Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. The Obtaining of the Mg-Zn-Ca-Zr Alloy Powder by a Mechanical Alloying Procedure
2.2. The Processing of the Mg-Zn-Ca-Zr Alloy Powder by a Selective Laser Melting (SLM) Procedure
2.3. The Microstructural and Mechanical Analysis of the Mg-Zn-Ca-Zr Alloy
3. Results and Discussions
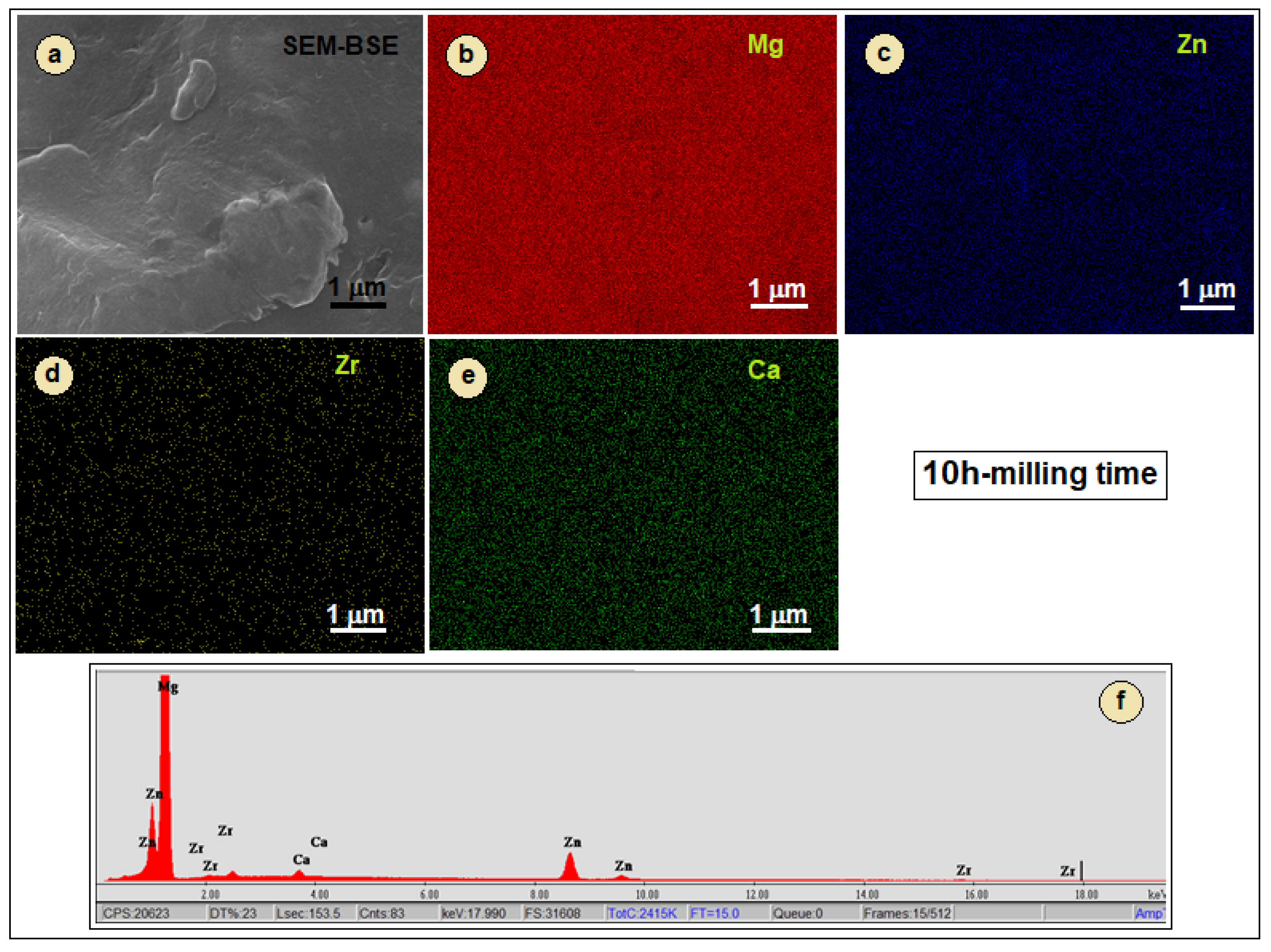
3.1. Microstructure Characterisation and Evolution
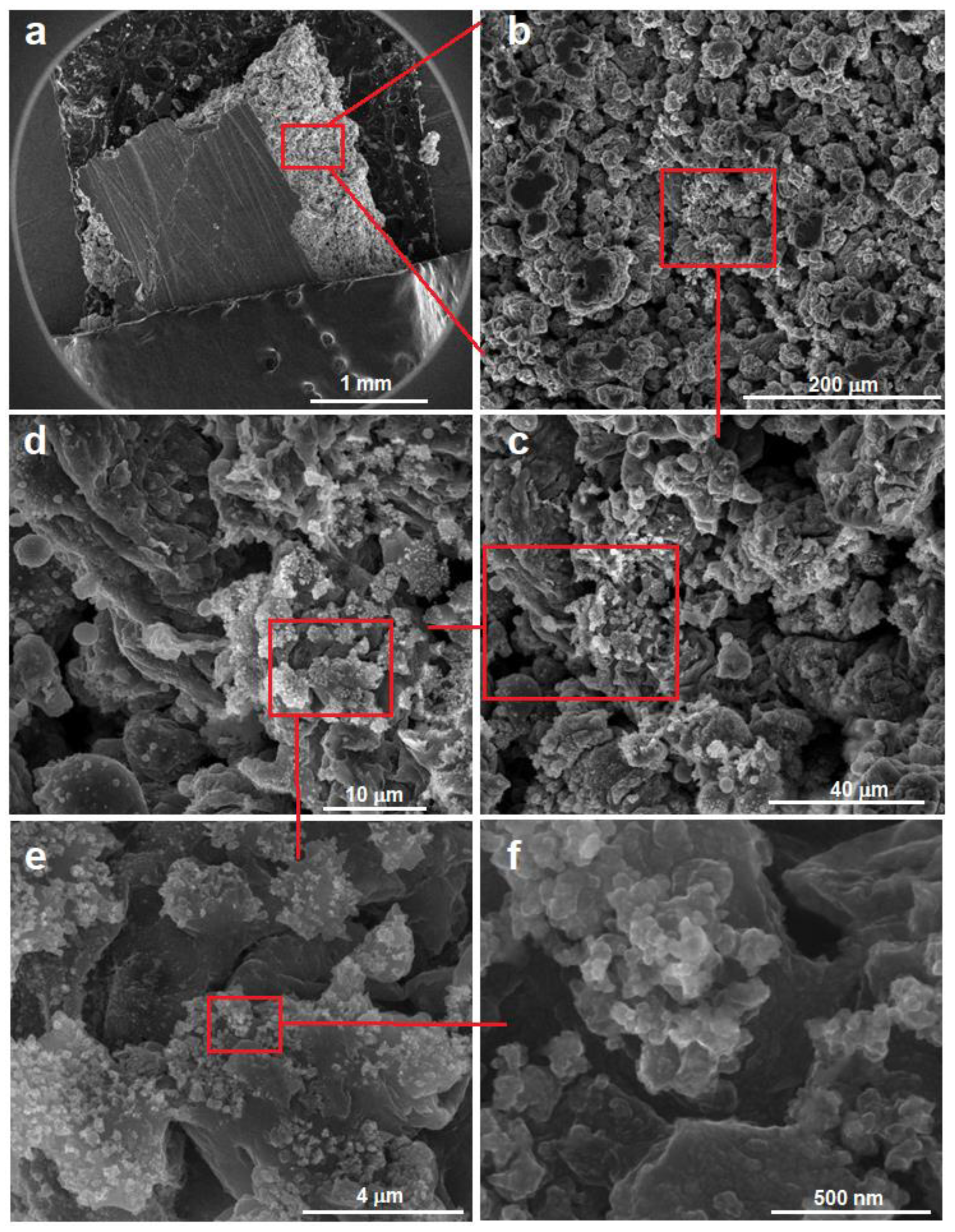
3.2. Preliminary Results for the SLM-Processed Sample from Powders Obtained by Mechanical Alloying with 10 h Milling Time
4. Conclusions
- (a)
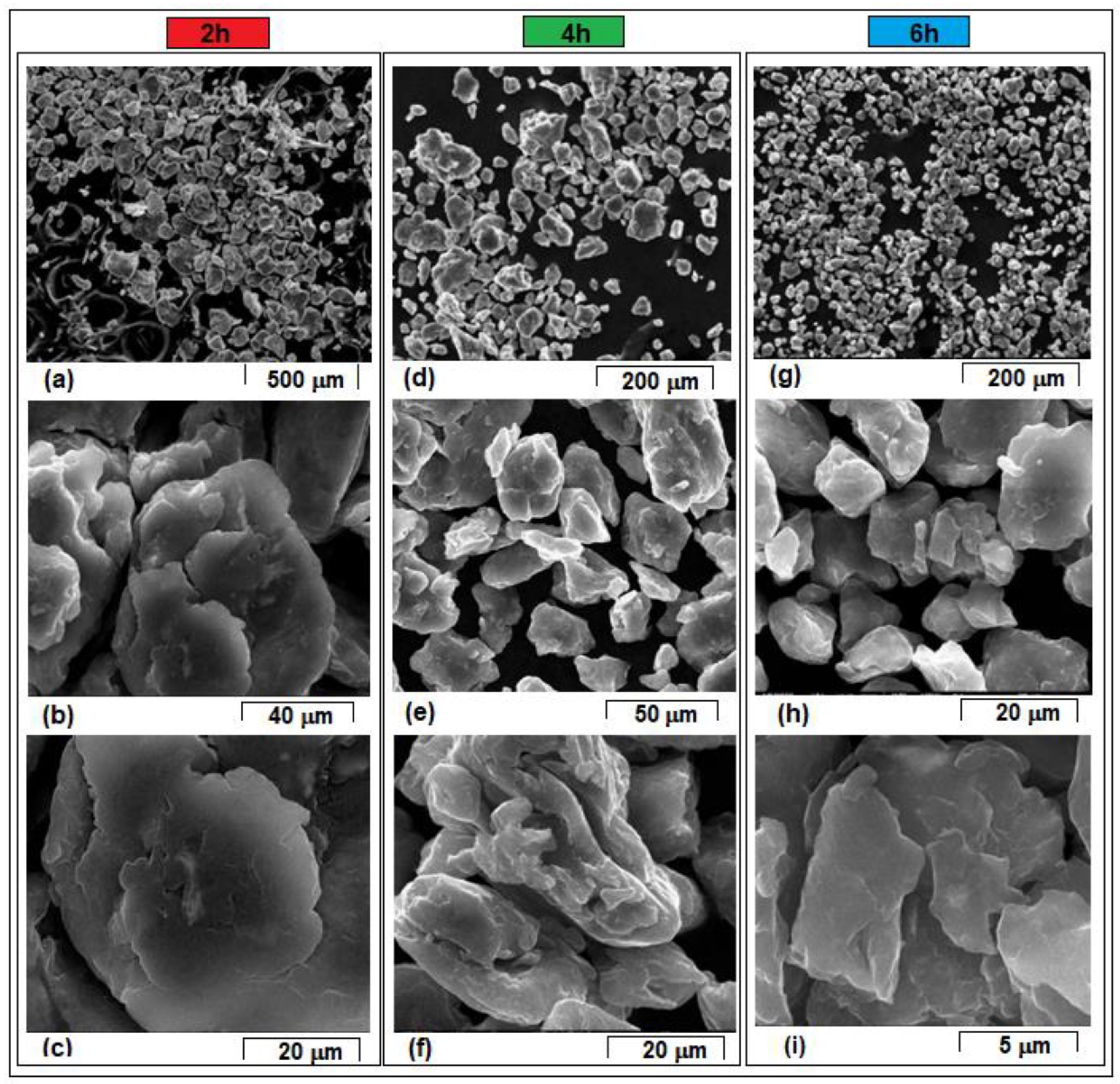
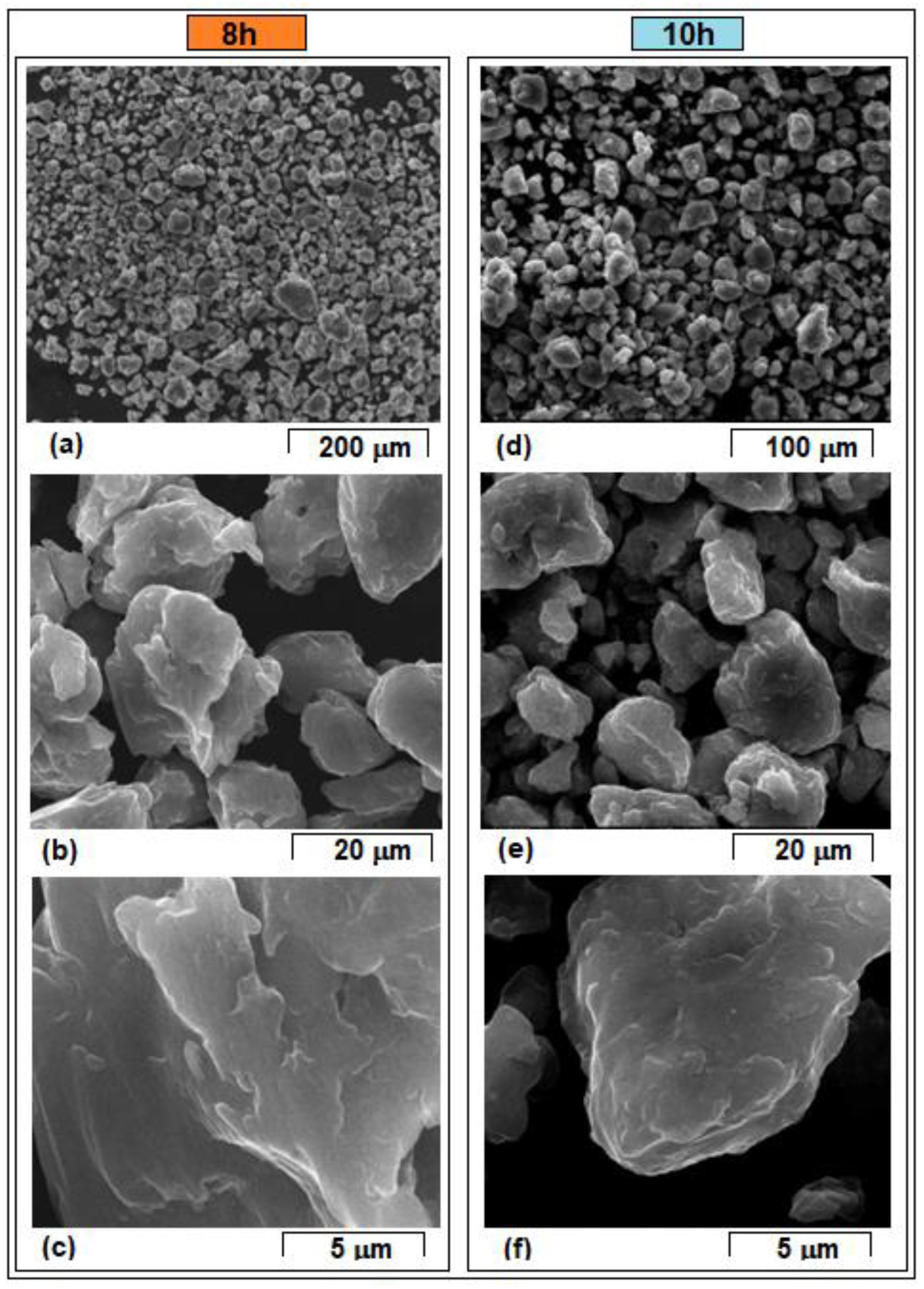
- The mechanical alloying method was applied to obtain the Mg-10Zn-0.5Zr-0.8Ca powder-alloy using different milling times. The morphologies of the milled powders reveal that the particles dimensions vary, with the average values ranging from 74.6 µm (after 2 h of milling process) to 16.2 µm (after 10 h of milling process).
- (b)
- During the milling process, the particles are subjected to repeated welding and fracturing. Strong plastic deformations occur, thus increasing the hardness, while at the same time the particle size decreases. The fluctuations in particle size are characteristic of the mechanical alloying process, as the particles are repeatedly subjected to cold welding, fracturing, and milling. This repetitive process results in a high homogeneity of chemical composition, which is supported by EDS analysis.
- (c)
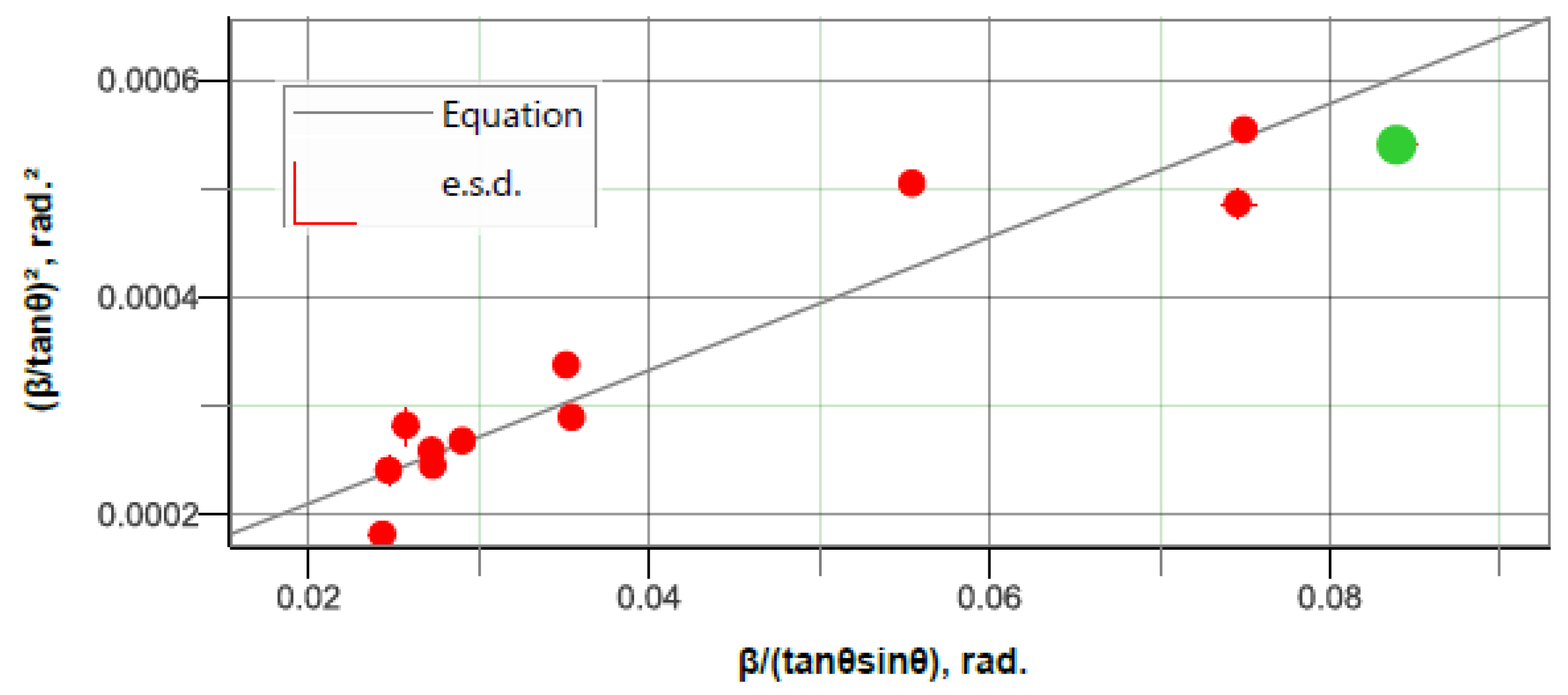
- It can be concluded that mechanical alloying has utility for achieving homogeneity of the alloy and microstructure refinement which is desirable for enhancing the alloy’s performances. However, the alloying chemical elements—Zn, Zr, and Ca—introduced in the Mg-based solid solution and possible local variations in the composition can create a distribution of d-spacing for the crystallographic plane, which conducts to a value of 0.236% for the lattice strain; in the studied case, it is presumed to be produced by residual stresses, dislocations, and any other defect that causes a nonuniform lattice distortion in the crystal.
- (d)
- The SLM trial has promising results, proven by SEM analysis for the microstructural aspects, and proven also by results obtained by mechanical tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witte, F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Badylak, S.F. Immunomodulatory biomaterials. Curr. Opin. Biomed. Eng. 2018, 6, 51–57. [Google Scholar]
- Hassan, S.F.; Islam, M.T.; Saheb, N.; Baig, M.M.A. Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials 2022, 15, 5669. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Kolawole, S.K.; Wang, J.; Su, X.; Tan, L.; Yang, K. Systems, Properties, Surface Modification and Applications of Biodegradable Magnesium-Based Alloys: A Review. Materials 2022, 15, 5031. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Shen, Z.; Miao, F.; Wang, J.; Sun, Y.; Zhu, S.; Zheng, Y.; Guan, S. A biodegradable magnesium alloy vascular stent structure: Design, optimisation and evaluation. Acta Biomater. 2022, 142, 402–412. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hung, F.Y.; Lin, Y.L.; Lin, C.Y. Biodegradation ZK50 magnesium alloy compression screws: Mechanical properties, biodegradable characteristics and implant test. J. Orthop. Sci. 2020, 25, 1107–1115. [Google Scholar]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Regen. Biomater. 2017, 4, 129–137. [Google Scholar]
- Yang, K.; Zhou, C.; Fan, H.; Fan, Y.; Jiang, Q.; Song, P.; Fan, H.; Chen, Y.; Zhang, X. Bio-Functional Design, Application and Trends in Metallic Biomaterials. Int. J. Mol. Sci. 2017, 19, 24. [Google Scholar] [CrossRef]
- Chang, G.; Regatte, R.R.; Schweitzer, M.E. Olympic fencers: Adaptations in cortical and trabecular bone determined by quantitative computed tomography. Osteoporos. Int. 2009, 20, 779–785. [Google Scholar]
- Okazaki, Y.; Gotoh, E.; Mori, J. Strength-Durability Correlation of Osteosynthesis Devices Made by 3D Layer Manufacturing. Materials 2019, 12, 436. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; Birgelen, C.V.; Christiansen, E.H.; Wijns, W.; Neumann, F.J.; Kaiser, C. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 months results of a prospective, multi centre, non-randomised, first-in-mantrial. Lancet 2015, 387, 31–39. [Google Scholar] [CrossRef]
- Plaass, C.; Von, F.C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.; Daniilidis, K. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies–3 years results of a randomized clinical trial. J. Orthop. Sci. 2018, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wen, P.; Guo, H.; Xia, D.; Zheng, Y.; Jauer, L.; Poprawe, R.; Voshage, M.; Schleifenbaum, J.H. Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta Biomater. 2019, 98, 3–22. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl 2017, 71, 1253–1266. [Google Scholar] [CrossRef]
- Salleh, E.M.; Ramakrishnan, S.; Hussain, Z. Synthesis of Biodegradable Mg-Zn Alloy by Mechanical Alloying: Effect of Milling Time. Procedia Chem. 2016, 19, 525–530. [Google Scholar] [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd Alloy after Mechanical Alloying. Materials 2021, 14, 226. [Google Scholar] [CrossRef]
- Lovašiová, P.; Lovaši, T.; Kubásek, J.; Jablonská, E.; Msallamová, Š.; Michalcová, A.; Vojtěch, D.; Suchý, J.; Koutný, D.; Ghassan Hamed Alzubi, E. Biodegradable WE43 Magnesium Alloy Produced by Selective Laser Melting: Mechanical Properties, Corrosion Behavior, and In-Vitro Cytotoxicity. Metals 2022, 12, 469. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Andani, M.T.; Shayesteh Moghaddam, N.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Abbasi, M.; Sajjadi, S.A.; Azadbeh, M. An investigation on the variations occurring during Ni3Al powder formation by mechanical alloying technique. J. Alloys Compd. 2010, 497, 171–175. [Google Scholar] [CrossRef]
- Neves, F.; Fernandes, F.M.B.; Martins, I.; Correia, J.B. Parametric optimization of Ti–Ni powder mixtures produced by mechanical alloying. J. Alloys Compd. 2011, 509, 271–274. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mat. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Liu, S.; Guo, H. Balling Behavior of Selective Laser Melting (SLM) Magnesium Alloy. Materials 2020, 13, 3632. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Man, H.C.; Gibson, I. Layer manufacturing of magnesium and its alloy structures for future applications. Virtual Phys. Prototyp. 2010, 5, 13–19. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Lau, M.L.; Man, H.C. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo degradation behaviour and biocompatibility of the magnesium alloy ZEK100 for use as a biodegradable bone implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr alloy. J. Alloys Compd. 2017, 691, 961–969. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Minárik, P.; Král, R.; Pešička, J.; Daniš, S.; Janeček, M. Microstructure characterization of LAE442 magnesium alloy processed by extrusion and ECAP. Mater. Charact. 2016, 112, 1–10. [Google Scholar]
- Minárik, P.; Jablonska, E.; Král, R.; Lipov, J.; Ruml, T.; Blawert, C.; Hadzima, B.; Chmelík, F. Effect of equal channel angular pressing on in vitro degradation of LAE442 magnesium alloy. Mater. Sci. Eng. C 2017, 73, 736–742. [Google Scholar] [CrossRef]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, T.; Nahan, K.; Guo, X.; Zhang, Z.; Dong, Z.; Chen, S.; Chou, D.T.; Hong, D.; Kumta, P.N.; et al. In vivo characterization of magnesium alloy biodegradation using electrochemical H2monitoring, ICP-MS, and XPS. Acta Biomater. 2017, 50, 556–565. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Li, H.; Liu, Y.; Bai, X.; Chau, W.; Zheng, Y.; Qin, L. Magnesium alloy-based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials 2018, 157, 86–97. [Google Scholar] [CrossRef]
- Wang, G.; Huang, G.; Chen, X.; Deng, Q.; Tang, A.; Jiang, B.; Pan, F. Effects of Zn addition on the mechanical properties and texture of extruded Mg-Zn-Ca-Ce magnesium alloy sheets. Mater. Sci. Eng. A 2017, 705, 46–54. [Google Scholar] [CrossRef]
- Yuasa, M.; Hayashi, M.; Mabuchi, M.; Chino, Y. Improved plastic anisotropy of Mg–Zn–Ca alloys exhibiting high-stretch formability: A first-principles study. Acta Mater. 2014, 65, 207–214. [Google Scholar] [CrossRef]
- Hrapkowicz, B.; Lesz, S.; Karolus, M.; Garbiec, D.; Wi´sniewski, J.; Rubach, R.; Gołombek, K.; Kremzer, M.; Popis, J. Microstructure and Mechanical Properties of Spark Plasma Sintered Mg-Zn-Ca-Pr Alloy. Metals 2022, 12, 375. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Alawad, M.O.; Aljohani, T.A.; El-Garaihy, W.H. Effect of ECAP Route Type on the Microstructural Evolution, Crystallographic Texture, Electrochemical Behavior and Mechanical Properties of ZK30 Biodegradable Magnesium Alloy. Materials 2022, 15, 6088. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on properties of selective laser melting Mg–9%Al powder mixture. Mater. Des. 2012, 34, 753–758. [Google Scholar] [CrossRef]
- Pawlak, A.; Chlebus, E. Process parameter optimization of Laser Micrometallurgy of AZ31 alloy. Interdiscip. J. Eng. Sci. 2015, 3, 10–15. [Google Scholar]
- Pawlak, A.; Szymczyk, P.E.; Kurzynowski, T.; Chlebus, E. Selective laser melting of magnesium AZ31B alloy powder. Rapid Prototyp. J. 2020, 26, 249–258. [Google Scholar] [CrossRef]
- Pawlak, A.; Rosienkiewicz, M.; Chlebus, E. Design of experiments approach in AZ31 powder selective laser melting process optimization. Arch. Civ. Mech. Eng. 2017, 17, 9–18. [Google Scholar] [CrossRef]
- He, C.; Bin, S.; Wu, P.; Gao, C.; Feng, P.; Yang, Y.; Liu, L.; Zhou, Y.; Zhao, M.; Yang, S.; et al. Microstructure evolution and biodegradation behavior of laser rapid solidified Mg–Al–Zn Alloy. Metals 2017, 7, 105. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Feng, P.; Guo, W.; Gao, C.; Wu, P.; Yang, Y.; Bin, S. Biodegradation mechanisms of selective laser-melted Mg–xAl–Zn alloy: Grain size and intermetallic phase. Virtual Phys. Prototyp. 2017, 13, 59–69. [Google Scholar] [CrossRef]
- Bahman, H.; Abdollah, A. Microstructure, mechanical properties, corrosion behavior and cytotoxicity of Mg-Zn-Al-Ca alloys as biodegradable materials. J. Alloys Compd. 2014, 607, 1–10. [Google Scholar]
- El-Garaihy, W.H.; Alateyah, A.I.; Alawad, M.O.; Aljohani, T.A. Improving the Corrosion Behavior and Mechanical Properties of Biodegradable Mg-Zn-Zr Alloys through ECAP for Usage in Biomedical Applications. In Magnesium Technology; Springer: Cham, Switzerland, 2022; pp. 259–269. [Google Scholar]
- Xu, L.; Liu, X.; Sun, K.; Fu, R.; Wang, G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials 2022, 15, 2613. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.; Guo, Y.B. Surface integrity of biodegradable Magnesium-Calcium orthopedic implant by burnishing. J. Mech. Behav. Biomed. Mater. 2011, 4, 1888–1904. [Google Scholar] [PubMed]
- Lee, C.D.; Kang, C.S.; Shin, K.S. Effect of galvanic corrosion between precipitate and matrix on corrosion behavior of as-cast magnesium-aluminum alloys. Met. Mater. Int. 2000, 6, 351–358. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Abbott, T.; Birbilis, N. The influence of zirconium additions on the corrosion of magnesium. Corros. Sci. 2014, 81, 27–35. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Abbott, T.; Birbilis, N. The influence of Mg-Zr master alloy microstructure on the corrosion of Mg. In Magnesium Technology 2013; The Minerals, Metals & Materials Society; Springer: Cham, Switzerland, 2013; pp. 157–162. [Google Scholar]
- Ding, Y.; Li, Y.; Lin, J.; Wen, C. Effects of zirconium and strontium on the biocorrosion of Mg-Zr-Sr alloys for biodegradable implant applications. J. Mater. Chem. B 2015, 3, 3714–3729. [Google Scholar] [CrossRef] [PubMed]
- Bobby Kannan, M.; Singh Raman, R.K. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef]
- Du, W.; Liu, K.; Ma, K.; Wang, Z.; Li, S. Effects of trace Ca/Sn addition on corrosion behaviors of biodegradable Mg–4Zn–0.2Mn alloy. J. Magnes. Alloys 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line Profiles of Neutron Powder-Diffraction Peaks for Structure Refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar]
- Kawano, S.; Iikubo, S.; Ohtani, H. Thermodynamic Stability of Mg-Based Laves Phases. Mater. Trans. 2018, 59, 890–896. [Google Scholar] [CrossRef]
- Ma, E. Alloys created between immiscible metals. Prog. Mater. Sci. 2005, 50, 413–509. [Google Scholar]

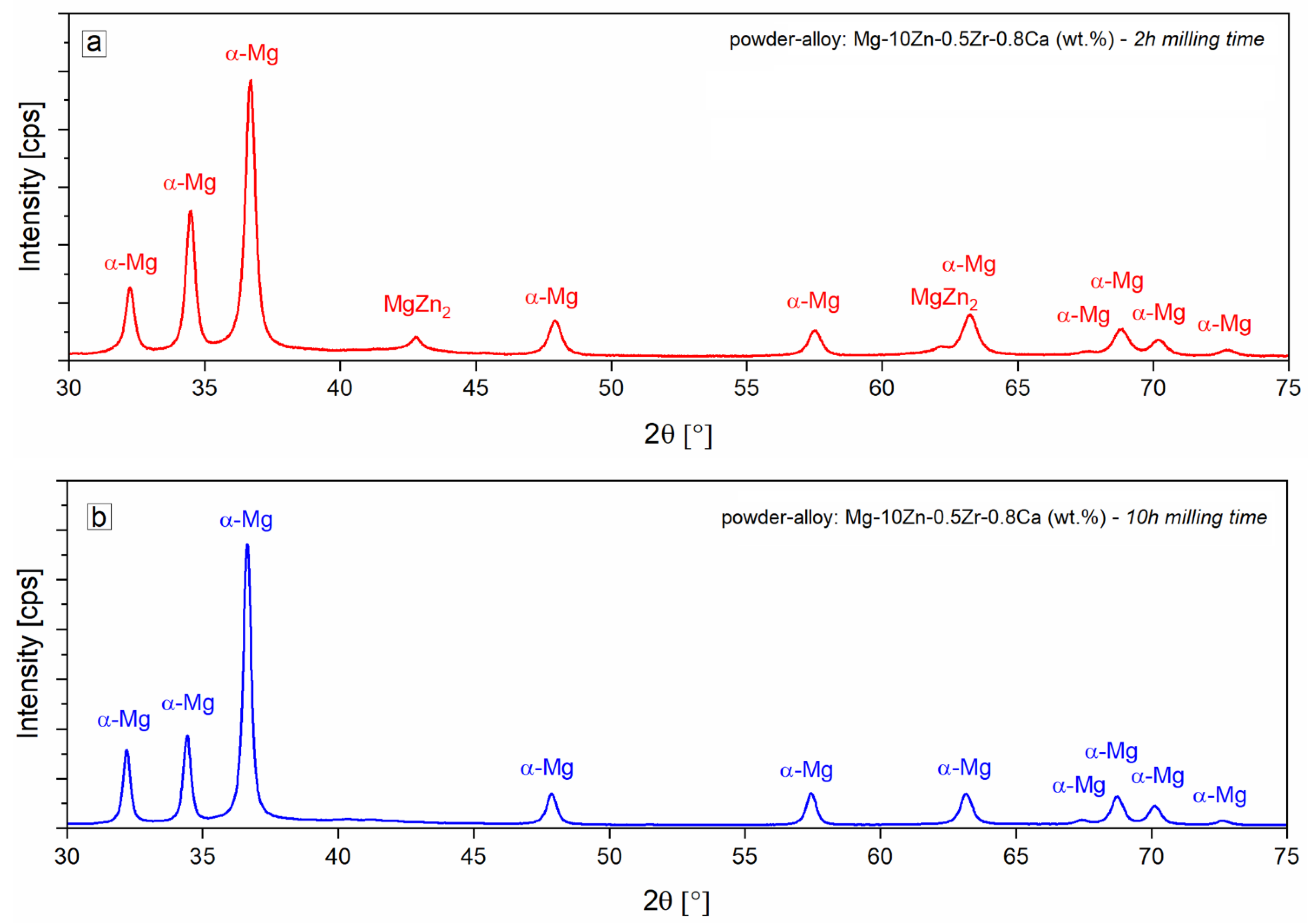





| 2Θ [°] | d [Å] | FWHM, [°] Full Width at Half Maximum of the Peak | Crystallite Size, [Å] | |
|---|---|---|---|---|
| 1 | 32.168 | 2.7804 | 0.301 | 287 |
| 2 | 34.408 | 2.6043 | 0.329 | 264 |
| 3 | 36.621 | 2.4518 | 0.3193 | 273.7 |
| 4 | 40.226 | 2.2401 | 0.49 | 181 |
| 5 | 47.839 | 1.8998 | 0.3961 | 229.1 |
| Milling Time [h] | |||||
|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 10 h | |
| Average particle size [µm] ± Standard Deviation | 74.6 µm ± 22.5 | 24.7 µm ± 18.8 | 16.6 µm ± 10.2 | 18.9 µm ± 12.4 | 16.2 µm ± 9.3 |
| Milling Time | Alloying Elements, [% wt.] | |||
|---|---|---|---|---|
| Mg | Zn | Zr | Ca | |
| 2 h | 89.6 ± 3.0 | 9.3 ± 1.7 | 0.32 ± 0.10 | 0.78 ± 0.20 |
| 4 h | 89.5 ± 3.0 | 9.4 ± 1.6 | 0.36 ± 0.10 | 0.74 ± 0.20 |
| 6 h | 89.1 ± 3.0 | 9.6 ± 1.6 | 0.48 ± 0.10 | 0.82 ± 0.20 |
| 8 h | 88.9 ± 3.0 | 9.8 ± 1.7 | 0.51 ± 0.10 | 0.79 ± 0.20 |
| 10 h | 88.7 ± 3.0 | 10.0 ± 1.7 | 0.51 ± 0.10 | 0.79 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raducanu, D.; Cojocaru, V.D.; Nocivin, A.; Hendea, R.E.; Ivanescu, S.; Stanciu, D.; Trisca-Rusu, C.; Serban, N.; Drob, S.I.; Campian, R.S. Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy. Crystals 2022, 12, 1641. https://doi.org/10.3390/cryst12111641
Raducanu D, Cojocaru VD, Nocivin A, Hendea RE, Ivanescu S, Stanciu D, Trisca-Rusu C, Serban N, Drob SI, Campian RS. Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy. Crystals. 2022; 12(11):1641. https://doi.org/10.3390/cryst12111641
Chicago/Turabian StyleRaducanu, Doina, Vasile Danut Cojocaru, Anna Nocivin, Radu Emil Hendea, Steliana Ivanescu, Doina Stanciu, Corneliu Trisca-Rusu, Nicolae Serban, Silviu Iulian Drob, and Radu Septimiu Campian. 2022. "Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy" Crystals 12, no. 11: 1641. https://doi.org/10.3390/cryst12111641
APA StyleRaducanu, D., Cojocaru, V. D., Nocivin, A., Hendea, R. E., Ivanescu, S., Stanciu, D., Trisca-Rusu, C., Serban, N., Drob, S. I., & Campian, R. S. (2022). Microstructure Evolution during Mechanical Alloying of a Biodegradable Magnesium Alloy. Crystals, 12(11), 1641. https://doi.org/10.3390/cryst12111641









