The Optimization of a Carbon Paper/MnO2 Composite Current Collector for Manufacturing a High-Performance Li–S Battery Cathode
Abstract
:1. Introduction
2. Materials and Methods
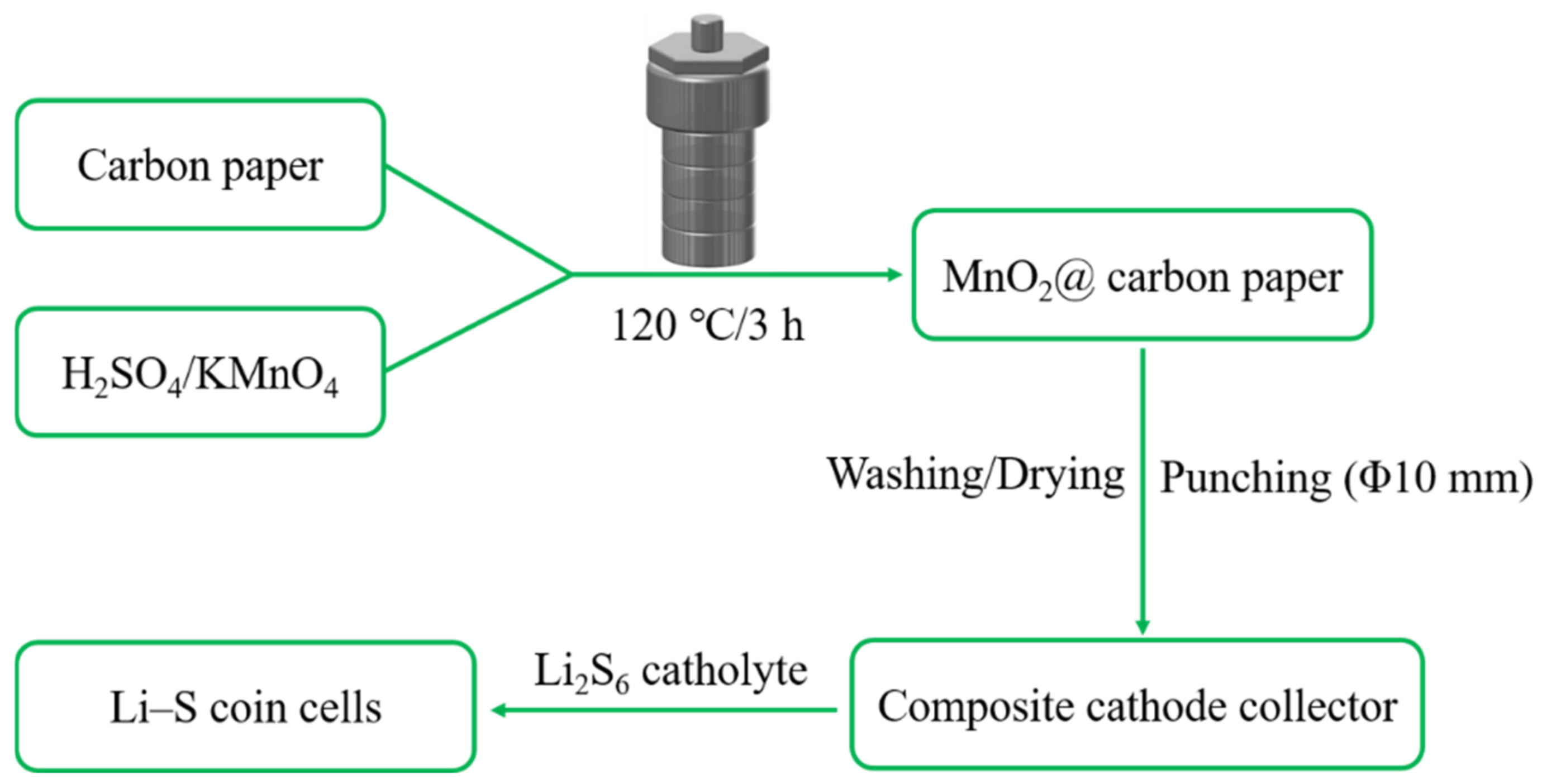
2.1. Synthesis of Carbon Paper/MnO2
2.2. Synthesis of Li2S6 Catholyte
2.3. Assembly of Lithium–Sulfur Battery
2.4. Structural Characterization
2.5. Electrochemical Characterization
3. Results and Discussion
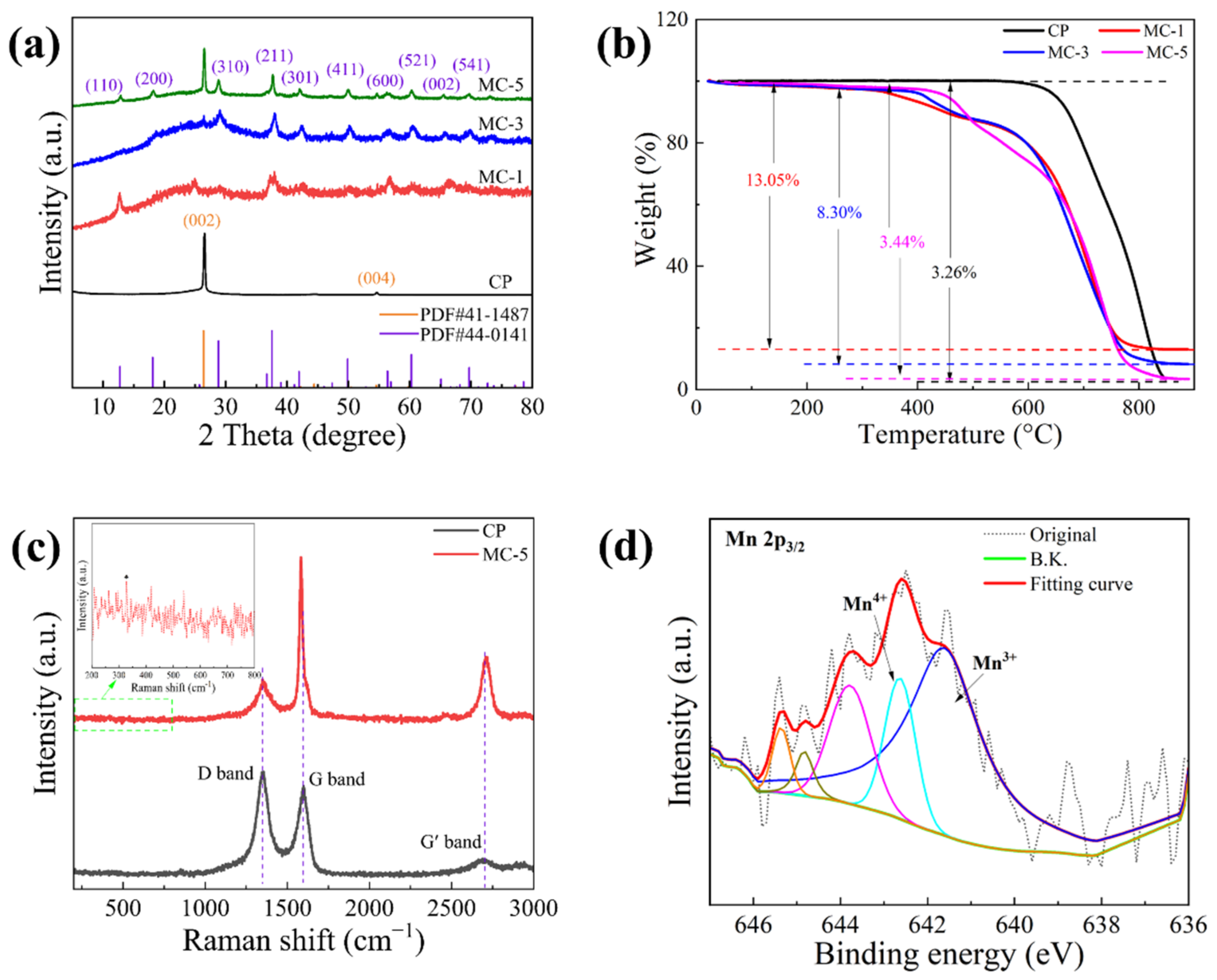
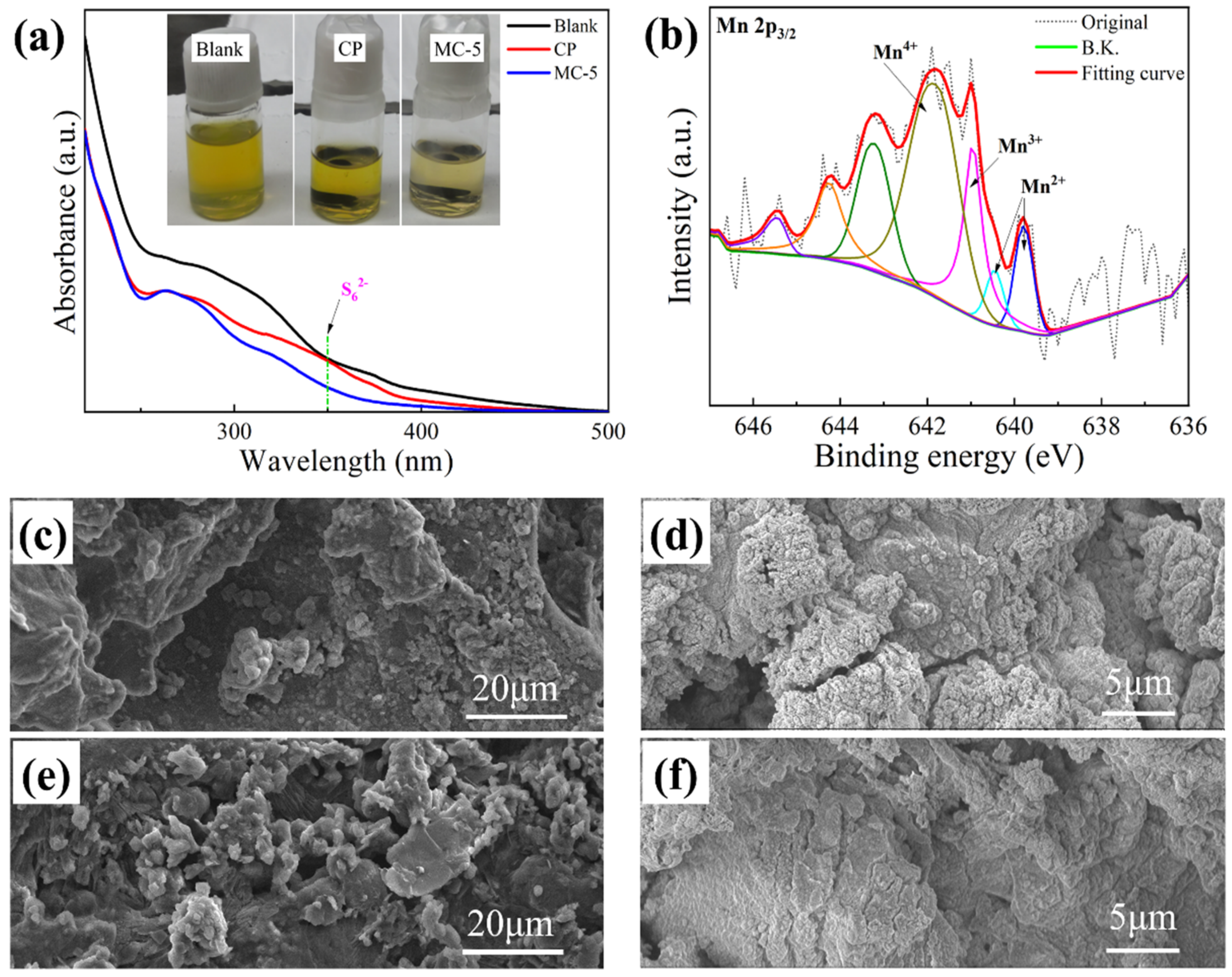
3.1. Structure and Morphology
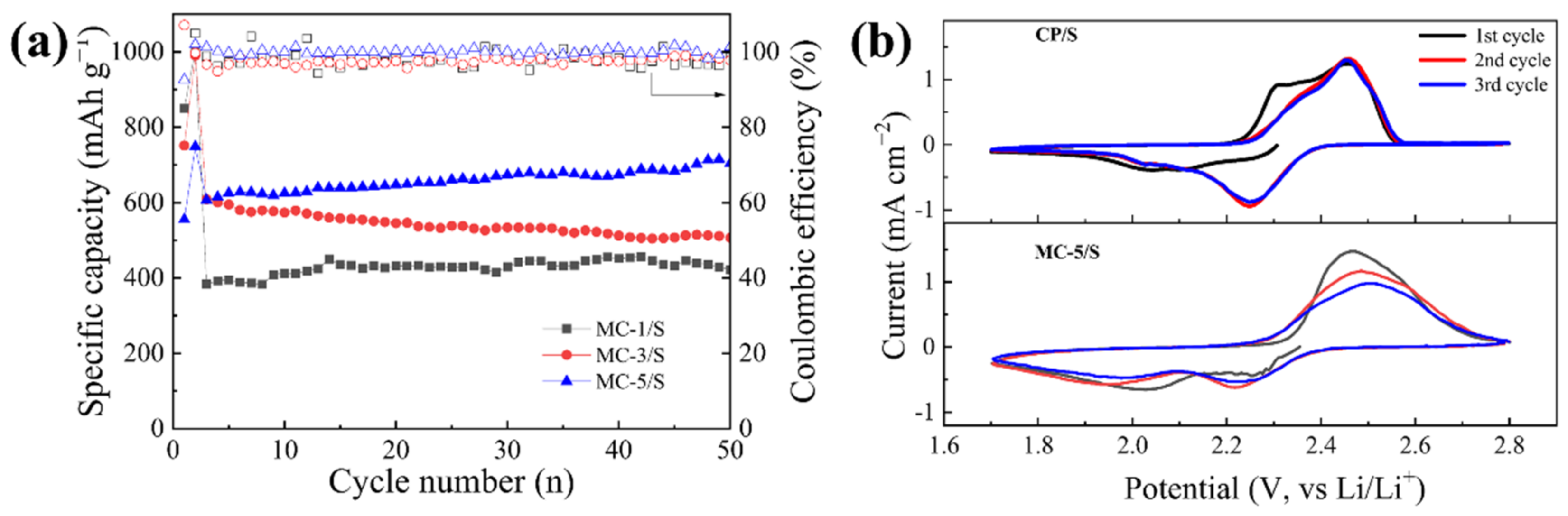
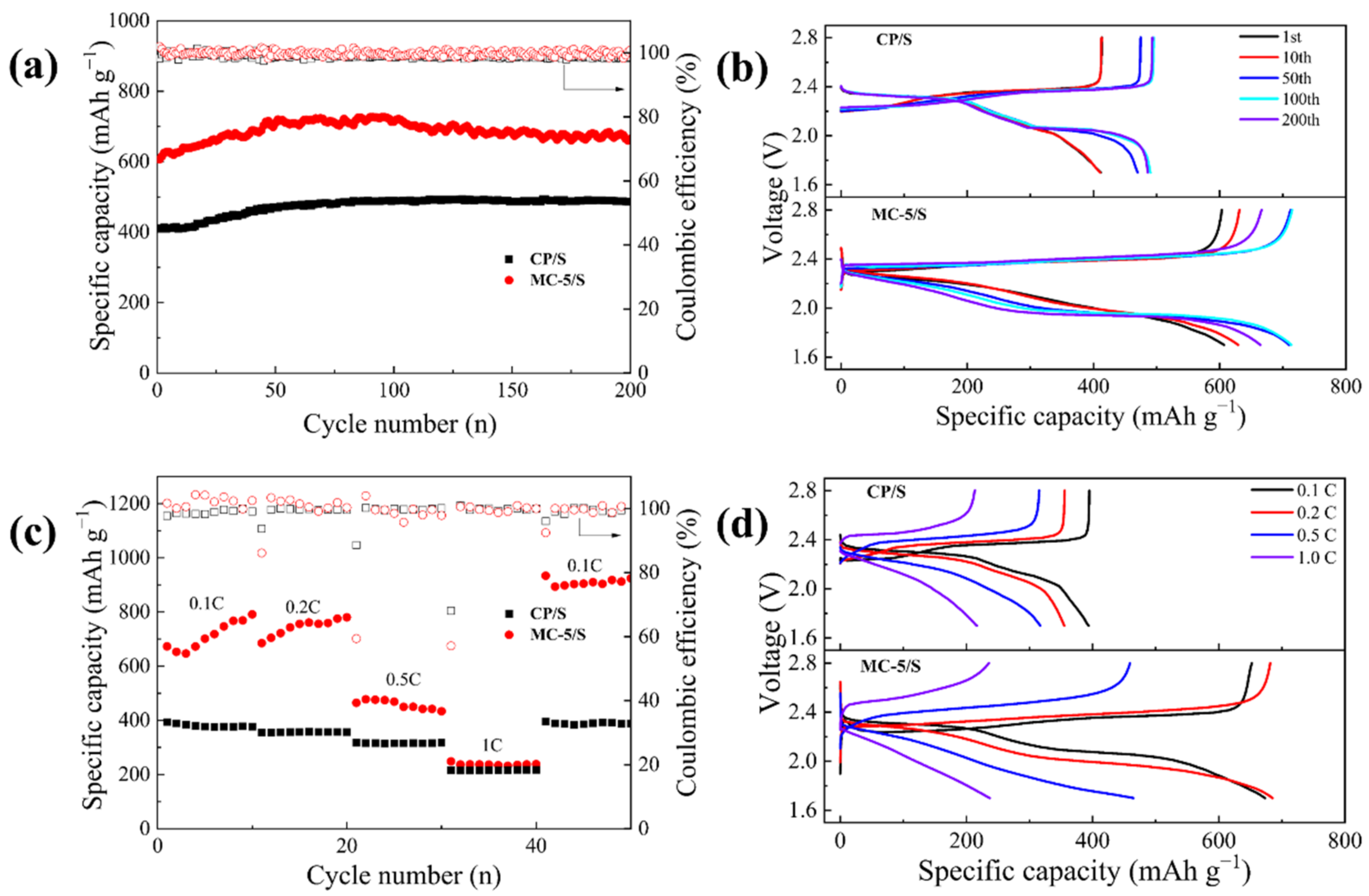
3.2. Electrochemical Performance
3.3. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nature 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Yang, H.X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.H.; Zu, C.X. Lithium–sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.Y.; Wu, J.F.; Li, G.R.; Liu, S.; Gao, X.P. To effectively drive the conversion of sulfur with electroactive niobium tungsten oxide microspheres for lithium–sulfur battery. Nano Energy 2020, 77, 105173. [Google Scholar] [CrossRef]
- McNulty, D.; Landgraf, V.; Trabesomger, S. The importance of sulfur host structural preservation for lithium–sulfur battery performance. J. Mater. Chem. A 2020, 8, 26085–26097. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.Z.; Chung, S.H.; Zu, C.X.; Su, Y.S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- She, Z.W.; Sun, Y.M.; Zhang, Q.F.; Cui, Y. Designing high-energy lithium–sulfur battery. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar]
- Ci, H.N.; Wang, M.L.; Sun, Z.T.; Wei, C.H.; Cai, J.S.; Lu, C.; Cui, G.; Liu, Z.F.; Sun, J.Y. Direct insight into sulfiphilicity-lithiophilicity design of bifunctional heteroatom-doped graphene mediator toward durable Li–S batteries. J. Energy Chem. 2022, 66, 474–482. [Google Scholar] [CrossRef]
- Laverde, J.; Rosero-Navarro, N.C.; Miura, A.; Buitrago-Sierra, R.; Tadanaga, K.; Lópes, D. Impact of sulfur infiltration time and its content in an N-doped mesoporous carbon for application in Li–S batteries. Batteries 2022, 8, 58. [Google Scholar] [CrossRef]
- Liang, X.; Kwok, C.Y.; Lodi-Marzano, F.; Pang, Q.; Cuisinier, M.; Huang, H.; Hart, C.J.; Houtarde, D.; Kaup, K.; Sommer, H.; et al. Tuning transition metal oxide-sulfur interactions for long life lithium sulfur batteries: The “goldilocks” principle. Adv. Energy Mater. 2016, 6, 501636. [Google Scholar] [CrossRef]
- Tao, X.Y.; Wang, J.G.; Liu, C.; Wang, H.T.; Yao, H.B.; Zheng, G.Y.; She, Z.W.; Cai, Q.X.; Li, W.Y.; Zhou, G.M.; et al. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium–sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef] [Green Version]
- Deng, D.R.; Xue, F.; Jia, Y.J.; Ye, J.C.; Bai, C.D.; Zheng, M.S.; Dong, Q.F. Co4N nanosheet assembled mesoporous sphere as a matrix for ultrahigh sulfur content lithium–sulfur batteries. ACS Nano 2017, 11, 6031–6039. [Google Scholar] [CrossRef]
- Ma, L.L.; Yu, L.J.; Liu, J.C.; Su, Y.Q.; Li, S.; Zang, X.H.; Meng, T.; Zhang, S.H.; Song, J.J.; Wang, J.Y.; et al. Construction of Ti4O7/TiN/carbon microdisk sulfur host with strong polar N-Ti-O bond for ultralong life lithium–sulfur battery. Energy Storage Mater. 2022, 44, 180–188. [Google Scholar] [CrossRef]
- He, J.R.; Hartmann, G.; Lee, M.; Hwang, G.S.; Chen, Y.; Manthiram, A. Freestanding 1T MoS2/Graphene heterostructure as a highly efficient electrocatalyst for lithium polysulfides in Li–S batteries. Energy Environ. Sci. 2019, 12, 344–350. [Google Scholar] [CrossRef]
- Zhang, C.F.; Ma, Y.L.; Zhang, X.T.; Abdolhosseinzadeh, S.; Sheng, H.W.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-dimensional transition metal carbides and nitrides (MXenes): Synthesis, properties, and electrochemical energy storage applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Ng, S.F.; Lau, M.Y.L.; Ong, W.J. Lithium–sulfur battery cathode design: Tailoring metal-based nanostructures for robust polysulfide adsorption and catalytic conversion. Adv. Mater. 2021, 33, 2008654. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.H.; Kang, Q.; Wang, D.S.; Li, Y.D. Single-atom catalysis enables long-life, high-energy lithium–sulfur batteries. Nano Res. 2020, 13, 1856–1866. [Google Scholar] [CrossRef]
- Zhang, H.B.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W. Surface modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2018, 28, 1807086. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Huang, Y.; Luo, L.; Fan, D.L.; Lu, Y.C.; Manthiram, A. Self-supported MoO2/MoS2 nano-sheets embedded in a carbon cloth as a binder-free substrate for high-energy lithium–sulfur batteries. Electrochim. Acta 2021, 367, 137482. [Google Scholar] [CrossRef]
- Zhang, H.; Ono, L.K.; Tong, G.Q.; Liu, Y.Q.; Qi, Y.B. Long-life lithium–sulfur batteries with high areal capacity based on coaxial CNT@TiN-TiO2 sponge. Nat. Commun. 2021, 12, 4738. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, W.C.; Zhang, Y.; Yu, X.F.; Zhang, B.S.; Li, F.; Lu, A.H. Paragenesis BN/CNTs hybrid as a monoclinic sulfur host for high rate and ultra-long life lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 24194–24200. [Google Scholar] [CrossRef]
- Yuan, J.J.; Huang, Z.; Song, Y.Z.; Li, M.Y.; Li, H.Y. In-situ crosslinked binder for high-stability S cathodes with greatly enhanced conduction and polysulfides anchoring. Chem. Eng. J. 2021, 426, 128705. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.N.; Bai, Z.Y.; Liu, W.W.; Liu, T.C.; Gim, J.; Jiang, G.P.; Yuan, Y.F.; Luo, D.; Yassar, R.S.; et al. A lithium–sulfur battery using a 2D current collector architecture with a large-sized sulfur host operated under high areal loading and low E/S ratio. Adv. Mater. 2018, 30, 1804271. [Google Scholar] [CrossRef]
- Xiao, P.T.; Sun, L.X.; Liao, D.K.; Agboola, P.; Imran, S.; Xu, Y. Unexpected effect of electrode architecture on high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 33269–33275. [Google Scholar] [CrossRef]
- Yang, B.Z.; Xie, P.; Luo, Q.; Li, Z.W.; Zhou, C.Y.; Yin, Y.H.; Liu, X.B.; Li, Y.S. Binder-free ω-Li3V2O5 catalytic network with multi-polarization center assists lithium–sulfur batteries for enhanced kinetics behavior. Adv. Funct. Mater. 2022, 32, 2110665. [Google Scholar] [CrossRef]
- Chung, S.H.; Chang, C.H.; Manthiram, A. Hierarchical sulfur electrodes as a testing platform for understanding the high-loading capability of Li–S batteries. J. Power Sources 2016, 334, 179–190. [Google Scholar] [CrossRef]
- Yen, Y.J.; Chung, S.H. Lean-electrolyte lithium–sulfur electrochemical cells with high-loading carbon nanotube/nanofiber–polysulfide cathodes. Chem. Commun. 2021, 57, 2009–2012. [Google Scholar] [CrossRef]
- Yu, C.H.; Yen, Y.J.; Chung, S.H. Nanoporosity of carbon–sulfur nanocomposites toward the lithium–sulfur battery electrochemistry. Nanomaterials 2021, 11, 1518. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chung, S.H. Advanced current collectors with carbon nanofoams for electrochemically stable lithium–sulfur cells. Nanomaterials 2021, 11, 2083. [Google Scholar] [CrossRef]
- Meng, L.; Li, Y.; Lin, Q.X.; Long, J.; Wang, Y.; Hu, J. Nitrogen and Oxygen dual self-doped flexible PPTA nanofiber carbon paper as an effective interlayer for lithium–sulfur batteries. ACS Appl. Energy Mater. 2021, 4, 8592–8603. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xu, P.; Liu, Q.B.; Yuan, D.; Zhu, S. Cobalt embedded in porous carbon fiber membranes for high-performance lithium–sulfur batteries. Carbon 2022, 187, 187–195. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, L.W.; Wang, Y.; Li, Q.; Chen, C.T.; Zhong, B.H.; Chen, Y.X.; Guo, X.D.; Wu, Z.G.; Liu, Y.; et al. Novel functional separator with self-assembled MnO2 layer via a simple and fast method in lithium–sulfur battery. J. Colloid Interface Sci. 2022, 606, 666–676. [Google Scholar] [CrossRef]
- Chen, Z.H.; Hu, Y.X.; Liu, W.; Yu, F.; Yu, X.F.; Mei, T.; Yu, L.; Wang, X.B. Three-dimensional engineering of sulfur/MnO2 composites for high-rate lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 38394–38404. [Google Scholar] [CrossRef]
- Pang, Z.Y.; Ma, Y.; Zhou, Y.; Miao, X.Y.; Kong, L.L.; Song, D.W.; Shi, X.X.; Zhang, H.Z.; Zhang, L.Q. Tailoring 3D carbon foam using CNTs and MnO2 to fabricate stable lithium/dissolved lithium polysulfide batteries. Langmuir 2021, 37, 4016–4024. [Google Scholar] [CrossRef]
- Yoshie, Y.; Hori, K.; Mae, T.; Noda, S. High-energy density Li–S battery with positive electrode of lithium polysulfides held by carbon nanotube sponge. Carbon 2021, 182, 32–41. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Pan, H.L.; Shao, Y.Y.; Yan, P.F.; Cheng, Y.W.; Han, K.S.; Nie, Z.M.; Wang, C.M.; Yang, J.H.; Li, X.L.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Khan, Z.; Park, S.; Hwang, S.M.; Yang, J.; Ko, H. Hierarchical urchin-shaped α-MnO2 on graphene-coated carbon microfibers: A binder-free electrode for rechargeable aqueous Na–air battery. NPG Asia Mater. 2016, 8, 294. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Fu, Y.; Lyu, L.; Lu, Z.; Zhou, L. In situ assembly of MnO2 nanosheets on sulfur-embedded multichannel carbon nanofiber composites as cathode for lithium–sulfur batteries. Sci. China Mater. 2020, 63, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselvo, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Binder, C.; Bendo, T.; Hammes, G.; Neves, G.; Binder, R.; Mello, J.D.; Klein, A.N. Structure and properties of in situ-generated two-dimensional turbostratic graphite nodules. Carbon 2017, 124, 685–692. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, L.F.; Chen, D.C.; Ji, X.; Jiang, Z.J.; Ding, D.; Liu, M.L. Phase evolution of an alpha MnO2-based electrode for pseudo-capacitors probed by in operando Raman spectroscopy. Nano Energy 2014, 9, 161–167. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.R.; Liu, S.; Gao, X.P. Hollow molybdate microspheres as catalytic hosts for enhancing the electrochemical performance of sulfur cathode under high sulfur loading and lean electrolyte. Adv. Funct. Mater. 2021, 31, 2010693. [Google Scholar] [CrossRef]
- Zhao, C.X.; Li, X.Y.; Zhao, M.; Chen, Z.X.; Song, Y.W.; Chen, W.J.; Liu, J.N.; Wang, B.; Zhang, X.Q.; Chen, C.M.; et al. Semi-immobilized molecular electrocatalysts for high-performance lithium–sulfur batteries. J. Am. Chem. Soc. 2021, 143, 19865–19872. [Google Scholar] [CrossRef]
- Sun, Z.H.; Zhang, J.Q.; Yin, L.C.; Hu, G.J.; Fang, R.P.; Cheng, H.M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium–sulfur batteries. Nat. Commun. 2017, 8, 14627. [Google Scholar] [CrossRef] [Green Version]
- He, J.R.; Bhargav, A.; Manthiram, A. High-energy-density, long-life lithium–sulfur batteries with practically necessary parameters enabled by low-cost Fe–Ni nanoalloy catalysts. ACS Nano 2021, 15, 8583–8591. [Google Scholar] [CrossRef]
- Wang, D.H.; Xie, D.; Yang, T.; Zhong, Y.; Wang, X.L.; Xia, X.H.; Gu, C.D.; Tu, J.P. Conversion from Li2SO4 to Li2S@C on carbon paper matrix: A novel integrated cathode for lithium-sulfur batteries. J. Power Sources 2016, 331, 475–480. [Google Scholar] [CrossRef]
- Radhika, G.; Subadevi, R.; Sivakumar, M. Sulfur nested with mixture of MnO2/AB composite as efficient host for high-performance Li–S batteries. J. Chem. Sci. 2020, 132, 53. [Google Scholar] [CrossRef]
- Zou, Q.L.; Lu, Y.C. Solvent-dictated lithium sulfur redox reactions: An Operando UV−vis spectroscopic study. J. Phys. Chem. Lett. 2016, 7, 1518–1525. [Google Scholar] [CrossRef]
- Rehman, S.; Tang, T.Y.; Ali, Z.S.; Huang, X.X.; Hou, Y.L. Integrated design of MnO2@carbon hollow nanoboxes to synergistically encapsulate polysulfides for empowering lithium sulfur batteries. Small 2017, 13, 1700087. [Google Scholar] [CrossRef]
- Cao, K.Z.; Liu, H.Q.; Li, Y.; Wang, Y.J.; Jiao, L.F. Encapsulating sulfur in δ-MnO2 at room temperature for Li–S battery cathode. Energy Storage Mater. 2017, 9, 78–84. [Google Scholar] [CrossRef]
- Wang, N.; Peng, S.K.; Cheng, X.; Wang, J.X.; Wang, C.; Qi, X.; Dai, S.L.; Yan, S.J. Construction of ultrathin MnO2 decorated graphene/carbon nanotube nanocomposites as efficient sulfur hosts for high-performance lithium–sulfur batteries. RSC Adv. 2019, 9, 6346–6355. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, J.T.; Lou, X.W. Hollow carbon nanofibers filled with MnO2 nanosheets as efficient sulfur hosts for lithium–sulfur batteries. Angew. Chem. Int. Ed. 2015, 54, 12886–12890. [Google Scholar] [CrossRef]
- Chen, H.; Dong, W.D.; Xia, F.J.; Zhang, Y.J.; Yan, M.; Song, J.P.; Zou, W.; Liu, Y.; Hu, Z.Y.; Liu, J.; et al. Hollow nitrogen-doped carbon/sulfur@MnO2 nanocomposite with structural and chemical dual-encapsulation for lithium–sulfur battery. Chem. Eng. J. 2020, 381, 122746. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Z.; Kong, L.; Zhang, H.; Deng, B.; Song, D.; Shi, X.; Ma, Y.; Zhang, L. The Optimization of a Carbon Paper/MnO2 Composite Current Collector for Manufacturing a High-Performance Li–S Battery Cathode. Crystals 2022, 12, 1596. https://doi.org/10.3390/cryst12111596
Pang Z, Kong L, Zhang H, Deng B, Song D, Shi X, Ma Y, Zhang L. The Optimization of a Carbon Paper/MnO2 Composite Current Collector for Manufacturing a High-Performance Li–S Battery Cathode. Crystals. 2022; 12(11):1596. https://doi.org/10.3390/cryst12111596
Chicago/Turabian StylePang, Zhiyuan, Linglong Kong, Hongzhou Zhang, Bin Deng, Dawei Song, Xixi Shi, Yue Ma, and Lianqi Zhang. 2022. "The Optimization of a Carbon Paper/MnO2 Composite Current Collector for Manufacturing a High-Performance Li–S Battery Cathode" Crystals 12, no. 11: 1596. https://doi.org/10.3390/cryst12111596
APA StylePang, Z., Kong, L., Zhang, H., Deng, B., Song, D., Shi, X., Ma, Y., & Zhang, L. (2022). The Optimization of a Carbon Paper/MnO2 Composite Current Collector for Manufacturing a High-Performance Li–S Battery Cathode. Crystals, 12(11), 1596. https://doi.org/10.3390/cryst12111596





