BMGs via conventional and flash annealing. J. Alloys Compd. 2020, 834, 154230. [Google Scholar] [CrossRef]
- Kosiba, K.; Scudino, S.; Kobold, R.; Kühn, U.; Greer, A.L.; Eckert, J.; Pauly, S. Transient nucleation and microstructural design in flash-annealed bulk metallic glasses. Acta Mater. 2017, 127, 416–425. [Google Scholar] [CrossRef]
- Li, C.J.; Tan, J.; Zhu, X.K.; Zhang, Y.; Stoica, M.; Kuhn, U.; Eckert, J. On the transformation-induced work-hardening behavior of Zr47.5Co47.5Al5 ultrafine-grained alloy. Intermetallics 2013, 35, 116–119. [Google Scholar] [CrossRef]
- Li, P.Y. Microstructure and mechanical properties of novel B2-type ductile Zr-Co-Cu alloys containing the B33 phase. Mater. Res. Express. 2017, 4, 086505. [Google Scholar] [CrossRef]
- Hossain, D.; Harris, I.R.; Barraclough, K.G. A study of ZrCo and related ternary phases represented by the general formula, Zr50Co50 xNix. J. Less Common Met. 1974, 37, 35–57. [Google Scholar] [CrossRef]
- François, A.; Veyssière, P. A TEM investigation of the deformation microstructure of CoZr and Co40Ni10Zr50 ordered alloys. Intermetallics 1994, 2, 9–22. [Google Scholar] [CrossRef]
- Wu, D.Y.; Song, K.K.; Gargarella, P.; Cao, C.D.; Li, R.; Kaban, I.; Eckert, J. Glass-forming ability, thermal stability of B2 CuZr phase, and crystallization kinetics for rapidly solidified Cu–Zr–Zn alloys. J. Alloys Compd. 2016, 664, 99–108. [Google Scholar] [CrossRef]
- Javid, F.A.; Mattern, N.; Pauly, S.; Eckert, J. Effect of Cobalt on Phase Formation, Microstructure, and Mechanical Properties of Cu50−xCoxZr50 (x = 2, 5, 10, 20 at.%) Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 43, 2631–2636. [Google Scholar] [CrossRef]
- Ishida, K.; Kainuma, R.; Ueno, N.; Nishizawa, T. Ductility enhancement in NiAl (B2)-base alloys by microstructural control. Metall. Trans. A 1991, 22, 441–446. [Google Scholar] [CrossRef]
- Zeumer, B.; Sauthoff, G. Deformation behaviour of intermetallic NiAl Ta alloys with strengthening Laves phase for high-temperature applications III. Effects of alloying with Cr. Intermetallics 1998, 6, 451–460. [Google Scholar] [CrossRef]
- McKamey, C.G.; Horton, J.A.; Liu, C.T. Effect of chromium on properties of Fe3Al. J. Mater. Res. 2011, 4, 1156–1163. [Google Scholar] [CrossRef]
- Nowak, K.; Kupka, M. High-temperature oxidation behaviour of B2 FeAl based alloy with Cr, Zr and B additions. Mater. Chem. Phys. 2012, 132, 902–908. [Google Scholar] [CrossRef]
- Aoki, K.; Izumi, O. Improvement in Room Temperature Ductility of the Intermetallic Compound Ni3Al by Ternary Trace Element Addition. J. Jpn. Inst. Met. 1979, 43, 358–359. [Google Scholar] [CrossRef] [Green Version]
- Wollmershauser, J.A.; Kabra, S.; Agnew, S.R. In situ neutron diffraction study of the plastic deformation mechanisms of B2 ordered intermetallic alloys: NiAl, CuZn, and CeAg. Acta Mater. 2009, 57, 213–223. [Google Scholar] [CrossRef]
- Mulay, R.P.; Agnew, S.R. The fracture behavior of B2 structured MgR intermetallics. Scr. Mater. 2009, 61, 1036–1039. [Google Scholar] [CrossRef]
- Russell, A.M.; Zhang, Z.; Lograsso, T.A.; Lo, C.C.H.; Pecharsky, A.O.; Morris, J.R.; Ye, Y.; Gschneidner, K.A.; Slager, A.J. Mechanical properties of single crystal YAg. Acta Mater. 2004, 52, 4033–4040. [Google Scholar] [CrossRef]
- Stumphy, B.; Mudryk, Y.; Russell, A.; Herman, D.; Gschneidner, K. Oxidation resistance of B2 rare earth–magnesium intermetallic compounds. J. Alloys Compd. 2008, 460, 363–367. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Wu, H.H.; Zhang, Z.Y.; Hui, X.D.; Chen, G.L.; Ma, D.; Wang, X.L.; Lu, Z.P. Formation of Cu–Zr–Al bulk metallic glass composites with improved tensile properties. Acta Mater. 2011, 59, 2928–2936. [Google Scholar] [CrossRef]
- Li, C.J.; Tan, J.; Wang, G.; Bednarčík, J.; Zhu, X.K.; Zhang, Y.; Stoica, M.; Kühn, U.; Eckert, J. Enhanced strength and transformation-induced plasticity in rapidly solidified Zr–Co–(Al) alloys. Scr. Mater. 2013, 68, 897–900. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Y.; Stoica, M.; Kuhn, U.; Mattern, N.; Pan, F.S.; Eckert, J. Study of mechanical property and crystallization of a ZrCoAl bulk metallic glass. Intermetallics 2011, 19, 567–571. [Google Scholar] [CrossRef]
- Dong, Q.; Pan, Y.J.; Tan, J.; Qin, X.M.; Li, C.J.; Gao, P.; Feng, Z.X.; Calin, M.; Eckert, J. A comparative study of glass-forming ability, crystallization kinetics and mechanical properties of Zr55Co25Al20 and Zr52Co25Al23 bulk metallic glasses. J. Alloys Compd. 2019, 785, 422–428. [Google Scholar] [CrossRef]
- Matsuda, M.; Hayashi, K.; Nishida, M. Ductility Enhancement in B2-Type Zr-Co-Ni Alloys with Martensitic Transformation. Mater. Trans. 2009, 50, 2335–2340. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.D.; Li, C.J.; Peng, Y.Z.; Gao, P.; Feng, Z.X.; Liu, Y.C.; Li, J.N.; Tao, J.M.; Yi, J.H. Fe-based metallic glass particles carry carbon nanotubes to reinforce Al matrix composites. Mater. Charact. 2022, 189, 112006. [Google Scholar] [CrossRef]
- Liu, J.; Fan, G.; Tan, Z.; Guo, Q.; Su, Y.; Li, Z.; Xiong, D.-B. Mechanical properties and failure mechanisms at high temperature in carbon nanotube reinforced copper matrix nanolaminated composite. Compos. Part A Appl. Sci. Manuf. 2019, 116, 54–61. [Google Scholar] [CrossRef]
- Deng, H.; Yi, J.; Xia, C.; Yi, Y. Mechanical properties and microstructure characterization of well-dispersed carbon nanotubes reinforced copper matrix composites. J. Alloys Compd. 2017, 727, 260–268. [Google Scholar] [CrossRef]





| Alloys | UTS (MPa) | UFS (%) |
|---|---|---|
| Zr50Co50 | 240 ± 20 | 6.99 |
| Zr48Co48Al4 | 304 ± 20 | 3.75 |
| Zr47.5Co47.5Al5 | 464 ± 20 | 3.93 |
| Zr47Co47Al6 | 396 ± 20 | 3.30 |
| Zr46.5Co46.5Al7 | 390 ± 20 | 3.28 |
| Zr46Co46Al8 | 363 ± 20 | 3.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Lu, W.; Li, C.; Gao, P.; You, X.; Tan, J. Effect of Al Content on the Microstructure and Tensile Properties of Zr-Co-Al Alloy Prepared by Rapid Solidification. Crystals 2022, 12, 1483. https://doi.org/10.3390/cryst12101483
Wu Z, Lu W, Li C, Gao P, You X, Tan J. Effect of Al Content on the Microstructure and Tensile Properties of Zr-Co-Al Alloy Prepared by Rapid Solidification. Crystals. 2022; 12(10):1483. https://doi.org/10.3390/cryst12101483
Chicago/Turabian StyleWu, Zixiang, Wenfei Lu, Caiju Li, Peng Gao, Xin You, and Jun Tan. 2022. "Effect of Al Content on the Microstructure and Tensile Properties of Zr-Co-Al Alloy Prepared by Rapid Solidification" Crystals 12, no. 10: 1483. https://doi.org/10.3390/cryst12101483





