Phase Sequence, Kinetics of Crystallization and Molecular Dynamics of the Chiral Liquid Crystalline Compound Forming a Hexatic Smectic Glass
Abstract
1. Introduction
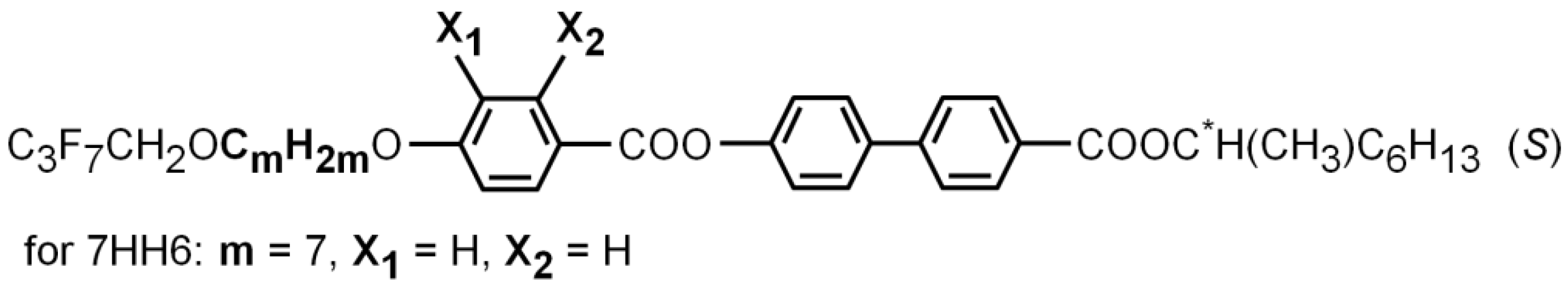
2. Materials and Methods
- (1)
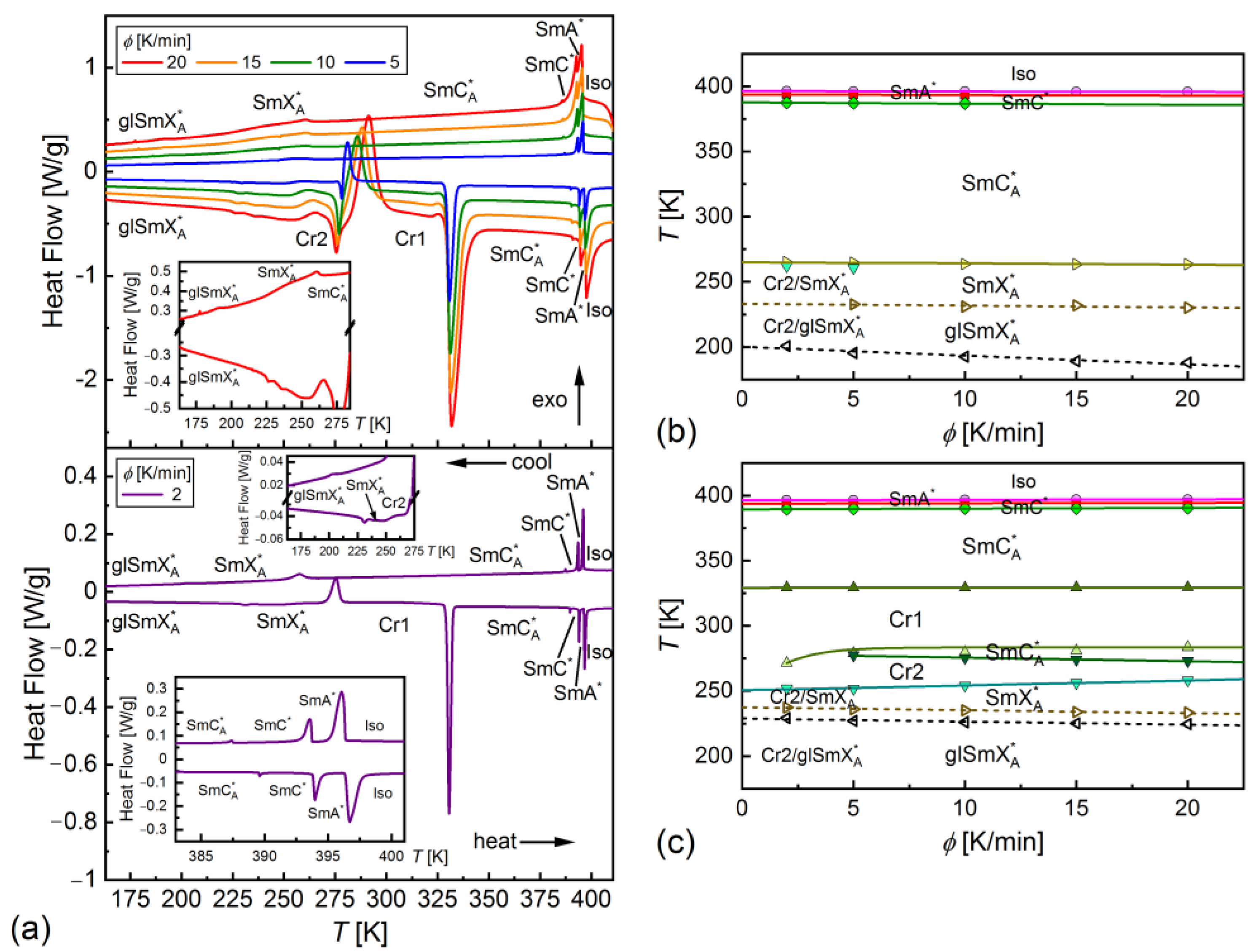
- Determination of the phase sequence—the sample was heated to the isotropic liquid phase, then cooled and subsequently heated at the temperature change rate 2, 5, 10, 15, 20 K/min in the 153–413 K temperature range;
- (2)
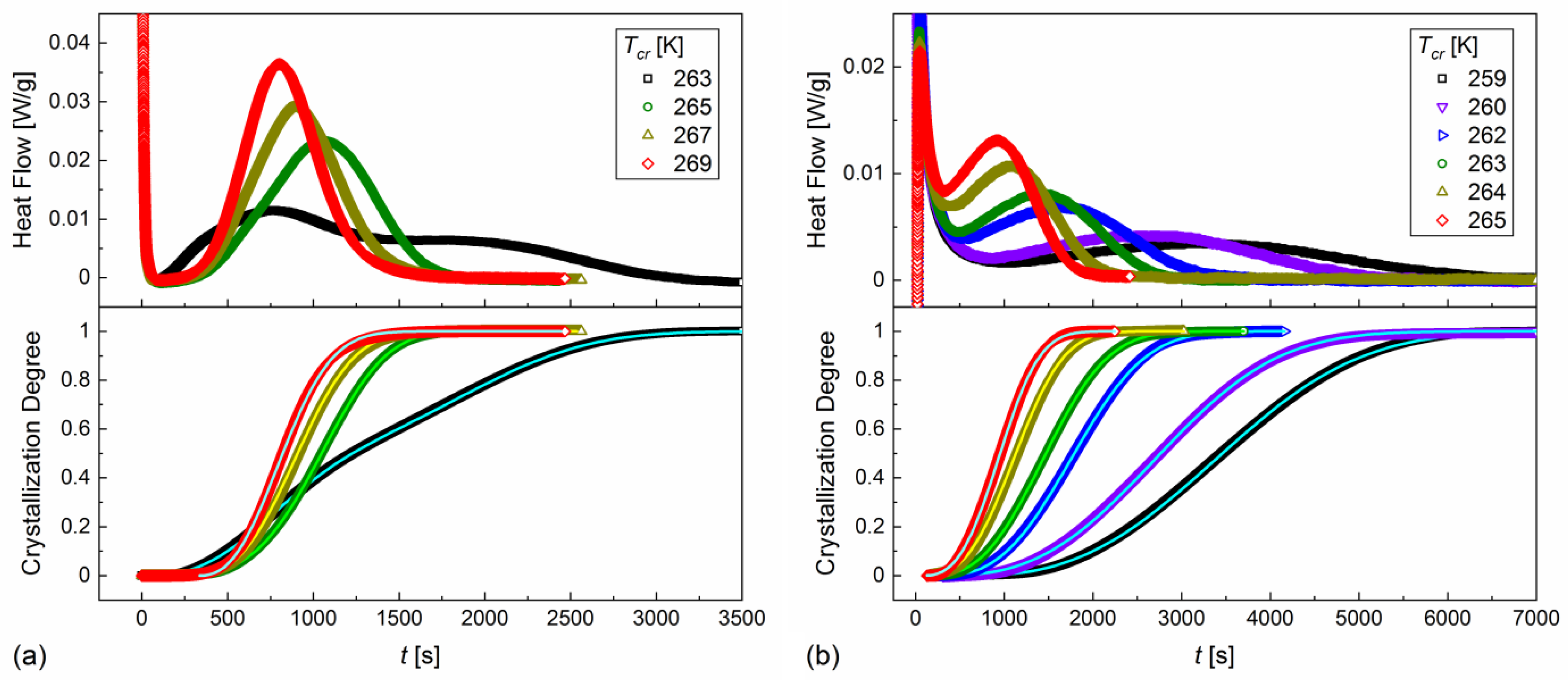
- Investigation of kinetics of melt crystallization—the sample was heated above the melting temperature and cooled at 2 K/min to the crystallization temperature from the 263–269 K range;
- (3)
- Investigation of kinetics of cold crystallization—the sample was heated above the melting temperature, cooled down at 10 K/min below the glass transition temperature and heated at 10 K/min to the crystallization temperature from the 259–265 K range.
3. Results and Discussion
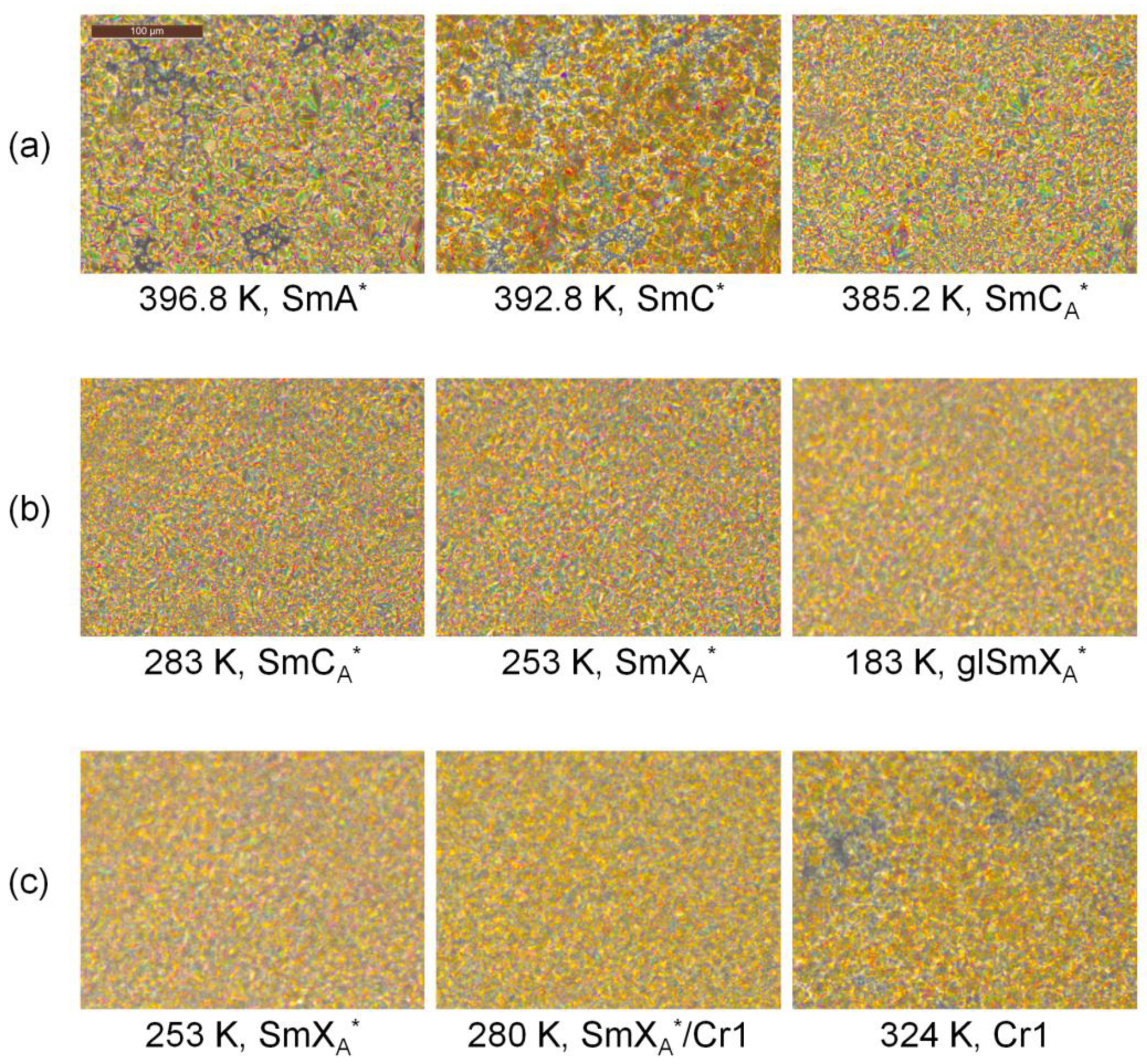
3.1. Phase Sequence
3.2. Molecular Dynamics
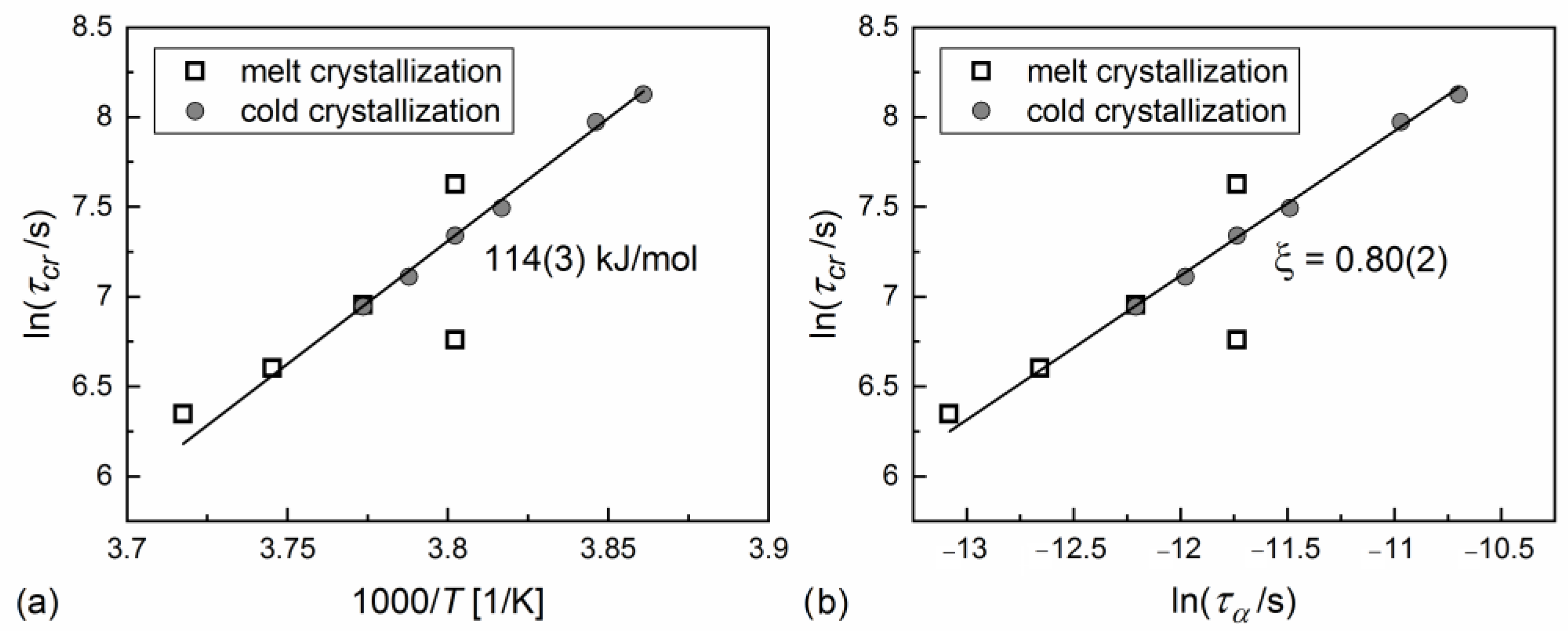
3.3. Kinetics of Crystallization
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rudquist, P. Smectic LCD Modes. In Handbook of Visual Display Technology; Chen, J., Cranton, W., Fihn, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Clark, N.A.; Lagerwall, S.T. Submicrosecond bistable electro-optic switching in liquid crystals. Appl. Phys. Lett. 1980, 36, 899–901. [Google Scholar] [CrossRef]
- Chandani, A.D.L.; Ouchi, Y.; Takezoe, H.; Fukuda, A.; Terashima, K.; Furukawa, K.; Kishi, A. Novel Phases Exhibiting Tristable Switching. Jpn. J. Appl. Phys. 1989, 28, L1261–L1264. [Google Scholar] [CrossRef]
- D’havé, K.; Rudquist, P.; Lagerwall, S.T.; Pauwels, H.; Drzewiński, W.; Dąbrowski, R. Solution of the dark state problem in antiferroelectric liquid crystal displays. Appl. Phys. Lett. 2000, 76, 3528–3530. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Gąsowska, J.; Otón, J.; Piecek, W.; Przedmojski, J.; Tykarska, M. High tilted antiferroelectric liquid crystalline materials. Displays 2004, 25, 9–19. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Drzewiński, W.; Dziaduszek, J.; Gąsowska, J.; Henderson, P.; Kula, P.; Otón, J.; Bennis, N. Orthoconic anti-ferroelectric liquid crystals containing biphenyl, terphenyl, or naphthyl mesogenic unit. Opto-Electron. Rev. 2007, 15, 32–36. [Google Scholar] [CrossRef]
- Czerwiec, J.M.; Ossowska-Chruściel, M.; Chruściel, J.; Wróbel, S. Room Temperature Ferroelectric Mixture of Chiral and Achiral Thioesters. Acta Phys. Pol. A 2010, 117, 549–552. [Google Scholar] [CrossRef]
- Fitas, J.; Marzec, M.; Tykarska, M.; Wróbel, S.; Dąbrowski, R. Ferroelectric Liquid Crystal for Use in a New Generation of LCDs. Acta Phys. Pol. A 2013, 124, 954–958. [Google Scholar] [CrossRef]
- Lalik, S.; Stefańczyk, O.; Dardas, D.; Górska, N.; Ohkoshi, S.-i.; Marzec, M. Modifications of FLC Physical Properties through Doping with Fe2O3 Nanoparticles (Part I). Materials 2021, 14, 4722. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, J.; Czupryński, K.; Skrzypek, K.; Filipowicz, M. Synthesis and Mesomorphic Properties of Chiral Esters Comprising Partially Fluorinated Alkoxyalkoxy Terminal Chains and a 1-methylheptyl Chiral Moiety. Mol. Cryst. Liq. Cryst. 2008, 495, 145[497]–157[509]. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, J.; Garbat, K.; Filipowicz, M.; Tykarska, M.; Rejmer, W.; Czupryński, K.; Spadło, A.; Bennis, N.; et al. Influence of alkoxy chain length and fluorosubstitution on mesogenic and spectral properties of high tilted antiferroelectric esters. J. Mater. Chem. 2011, 21, 2144–2153. [Google Scholar] [CrossRef]
- Vertogen, G.; de Jeu, W.H. Thermotropic Liquid Crystals: Fundamentals; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Kolek, Ł.; Massalska-Arodź, M.; Majda, D.; Wantusiak, B.; Zalewski, S.; Kula, P. Studies of Phase Diagram of a Liquid Crystal with 4-[2-(3-Fluorophenyl)ethyl]biphenyl Core of Molecules. Acta Phys. Pol. A 2012, 122, 370–374. [Google Scholar] [CrossRef]
- Kolek, Ł.; Massalska-Arodź, M.; Paluch, M.; Adrjanowicz, K.; Rozwadowski, T.; Majda, D. Dynamics in ferro- and antiferroelectric phases of a liquid crystal with fluorinated molecules as studied by dielectric spectroscopy. Liq. Cryst. 2013, 40, 1082–1088. [Google Scholar] [CrossRef]
- Kolek, Ł.; Massalska-Arodź, M.; Adrjanowicz, K.; Rozwadowski, T.; Dychtoń, K.; Drajewicz, M.; Kula, P. Molecular dynamics and cold crystallization process in a liquid-crystalline substance with para-, ferro- and antiferro-electric phases as studied by dielectric spectroscopy and scanning calorimetry. J. Mol. Liq. 2020, 297, 111913. [Google Scholar] [CrossRef]
- Kolek, Ł.; Massalska-Arodź, M.; Majda, D.; Suchodolska, B.; Zalewski, S. Studies of Phase Diagram and Glass Transitions of a Liquid Crystal with Ferro- and Antiferroelectric Phases. Acta Phys. Pol. A 2013, 124, 909–912. [Google Scholar] [CrossRef]
- Deptuch, A.; Piwowarczyk, M.; Jasiurkowska-Delaporte, M.; Kim, J.; Urbańska, M.; Skolarczyk, M.; Jaworska-Gołąb, T.; Marzec, M. Fluorosubstitution of the Molecular Core in Chiral Esters with Short Terminal Carbon Chains: Influence on Physical Properties. Crystals 2022, 12, 1028. [Google Scholar] [CrossRef]
- Deptuch, A.; Jasiurkowska-Delaporte, M.; Zając, W.; Juszyńska-Gałązka, E.; Drzewicz, A.; Urbańska, M. Investigation of crystallization kinetics and its relationship with molecular dynamics for chiral fluorinated glassforming smectogen 3F5HPhH6. Phys. Chem. Chem. Phys. 2021, 23, 19795–19810. [Google Scholar] [CrossRef]
- Drzewicz, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Deptuch, A.; Gałązka, M.; Zając, W.; Drzewiński, W. On relaxation and vibrational dynamics in the thermodynamic states of a chiral smectogenic glass-former. Phys. Chem. Chem. Phys. 2022, 24, 4595–4612. [Google Scholar] [CrossRef]
- Dierking, I. Experimental investigations of a chiral smectic glass-forming liquid crystal. Liq. Cryst. 2008, 35, 1015–1022. [Google Scholar] [CrossRef]
- Gujral, A.; Yu, L.; Ediger, M.D. Anisotropic organic glasses. Curr. Opin. Solid State Mater. Sci. 2018, 22, 49–57. [Google Scholar] [CrossRef]
- Teerakapibal, R.; Huang, C.; Gujral, A.; Ediger, M.D.; Yu, L. Organic Glasses with Tunable Liquid-Crystalline Order. Phys. Rev. Lett. 2018, 120, 055502. [Google Scholar] [CrossRef]
- Maltisovs, M.; Pikulins, D.; Krumins, K.; Ozols, A. Identifying defects in bistable smectic-A liquid crystal displays after extended period of functional testing. In Proceedings of the 2020 IEEE 61th International Scientific Conference on Power and Electrical Engineering of Riga Technical University (RTUCON), Riga, Latvia, 5–7 November 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Wu, J.; Usui, T.; Hanna, J.-i. Synthesis of a novel smectic liquid crystalline glass and characterization of its charge carrier transport properties. J. Mater. Chem. 2011, 21, 8045–8051. [Google Scholar] [CrossRef]
- Deptuch, A.; Jaworska-Gołąb, T.; Marzec, M.; Pociecha, D.; Fitas, J.; Żurowska, M.; Tykarska, M.; Hooper, J. Mesomorphic phase transitions of 3F7HPhF studied by complementary methods. Phase. Trans. 2018, 91, 186–198. [Google Scholar] [CrossRef]
- Deptuch, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Zając, W.; Urbańska, M. Molecular Dynamics and Kinetics of Isothermal Cold Crystallization in the Chiral Smectogenic 3F7HPhH6 Glassformer. Crystals 2021, 11, 1487. [Google Scholar] [CrossRef]
- Deptuch, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Zając, W.; Urbańska, M. Correction: Molecular Dynamics and Kinetics of Isothermal Cold Crystallization in the Chiral Smectogenic 3F7HPhH6 Glassformer. Crystals, 2022; accepted. [Google Scholar]
- Deptuch, A.; Drzewicz, A.; Dziurka, M.; Górska, N.; Hooper, J.; Jaworska-Gołąb, T.; Juszyńska-Gałązka, E.; Marzec, M.; Piwowarczyk, M.; Srebro-Hooper, M.; et al. Influence of fluorosubstitution on physical properties of the smectogenic chiral ester. Mater. Res. Bull. 2022, 150, 111756. [Google Scholar] [CrossRef]
- Deptuch, A.; Jasiurkowska-Delaporte, M.; Urbańska, M.; Baran, S. Kinetics of cold crystallization in two liquid crystalline fluorinated homologues exhibiting the vitrified smectic CA* phase. J. Mol. Liq. 2022, 368, 120612. [Google Scholar] [CrossRef]
- Deptuch, A.; Juszyńska-Gałązka, E.; Drzewicz, A.; Urbańska, M. Comparative study of crystallization of two fluorinated chiral liquid crystalline compounds differing by fluorosubstitution of the molecular core. J. Cryst. Growth 2022, 593, 126771. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Levstik, A.; Carlsson, T.; Filipic, C.; Zeks, B. Goldstone mode and soft mode at the smectic-A–smectic-C/emph>phase transition studied by dielectric relaxation. Phys. Rev. A 1987, 35, 3527–3534. [Google Scholar] [CrossRef]
- Filipič, C.; Carlsson, T.; Levstik, A.; Žekš, B.; Blinc, R. Dielectric properties near the smecticC*—Smectic-A phase transition of some ferroelectric liquid-crystalline systems with a very large spontaneous polarization. Phys. Rev. A 1988, 38, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Buivydas, M.; Gouda, F.; Andersson, G.; Lagerwall, S.T.; Stebler, B.; Bomelburg, J.; Heppke, G.; Gestblom, B. Collective and non-collective excitations in antiferroelectric and ferrielectric liquid crystals studied by dielectric relaxation spectroscopy and electro-optic measurements. Liq. Cryst. 1997, 23, 723–739. [Google Scholar] [CrossRef]
- Deptuch, A.; Lalik, S.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Urbańska, M.; Marzec, M. Comparative study of electrooptic, dielectric, and structural properties of two glassforming antiferroelectric mixtures with a high tilt angle. Phys. Rev. E 2022, 105, 024705. [Google Scholar] [CrossRef] [PubMed]
- Johari, G.P.; Goodby, J.W. Dielectric relaxations in a supercooled liquid and glassy smectic phase. J. Chem. Phys. 1982, 77, 5165–5172. [Google Scholar] [CrossRef]
- Böhmer, R.; Ngai, C.; Angell, C.A.; Plazek, D.J. Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 1993, 99, 4201–4209. [Google Scholar] [CrossRef]
- Tanaka, H. Relationship among glass-forming ability, fragility, and short-range bond ordering of liquids. J. Non-Cryst. Solids 2005, 351, 678–690. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avramov, I.; Avramova, K.; Rüssel, C. New method to analyze data on overall crystallization kinetics. J. Cryst. Growth. 2005, 285, 394–399. [Google Scholar] [CrossRef]
- Velisaris, C.N.; Seferis, J.C. Crystallization kinetics of polyetheretherketone (peek) matrices. Polym. Eng. Sci. 1986, 26, 1574–1581. [Google Scholar] [CrossRef]
- Ediger, M.D.; Harrowell, P.; Yu, L. Crystal growth kinetics exhibit a fragility-dependent decoupling from viscosity. J. Chem. Phys. 2008, 128, 034709. [Google Scholar] [CrossRef] [PubMed]
- Exner, G.; Pérez, E.; Krasteva, M. Structure and Phase Transitions of Polymer Liquid Crystals, Revealed by Means of Differential Scanning Calorimetry, Real-Time Synchrotron WAXD, MAXS and SAXS and Microscopy. In Liquid Crystalline Polymers; Thakur, V.K., Kessler, M.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Osiecka, N.; Massalska-Arodź, M.; Galewski, Z.; Chłędowska, K.; Wróbel, S.; Morito, T.; Yamamura, Y.; Saito, K. Dynamics and Phase Transitions of 4-Bromobenzylidene-4′-pentyloxyaniline and 4-Bromobenzylidene-4′-hexyloxyaniline as Studied by Dielectric Spectroscopy. Acta Phys. Pol. A 2013, 124, 913–916. [Google Scholar] [CrossRef]


| Transition | [K] | [K] | [kJ/mol] | [kJ/mol] |
|---|---|---|---|---|
| Iso/SmA* | 396.4 (396.4) | 396.7 (396.1) | 4.72 (4.68) | 7.05 (6.99) |
| SmA*/SmC* | 393.8 (393.7) | 394.0 (393.6) | 1.59 (1.68) | 2.39 (2.52) |
| SmC*/SmCA* | 389.5 (387.5) | 389.6 (387.4) | 0.21 (0.16) | 0.32 (0.23) |
| SmCA*/SmXA* | (263.5) | (260.0) | (0.34) | (0.65) |
| [K] | [s] | [s] | |
|---|---|---|---|
| melt crystallization | |||
| 263 | 0, 0 (fixed) | 2050(1), 863(1) | 3.55(1), 2.61(1) |
| 265 | 99(1) | 1049(1) | 3.61(1) |
| 267 | 259(2) | 738(2) | 2.86(2) |
| 269 | 328(2) | 571(2) | 2.51(1) |
| cold crystallization | |||
| 259 | 492(4) | 3384(4) | 2.78(1) |
| 260 | 219(2) | 2899(2) | 2.80(1) |
| 262 | 211(2) | 1794(2) | 2.86(1) |
| 263 | 118(2) | 1540(2) | 2.82(1) |
| 264 | 52(2) | 1224(2) | 2.90(1) |
| 265 | 36(2) | 1037(3) | 3.00(1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deptuch, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Urbańska, M. Phase Sequence, Kinetics of Crystallization and Molecular Dynamics of the Chiral Liquid Crystalline Compound Forming a Hexatic Smectic Glass. Crystals 2022, 12, 1583. https://doi.org/10.3390/cryst12111583
Deptuch A, Jasiurkowska-Delaporte M, Juszyńska-Gałązka E, Drzewicz A, Urbańska M. Phase Sequence, Kinetics of Crystallization and Molecular Dynamics of the Chiral Liquid Crystalline Compound Forming a Hexatic Smectic Glass. Crystals. 2022; 12(11):1583. https://doi.org/10.3390/cryst12111583
Chicago/Turabian StyleDeptuch, Aleksandra, Małgorzata Jasiurkowska-Delaporte, Ewa Juszyńska-Gałązka, Anna Drzewicz, and Magdalena Urbańska. 2022. "Phase Sequence, Kinetics of Crystallization and Molecular Dynamics of the Chiral Liquid Crystalline Compound Forming a Hexatic Smectic Glass" Crystals 12, no. 11: 1583. https://doi.org/10.3390/cryst12111583
APA StyleDeptuch, A., Jasiurkowska-Delaporte, M., Juszyńska-Gałązka, E., Drzewicz, A., & Urbańska, M. (2022). Phase Sequence, Kinetics of Crystallization and Molecular Dynamics of the Chiral Liquid Crystalline Compound Forming a Hexatic Smectic Glass. Crystals, 12(11), 1583. https://doi.org/10.3390/cryst12111583








