Nanofiber Sr2Fe1.5Mo0.5O6-δ Electrodes Fabricated by the Electrospinning Method for Solid-Oxide Cells
Abstract
:1. Introduction
2. Experimental Section
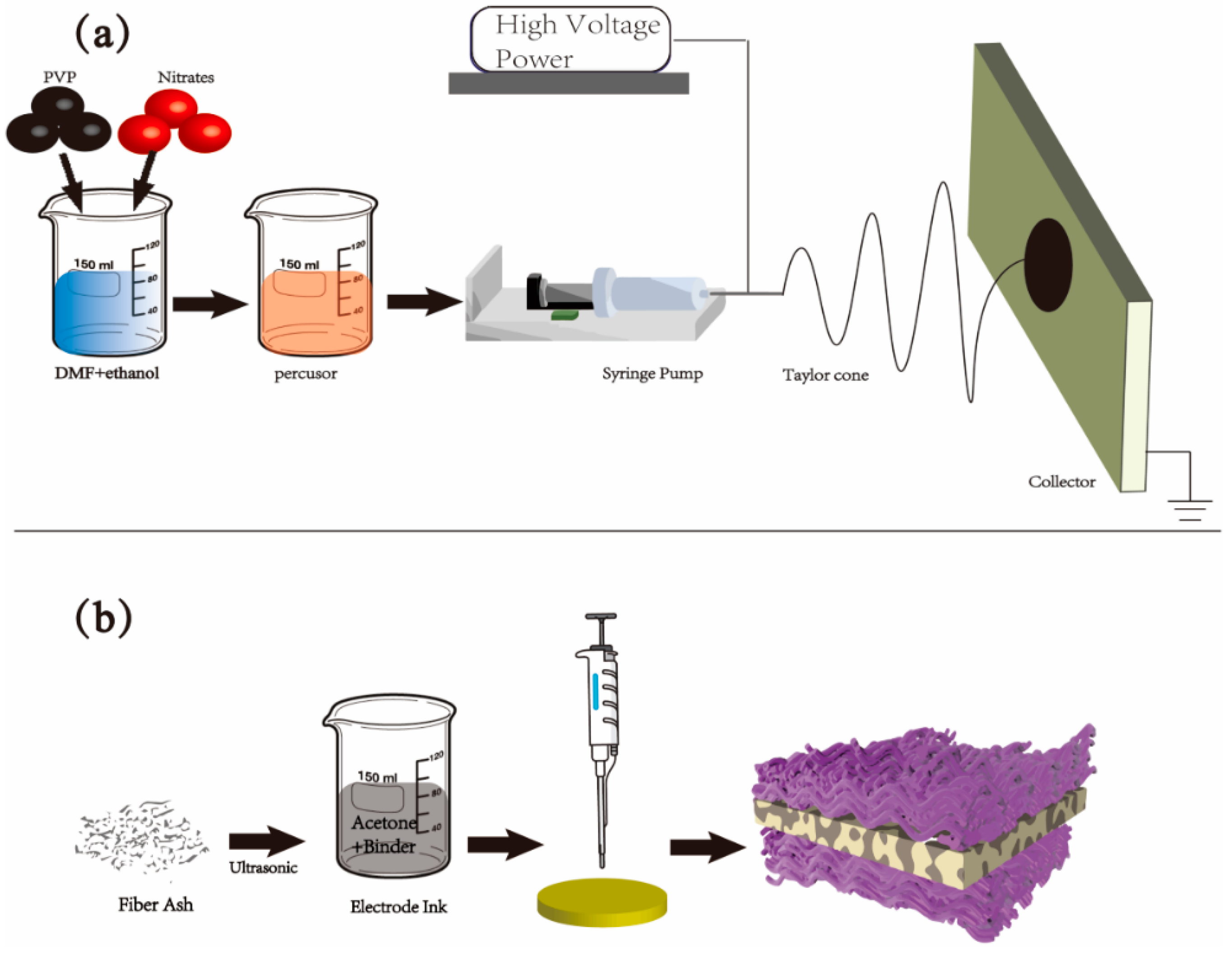
2.1. Material and Cell Preparation
2.2. Measurement
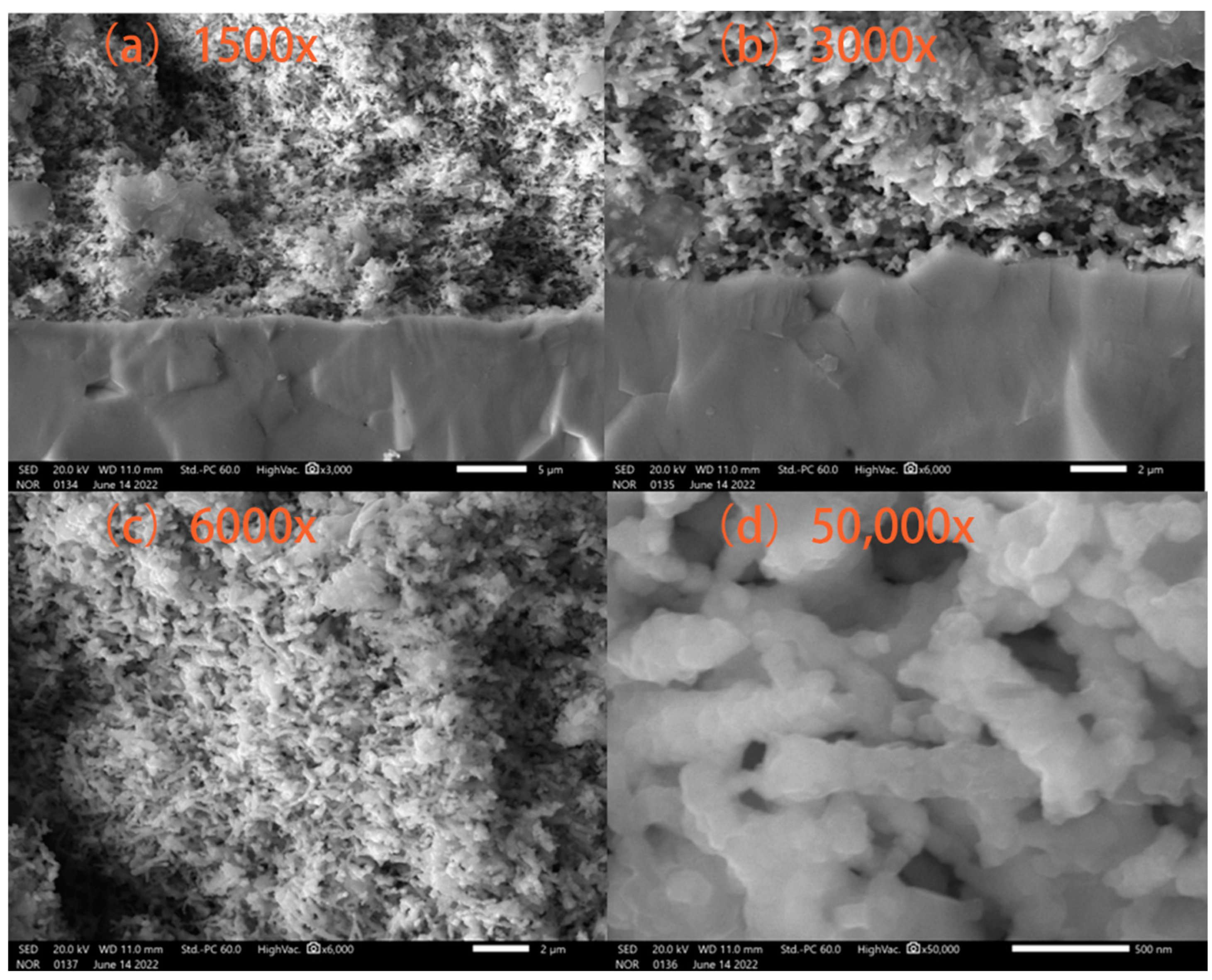
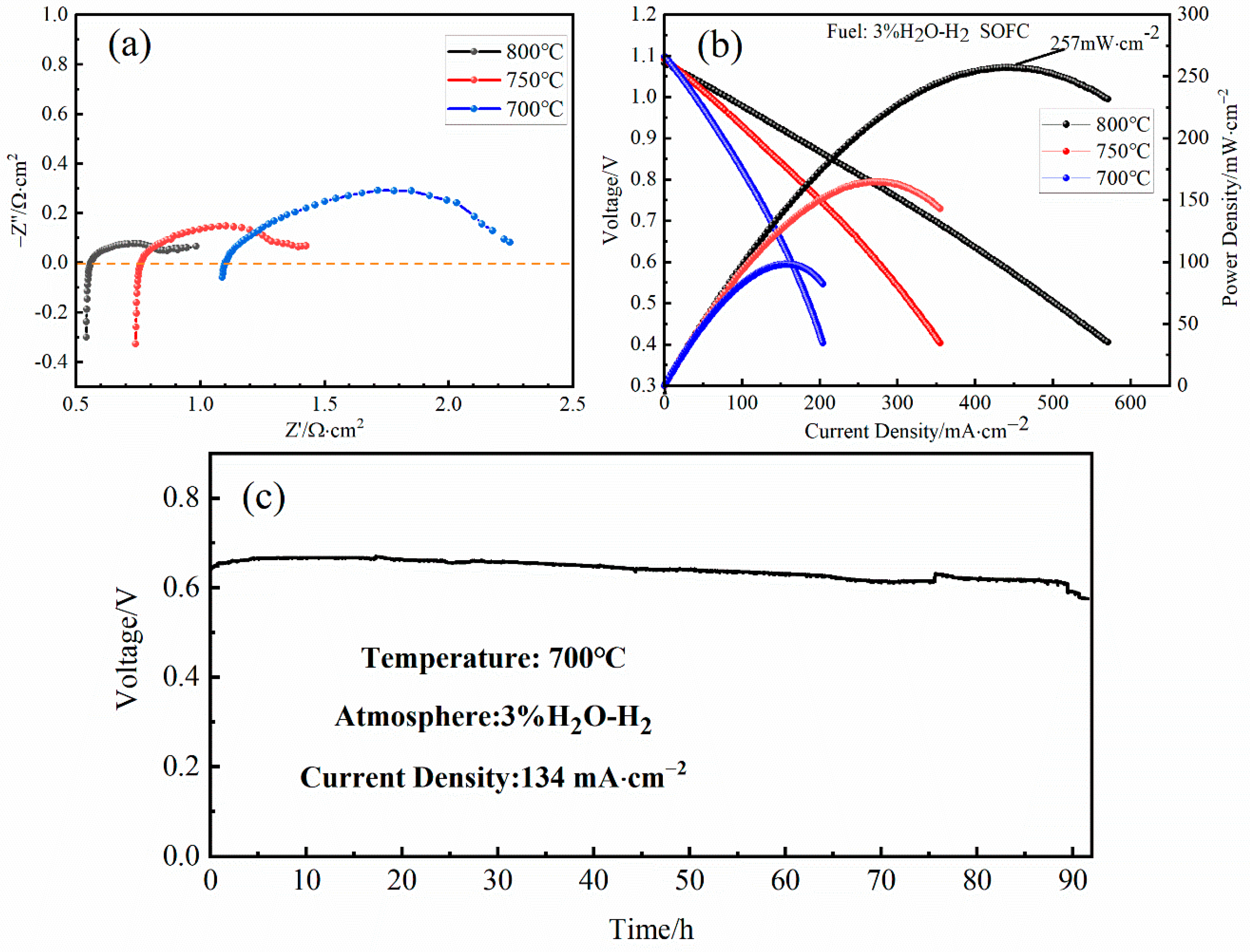
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isenberg, A.O. Energy conversion via solid oxide electrolyte electrochemical cells at high temperatures. Solid State Ion. 1981, 3–4, 431–437. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. A modeling study on concentration overpotentials of a reversible solid oxide fuel cell. J. Power Sources 2006, 163, 460–466. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Marrero-López, D.; Canales-Vázquez, J.; Irvine, J.T.S. Symmetric and reversible solid oxide fuel cells. RSC Adv. 2011, 1, 1403–1414. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Sun, Z. High performance and stability of nanocomposite oxygen electrode for solid oxide cells. Int. J. Hydrogen Energy 2020, 45, 5554–5564. [Google Scholar] [CrossRef]
- Liu, C.; Pu, J.; Chen, X.; Ma, Z.; Ding, X.; Zhou, J.; Wang, S. Influence of anode’s microstructure on electrochemical performance of solid oxide direct carbon fuel cells. Int. J. Hydrogen Energy 2020, 45, 11784–11790. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Ni, M. Simulation of sintering kinetics and microstructure evolution of composite solid oxide fuel cells electrodes. Int. J. Hydrogen Energy 2012, 37, 3392–3402. [Google Scholar] [CrossRef] [Green Version]
- Kenney, B.; Valdmanis, M.; Baker, C.; Pharoah, J.G.; Karan, K. Computation of TPB length, surface area and pore size from numerical reconstruction of composite solid oxide fuel cell electrodes. J. Power Sources 2009, 189, 1051–1059. [Google Scholar] [CrossRef]
- Chen, D.; Lin, Z.; Zhu, H.; Kee, R.J. Percolation theory to predict effective properties of solid oxide fuel-cell composite electrodes. J. Power Sources 2009, 191, 240–252. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Hou, Z.; Shi, C.; Zhang, G.; Zeng, F.; Zhou, J.; Wang, S. La1-xCaxFeO3-δ air electrode fabricated by glycine-nitrate combustion method for solid oxide electrolysis cell. Ceram. Int. 2021, 47, 32318–32323. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Li, W.; Hou, Z.; Shi, C.; Zhang, G.; Zhou, J.; Wang, S. A promising strontium and cobalt-free air electrode Pr1-xCaxFeO3-δ for solid oxide electrolysis cell. Int. J. Hydrogen Energy 2021, 46, 30230–30238. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Hussain, A.M.; Song, D.; Miura, Y.; Chen, Y.; Luo, Z.; Kane, N.; Niu, Y.; Dale, N.; et al. High-Performance, Thermal Cycling Stable, Coking-Tolerant Solid Oxide Fuel Cells with Nanostructured Electrodes. ACS Appl. Mater. Interfaces 2021, 13, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the Electrochemical Performance of Fe-Based Layered Double Perovskite Cathodes by Zn2+ Doping for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, Y.; Zheng, Y.; Li, W.; Chen, X.; Sun, X.; Chen, X.; Zhou, J. La0.75Sr0.25Cr0.5Mn0.5O3- as cathode for electrolysis and co-electrolysis of CO2 and H2O in solid oxide electrolysis cell. Ceram. Int. 2021, 47, 23350–23361. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, J.; Lee, W. One-step fabrication of composite nanofibers for solid oxide fuel cell electrodes. J. Power Sources 2019, 434, 226749. [Google Scholar] [CrossRef]
- Parbey, J.; Wang, Q.; Yu, G.; Zhang, X.; Li, T.; Andersson, M. Progress in the use of electrospun nanofiber electrodes for solid oxide fuel cells: A review. Rev. Chem. Eng. 2020, 36, 879–931. [Google Scholar] [CrossRef]
- Enrico, A.; Zhang, W.; Lund Traulsen, M.; Sala, E.M.; Costamagna, P.; Holtappels, P. La0.6Sr0.4Co0.2Fe0.8O3-δ nanofiber cathode for intermediate-temperature solid oxide fuel cells by water-based sol-gel electrospinning: Synthesis and electrochemical behaviour. J. Eur. Ceram. Soc. 2018, 38, 2677–2686. [Google Scholar] [CrossRef] [Green Version]
- Hieu, N.T.; Park, J.; Tae, B. Synthesis and characterization of nanofiber-structured Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite oxide used as a cathode material for low-temperature solid oxide fuel cells. Mater. Sci. Eng. B 2012, 177, 205–209. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, Y.; Zhang, Y.; Yan, R.; Ding, D.; Zhao, B.; Yoo, S.; Dang, D.; Hu, R.; Yang, C.; et al. A Highly Efficient and Robust Nanofiber Cathode for Solid Oxide Fuel Cells. Adv. Energy Mater. 2016, 7, 1601890. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3-delta/CeO2 Heterostructured Composite Nanofibers as a Highly Active and Robust Cathode Catalyst for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, T.; Zhou, S.; Wu, Y.; Chen, H.; Ou, X.; Ling, Y. Characterization of Sr2Fe1.5Mo0.5O6-delta-Gd0.1Ce0.9O1.95 symmetrical electrode for reversible solid oxide cells. Ceram. Int. 2019, 45, 10969–10975. [Google Scholar] [CrossRef]
- Rath, M.K.; Lee, K.-T. Superior electrochemical performance of non-precious Co-Ni-Mo alloy catalyst-impregnated Sr2FeMoO6-delta as an electrode material for symmetric solid oxide fuel cells. Electrochim. Acta 2016, 212, 678–685. [Google Scholar] [CrossRef]
- Han, Z.; Dong, H.; Wu, Y.; Yang, Y. Locating the rate-limiting step of hydrogen conversion on Sr2Fe1.5Mo0.5O6 (0 0 1) surface: Implications for efficient SOFC anode design. Appl. Surf. Sci. 2022, 595, 153513. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Pavone, M.; Carter, E.A. Effect of Antisite Defects on the Formation of Oxygen Vacancies in Sr2FeMoO6: Implications for Ion and Electron Transport. Chem. Mater. 2011, 23, 4525–4536. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Wang, Y.; Li, Y. Sr2Fe2−Mo O6—Perovskite as an anode in a solid oxide fuel cell: Effect of the substitution ratio. Catal. Today 2016, 259, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Pu, X.; Wan, Y.; Zhu, K.; Xia, C. Research progress in effect of element doping on electrochemical properties of Sr_2Fe_(1.5)Mo_(0.5)O_(6-delta) based anode materials. J. Mater. Eng. 2021, 49, 1–13. [Google Scholar]
- Porotnikova, N.M.; Osinkin, D.A. Recent advances in heteroatom substitution Sr2Fe1.5Mo0.5O6-δ oxide as a more promising electrode material for symmetrical solid-state electrochemical devices: A review. Electrochem. Mater. Technol. 2022, 1, 20221003. [Google Scholar]
- Li, M.; Ni, M.; Su, F.; Xia, C. Proton conducting intermediate-temperature solid oxide fuel cells using new perovskite type cathodes. J. Power Sources 2014, 260, 197–204. [Google Scholar] [CrossRef]
- Tian, C.; Cheng, J.; Yang, J. A highly active cathode material of Cu-doped Sr2Fe1.5Mo0.5O6 for symmetrical solid oxide fuel cells. J. Mater. Sci.-Mater. Electron. 2021, 32, 1258–1264. [Google Scholar] [CrossRef]
- Qiu, P.; Sun, S.; Li, J.; Jia, L. A review on the application of Sr2Fe1.5Mo0.5O6-based oxides in solid oxide electrochemical cells. Sep. Purif. Technol. 2022, 298, 121581. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A Novel Electrode Material for Symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef]
- Yang, G.; Feng, J.; Sun, W.; Dai, N.; Hou, M.; Hao, X.; Qiao, J.; Sun, K. The characteristic of strontium-site deficient perovskites SrxFe1.5Mo0.5O6-delta (x = 1.9–2.0) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Sources 2014, 268, 771–777. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Li, Y. Direct CH4 fuel cell using Sr2FeMoO6 as an anode material. J. Power Sources 2011, 196, 6104–6109. [Google Scholar] [CrossRef]
- Parbey, J.; Xu, M.; Lei, J.; Espinoza-Andaluz, M.; Li, T.S.; Andersson, M. Electrospun fabrication of nanofibers as high-performance cathodes of solid oxide fuel cells. Ceram. Int. 2020, 46, 6969–6972. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Nechache, A.; Mansuy, A.; Petitjean, M.; Mougin, J.; Mauvy, F.; Boukamp, B.A.; Cassir, M.; Ringuedé, A. Diagnosis of a cathode-supported solid oxide electrolysis cell by electrochemical impedance spectroscopy. Electrochim. Acta 2016, 210, 596–605. [Google Scholar] [CrossRef]
- Pototskaya, V.V.; Gichan, O.I. The Gerischer finite length impedance: A case of unequal diffusion coefficients. J. Electroanal. Chem. 2019, 852, 113511. [Google Scholar] [CrossRef]
- Ivers-Tiffee, E.; Weber, A. Evaluation of electrochemical impedance spectra by the distribution of relaxation times. J. Ceram. Soc. Jpn. 2017, 125, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Osinkin, D.A. An approach to the analysis of the impedance spectra of solid oxide fuel cell using the DRT technique. Electrochim. Acta 2021, 372, 137858. [Google Scholar] [CrossRef]
- Oz, A.; Gelman, D.; Tsur, Y.; Singh, K.; Thangadurai, V. Evolutionary Programming Based Approach for SOFC Cathode Characterization: A Case Study on Co-Free Mixed Conducting Perovskites. ECS Trans. 2017, 78, 2099–2108. [Google Scholar] [CrossRef]
- Jing, C.; Bo, Y.U.; Wen-Qiang, Z.; Xue, W. SOC Stack Impedance Characterization and Identification Based on DRT and ADIS Methods. J. Inorg. Mater. 2016, 31, 1279–1288. [Google Scholar] [CrossRef]
- Sumi, H.; Shimada, H.; Yamaguchi, Y.; Mizutani, Y.; Okuyama, Y.; Amezawa, K. Comparison of electrochemical impedance spectra for electrolyte-supported solid oxide fuel cells (SOFCs) and protonic ceramic fuel cells (PCFCs). Sci. Rep. 2021, 11, 10622. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Feng, C.; Shao, S.; Li, X.; Huang, X.; Cao, J. An electrochemical assessment of pristine SrCoO3-δ using the distribution of relaxation times analysis. Asia-Pac. J. Chem. Eng. 2022, 17, e2784. [Google Scholar] [CrossRef]
- Boukamp, B.A. Derivation of a Distribution Function of Relaxation Times for the (fractal) Finite Length Warburg. Electrochim. Acta 2017, 252, 154–163. [Google Scholar] [CrossRef]


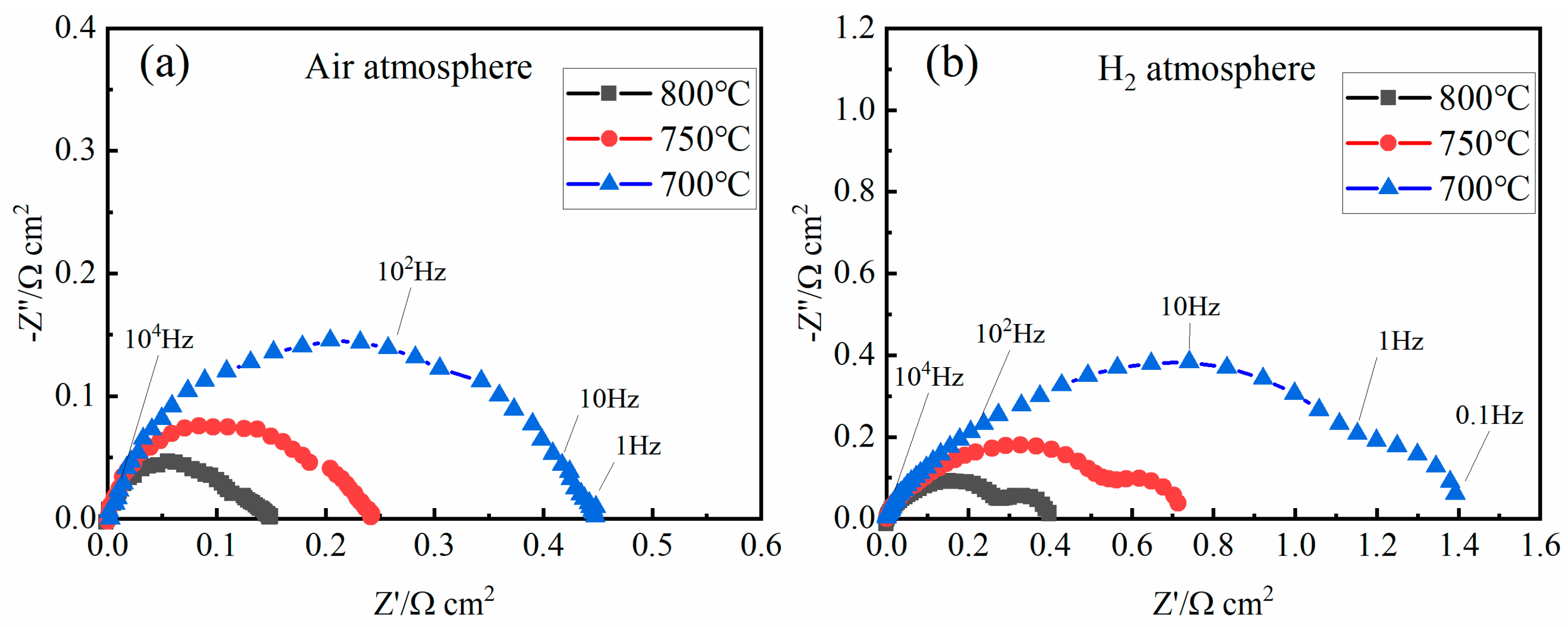
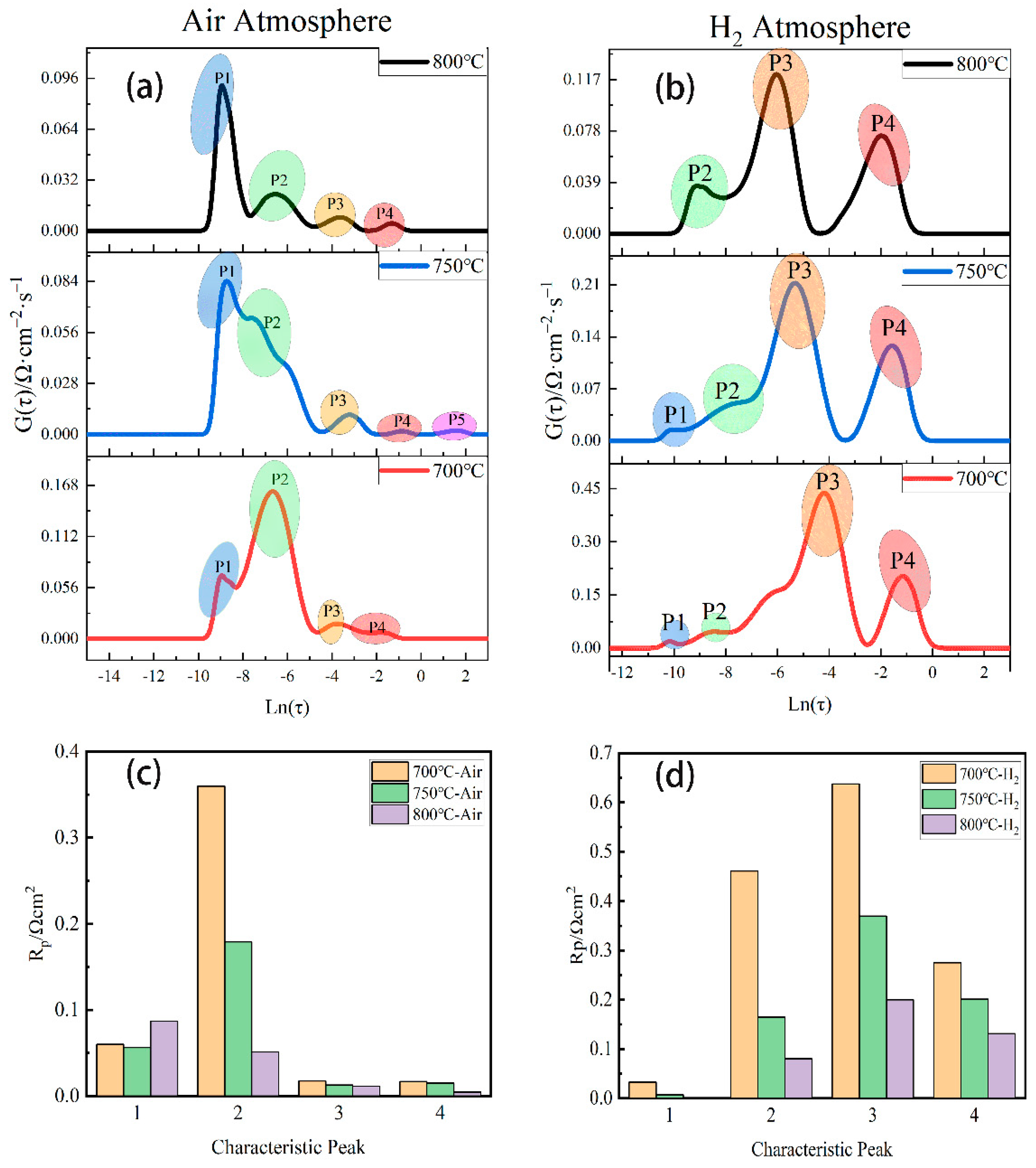
| Resistance (Ω∙cm2) | 800 °C | 750 °C | 700 °C |
|---|---|---|---|
| Rp-H2 | 0.4 | 0.72 | 1.4 |
| Rp-Air | 0.14 | 0.24 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Leng, Z.; Ling, Y.; Bai, H.; Li, S.; Zhou, J.; Wang, S. Nanofiber Sr2Fe1.5Mo0.5O6-δ Electrodes Fabricated by the Electrospinning Method for Solid-Oxide Cells. Crystals 2022, 12, 1624. https://doi.org/10.3390/cryst12111624
Zhang B, Leng Z, Ling Y, Bai H, Li S, Zhou J, Wang S. Nanofiber Sr2Fe1.5Mo0.5O6-δ Electrodes Fabricated by the Electrospinning Method for Solid-Oxide Cells. Crystals. 2022; 12(11):1624. https://doi.org/10.3390/cryst12111624
Chicago/Turabian StyleZhang, Bo, Zhizhong Leng, Yihan Ling, Hu Bai, Sha Li, Juan Zhou, and Shaorong Wang. 2022. "Nanofiber Sr2Fe1.5Mo0.5O6-δ Electrodes Fabricated by the Electrospinning Method for Solid-Oxide Cells" Crystals 12, no. 11: 1624. https://doi.org/10.3390/cryst12111624






