Morphology and Growth Mechanism of β-Rhombohedral Boron and Pentagonal Twins in Cu Alloy
Abstract
1. Introduction
2. Experimental Section
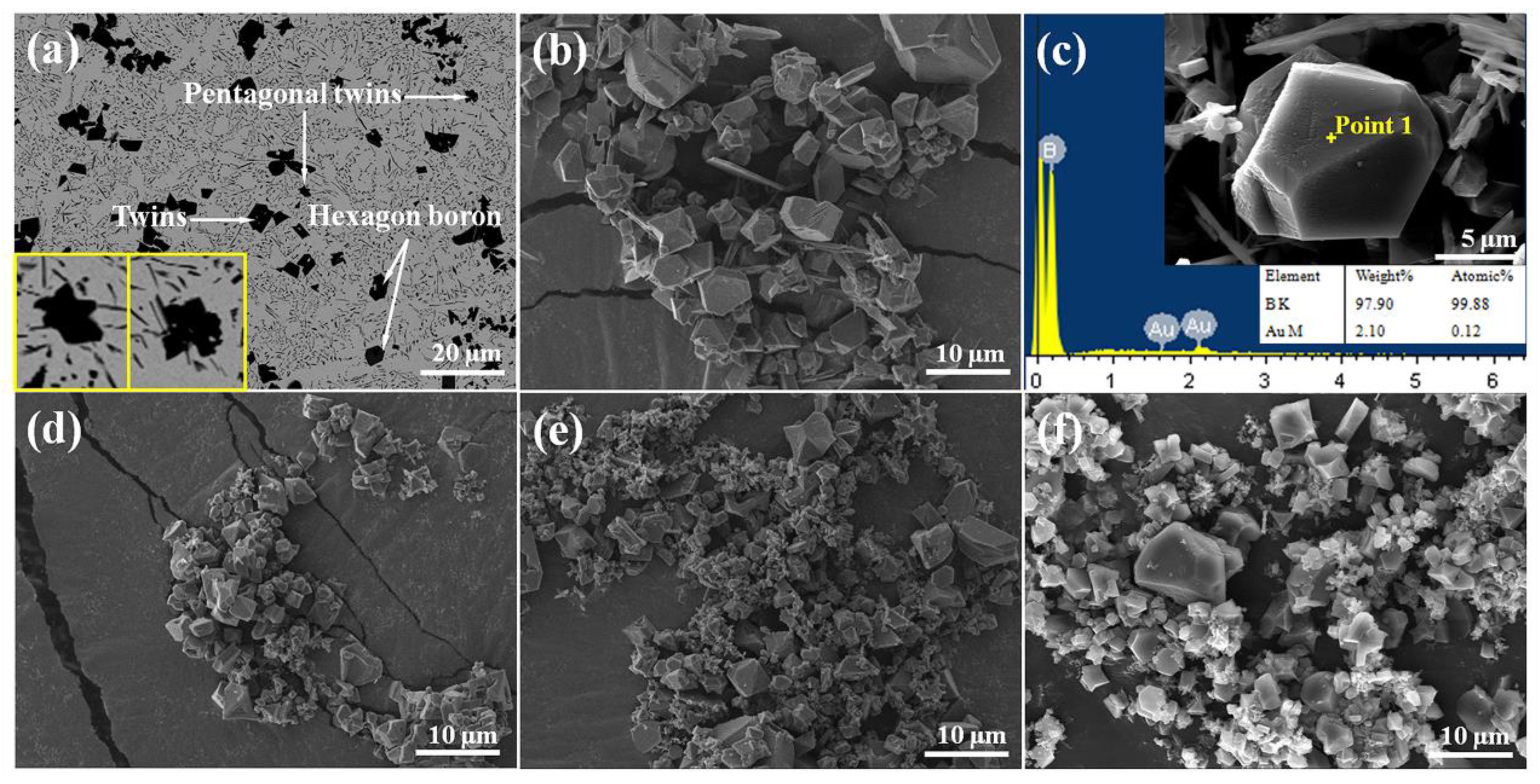
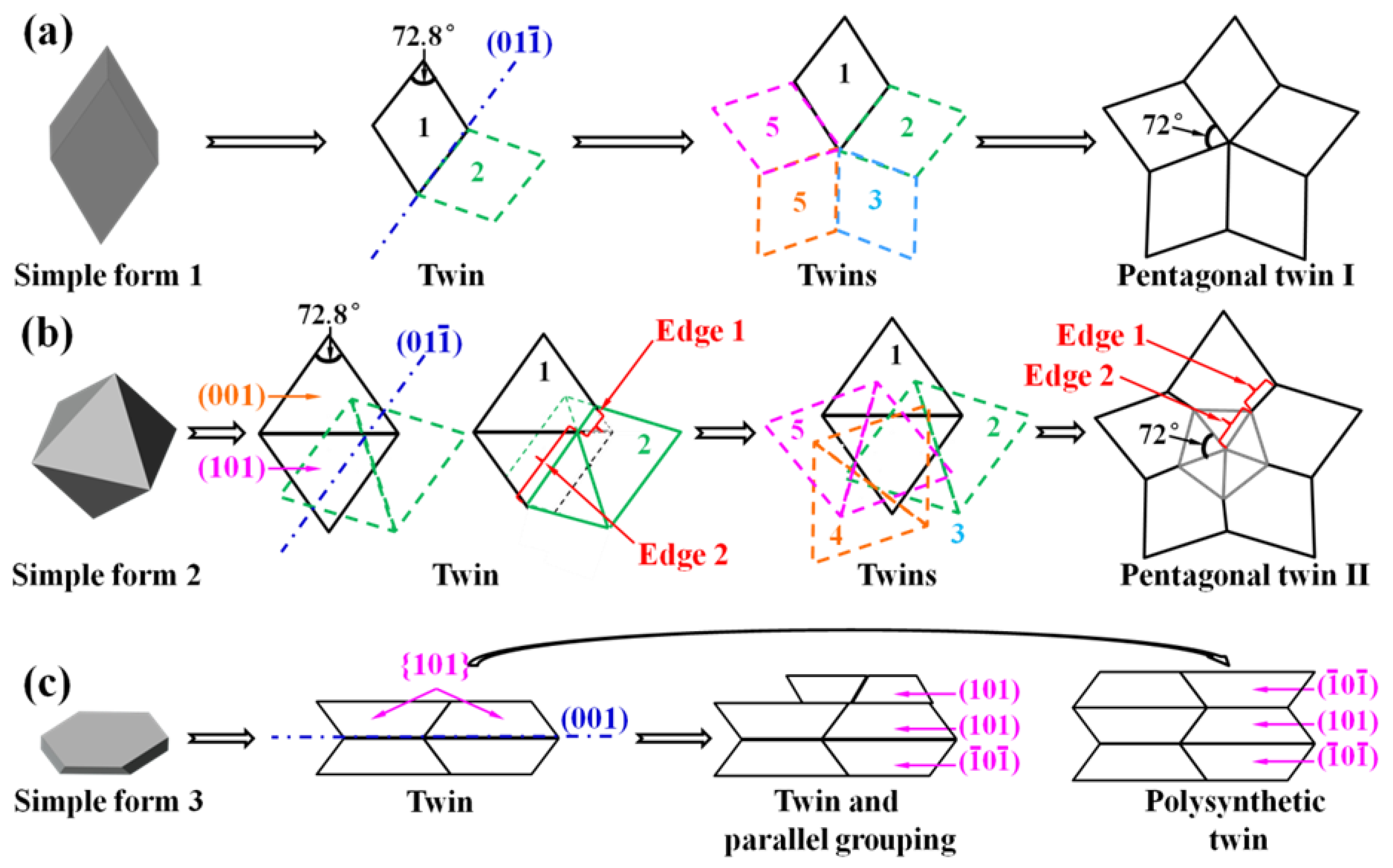
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojha, P.K.; Karmakar, S. Boron for liquid fuel Engines-A review on synthesis, dispersion stability in liquid fuel, and combustion aspects. Prog. Aerosp. Sci. 2018, 100, 18–45. [Google Scholar] [CrossRef]
- Rolf, F.B.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar]
- Kalot, G.; Godard, A.; Busser, B.; Pliquett, J.; Sancey, L. Aza-BODIPY: A new vector for enhanced theranostic boron neutron capture therapy applications. Cells 2020, 9, 1953. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, R.J.; Conway, A.M.; Reinhardt, C.E.; Graff, R.T.; Wang, T.F.; Deo, N.; Cheung, C.L. 6:1 aspect ratio silicon pillar based thermal neutron detector filled with B10. Appl. Phys. Lett. 2008, 93, 133502. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Li, Y.F.; Chen, H.W.; Sun, Z.X.; Wang, N.; Qin, J.Y.; Li, H.; Bian, X.F.; Liu, X.F. Growth of single crystalline boron nanotubes in a Cu alloy. CrystEngComm 2017, 19, 4510–4518. [Google Scholar] [CrossRef]
- Patel, R.; Chou, T.; Iqbal, Z. Synthesis of Boron Nanowires, Nanotubes, and Nanosheets. J. Nanomater. 2015, 2015, 243925. [Google Scholar] [CrossRef]
- Bai, H.; Zou, H.H.; Chen, G.X.; Yu, J.H.; Nishimura, K.; Dai, W.; Jiang, N. Nucleation and growth of boron nanowires on diamond particles. Appl. Surf. Sci. 2014, 313, 132–137. [Google Scholar] [CrossRef]
- Yang, Q.; Sha, J.; Wang, L.; Su, Z.; Ma, X.; Wang, J.; Yang, D. Morphology and diameter controllable synthesis of boron nanowires. J. Mater. Sci. 2006, 41, 3547–3552. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Hui, C.; Tian, J.; Bao, L.; Shen, C.; Gao, H.J. Field emission properties of patterned boron nanocones. Nanotechnology 2010, 21, 325705. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xie, Y.; Peng, Q.; Chen, Y.P. Phonon transport in single-layer boron nanoribbons. Nanotechnology 2016, 27, 445703. [Google Scholar] [CrossRef]
- Xu, T.T.; Zheng, J.; Wu, N.; Nicholls, A.W.; Roth, J.; Dikin, D.A.; Ruoff, R.S. Crystalline Boron Nanoribbons: Synthesis and Characterization. Nano Lett. 2004, 4, 963–968. [Google Scholar] [CrossRef]
- Yuan, W.T.; Wu, Y.Y.; Zhang, G.D.; Wu, C.C.; Zhao, S.; Liu, X.F. Study on spheroidization and the growth mechanism of eutectic boron in Cu-B alloys. CrystEngComm 2020, 22, 6993–7001. [Google Scholar] [CrossRef]
- Penev, E.S.; Kutana, A.; Yakobson, B.I. Can two-dimensional boron superconduct? Nano Lett. 2016, 16, 2522–2526. [Google Scholar] [CrossRef]
- Oganov, A.R.; Chen, J.; Gatti, C.; Ma, Y.; Ma, Y.; Glass, C.W.; Liu, Z.; Yu, T.; Kurakevych, O.O.; Solozhenko, V.L. Lonic high-pressure form of elemental boron. Nature 2009, 457, 863–867. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Reddy, K.M.; Xie, K.Y.; Hemker, K.J.; Goddard, W.A. New ground-state crystal structure of elemental boron. Phys. Rev. Lett. 2016, 117, 085501. [Google Scholar] [CrossRef] [PubMed]
- Hayami, W.; Otani, S. The role of surface energy in the growth of boron crystals. J. Phys. Chem. C 2007, 111, 688–692. [Google Scholar] [CrossRef]
- Hayami, W.; Otani, S. Surface energy and growth mechanism of β-tetragonal boron crystal. J. Phys. Chem. C 2007, 111, 10394–10397. [Google Scholar] [CrossRef]
- Hughes, R.E.; Kennard, C.; Sullenger, D.B.; Weakliem, H.A.; Hoard, J.L. The structure of β-rhombohedral boron. J. Am. Chem. Soc. 1963, 85, 361–362. [Google Scholar] [CrossRef]
- Fujimori, M.; Nakata, T.; Nakayama, T.; Nishibori, E.; Kimura, K.; Takata, M.; Sakata, M. Peculiar covalent bonds in α-rhombohedral boron. Phys. Rev. Lett. 1999, 82, 4452–4455. [Google Scholar] [CrossRef]
- Shirai, K. Phase diagram of boron crystals. Jpn. J. Appl. Phys. 2017, 56, 05FA06. [Google Scholar] [CrossRef]
- Widom, M.; Mihalkovic, M. Symmetry-broken crystal structure of elemental boron at low temperature. Phys. Rev. B 2008, 77, 064113. [Google Scholar] [CrossRef]
- Bykova, E.; Parakhonskiy, G.; Dubrovinskaia, N.; Chernyshov, D.; Dubrovinsky, L. The crystal structure of aluminum doped β-rhombohedral boron. J. Solid State Chem. 2012, 194, 188–193. [Google Scholar] [CrossRef]
- Sun, Z.X.; Wu, Y.Y.; Han, X.X.; Zhang, G.J.; Liu., X.F. Growth mechanisms of alpha-boron and beta-boron in a copper melt at ambient pressure and its stabilities. CrystEngComm 2017, 19, 3947–3954. [Google Scholar] [CrossRef]
- Frenking, G.; Holzmann, N. Chemistry: A boron-boron triple bond. Science 2012, 336, 1394–1395. [Google Scholar] [CrossRef]
- He, J.L.; Wu, E.D.; Wang, H.T.; Liu, R.P.; Tian, Y.J. Lonicities of boron-boron bonds in B12 icosahedra. Phys. Rev. Lett. 2005, 94, 015504. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Y.Y.; Sun, Z.X.; Zhou, B.; Liu, X.F. Superhard copper matrix composite reinforced by ultrafine boron for wear-resistant bearings. ACS Appl. Nano Mater. 2018, 1, 5382–5388. [Google Scholar] [CrossRef]
- Fu, X. Uncovering the internal structure of five-fold twinned nanowires through 3D electron diffraction mapping. Chin. Phys. B 2020, 29, 068101. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, P.; You, Q.; Ye, S. Five-fold twinned pentagonal gold nanocrystal structure exclusively bounded by {110} facets. CrystEngComm 2013, 15, 2350–2353. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. The B-Cu (Boron-Copper) system. Bull. Alloy Phase Diagr. 1982, 3, 45–48. [Google Scholar] [CrossRef]
- Nie, J.F.; Wu, Y.Y.; Li, P.T.; Li, H.; Liu, X.F. Morphological evolution of TiC from octahedron to cube induced by elemental nickel. CrystEngComm 2012, 14, 2213–2221. [Google Scholar] [CrossRef]
- Werheit, H. Comment on “New ground-state crystal structure of elemental boron”. Phys. Rev. Lett. 2017, 118, 089601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, X.J.; Sun, Y.Q.; Liu, D.L.; Li, C.C.; Cai, W.P.; Li, Y. A universal route with fine kinetic control to a family of penta-twinned gold nanocrystals. Chem. Sci. 2021, 12, 12631–12639. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Yuan, W.; Wen, Y.; Wei, Z.; Gao, T.; Wu, Y.; Liu, X. Morphology and Growth Mechanism of β-Rhombohedral Boron and Pentagonal Twins in Cu Alloy. Crystals 2022, 12, 1516. https://doi.org/10.3390/cryst12111516
Han J, Yuan W, Wen Y, Wei Z, Gao T, Wu Y, Liu X. Morphology and Growth Mechanism of β-Rhombohedral Boron and Pentagonal Twins in Cu Alloy. Crystals. 2022; 12(11):1516. https://doi.org/10.3390/cryst12111516
Chicago/Turabian StyleHan, Junqing, Wentao Yuan, Yihan Wen, Zuoshan Wei, Tong Gao, Yuying Wu, and Xiangfa Liu. 2022. "Morphology and Growth Mechanism of β-Rhombohedral Boron and Pentagonal Twins in Cu Alloy" Crystals 12, no. 11: 1516. https://doi.org/10.3390/cryst12111516
APA StyleHan, J., Yuan, W., Wen, Y., Wei, Z., Gao, T., Wu, Y., & Liu, X. (2022). Morphology and Growth Mechanism of β-Rhombohedral Boron and Pentagonal Twins in Cu Alloy. Crystals, 12(11), 1516. https://doi.org/10.3390/cryst12111516







