1. Introduction
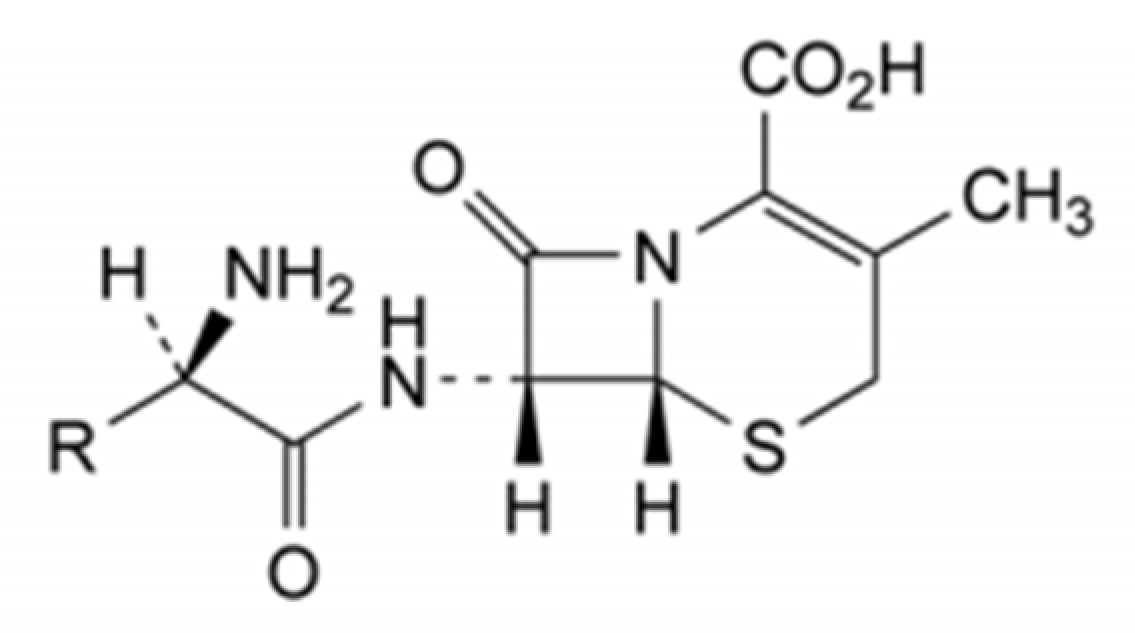
Cephalexin is a broad-spectrum antibiotic, which can achieve bactericidal effect by inhibiting the synthesis of cell wall. It is a semi synthetic cephalosporin with the largest clinical use at present. For sustainable development, it is of great significance to improve its production process with regard to environmental protection, cost reduction, and quality control. Molecular structure and molecular information for cefradin, cephalexin and cefadroxil are showed in
Figure 1 and
Table 1.
Industrially, the production of cephalexin includes cefalexin acylation synthesis and recovery of cephalexin mother liquor. The nucleus 7-amino deacetoxy cephalosporin acid (7-ADCA) is always used as the raw material, with acylation reaction of the side chain phenylglycine (PG) to obtain cephalexin. The acylation process includes chemical method and enzymatic catalysis method. At present, the enzyme catalyzed synthesis is frequently used for industrial production [
1,
2]. The recovery of cephalexin mother liquor can also be divided into complexation method and enzyme-catalyzed hydrolysis method, as seen in
Figure 1. Due to the immature enzyme-catalyzed hydrolysis technology, complex method is still dominant in industrial production at present. The complexation method of the cephalexin mother liquor involves using 2-naphthol etc., to complex the cephalexin in the crystallization mother liquor, precipitating the cephalexin complex, and then decomplexing to prepare an aqueous cephalexin solution, which is applied to the cephalexin preparation process. This process involves not only carcinogens such as complexing agent naphthol [
3,
4,
5,
6,
7,
8,
9,
10], but also various organic solvents such as methanol and dichloromethane [
3,
4,
5,
6,
7,
8,
9]. A large amount of volatile organic compounds (VOC) was produced in the production process, causing great environmental pollution.
Due to the requirements of sustainable development, it is urgent to improve the enzymatic hydrolysis technology to recover 7-aminodeacetoxycephalosporin acid (7-ADCA) and phenylglycine from the cephalexin mother liquor, and 7-ADCA was reused as a raw material for the preparation of cephalexin. Preparation process of cephalexin of enzymatic catalysis method and complex method are shown in
Figure 2.
Penicillin acyltransferase (PGA) can be also used in cephalexin-catalyzed hydrolysis technology. PGA has been used in the preparation of antibiotic mother nucleus 6-aminopenicillin acid (6-APA) as early as the 1960s [
11,
12,
13,
14], and 7-ADCA in the 1970s [
15].
Regarding the enzymatic preparation of 7-ADCA, Nys, P S. et al. have studied the kinetics of 7-phenylacetaminodeacetoxycephalosporin acid (7-PADCA) catalyzed by immobilized penicillinase in 1980 [
16]. In 2000, Velasco, J. et al. used deacetoxycephalosporin C (DAOC) deacylation to prepare 7-ADCA [
17]. Deacylation was carried out through two final enzyme biotransformation catalyzed by D-amino acid oxidase (DAO) and glutaryl acylase (GLA). In 2002, Schroen, CGPH et al. used immobilized enzymes to hydrolyze hexadyl 7-ADCA to prepare 7-ADCA [
18]. However, there are a few reports on the use of cephalexin mother liquor as the raw material, and how to recover high-quality 7-ADCA is still a key technical problem to be solved urgently. This study is first to investigate the thermodynamics and kinetics of the hydrolysis reaction catalyzed by cephalexin.
PGA used in the cephalexin catalyzed hydrolysis in this study was derived from the penicillin acyltransferase of Escherichia coli ATCC11105, and was obtained through mutation screening. The enzyme-catalyzed hydrolysis reaction activity after mutation is higher, the rate is faster, the enzyme conversion rate is higher, the substrate concentration tolerance is stronger, the residue is less, and the operation process is robust.
Crystallization is a key process for separation and purification of drugs, which is also widely used in the production of bulk commercial chemicals [
19].
The hydrolysate process is catalyzed by cephalexin containing 7-ADCA and PG. There is a large difference between solubility of 7-ADCA and PG at pH 6–9 [
20,
21]. Because the solubility of PG is much lower than that of 7-ADCA, in the mixture of 7-ADCA and PG, PG can be first removed by crystallization through pH adjustment. Then 7-ADCA can be obtained by crystallization through additional pH adjustment.
In order to design an efficient crystallization process of 7-ADCA and PG with low cephalexin residues, the influence factors on the hydrolysis of 7-ADCA and PG are necessary to be systematically investigated at different pH and solubility, as well as catalyzation by the cephalexin. These are particularly important to obtain 7-ADCA crystal with good morphology and high purity.
For thermodynamics, Jauregi, P et al. reported the solubility of PG at different pH in 2002 [
20]. In order to unify the measurement conditions, the solubility of 7-ADCA and PG at different temperatures and pH was determined, and the temperature-dependent models were studied using the Apelblat equation. In addition, Tsuji equation and Nezhad equation were used to study the pH-dependent model, and Akaike Information Criterion (AIC) was used to analyze and compare the fitting results of the two models.
For process kinetics, reaction rates at different substrate concentrations were measured. The Lineweaver–Burk double reciprocal equation was used to show correlations. The Michaelis constant Km and the maximum reaction rate Vmax were obtained. The hydrolysis reactions of cefradine and cefadroxil were also studied. In the study of the influencing factors of the hydrolysis reaction catalyzed by cephalexin, the effects of different additives, particle size of immobilized enzyme, methanol concentration, and organic solvent on the hydrolysis reaction rate of cephalexin were investigated.
Finally, based on the results of thermodynamics and kinetics of the hydrolysis reaction catalyzed by cephalexin, the process of preparing 7-ADCA by hydrolysis catalyzed by cephalexin was optimized. The obtained 7-ADCA crystals were characterized by XRD, thermal analysis, infrared characterization, SEM, and the purity of 7-ADCA crystals was determined by HPLC. The results proved the high-quality of 7-ADCA crystals.
2. Materials and Methods
2.1. Experimental Materials
Cephalexin (429112204023, content 100.5%, moisture 6.0%%), cefradine (229022205011, content 96.9%, moisture 3.9%%), cefadroxil (D1132205018, content 99.6%, moisture 4.9%), 7-ADCA (10002207021, content 99.0%) provided by North China Pharmaceutical Co. Ltd. (Shijiazhuang, China), PG (B220318042, content 99.82%) provided by Hebei Huaxu Chemical Co. Ltd. (Hebei, China), both concentrated sulfuric acid and concentrated ammonia are analytically pure and purchased from Shijiazhuang reagent factory; purified water is secondary purified water made in the laboratory; the immobilized penicillin acylase (470 U/g, wet weight, at pH8.0 and 28 °C) was donated to Hunan Flag Biotechnology Co., Ltd. (Hunan, China)
Instrument: HPLC: Agilent 1260; pH meter: DELTA 320, METTLER TOLEDO Instruments Co., Ltd. (Greifensee, Switzerland); ME204E electronic analytical balance, METTLER TOLEDO Instruments Co., Ltd. (Greifensee, Switzerland).
HPLC analysis: Substrates and products were identified and analyzed by HPLC (Agilent 1260) and a UV detector (220 nm) with a ODS C18 column (4.6 × 250 nm, 5 μm). For the analysis of cephalexin hydrolysis reactions, the eluent composed of water, methanol, 3.86% sodium acetate solution, 4% acetic acid solution (3710:1200:75:15, v/v) was used. In the case of cephalexin hydrolysis reaction, the flow rate of the eluent was 1.0 mL/min, and 0.2 mL reaction solution was carefully removed with a tube, diluted to 50 mL with the eluent, and then 20 μL was injected. The temperature of the column was 298 K.
2.2. Experimental Set-Up and Process
2.2.1. Experimental Set-Up
The experimental set-up with two peristatic pumps, reaction bottle, electric mixer is shown in
Figure 3.
2.2.2. Experimental Process
Solubility Determination of 7-ADCA and Phenylglycine
A total of 100 mL purified water was added into a 250 mL four-necked flask, and the solubility of 7-ADCA and phenylglycine was determined at different pH and temperature.
The solubility of 7-ADCA and phenylglycine was determined and the solution was observed by the laser monitoring technology. The pre-weighted solute (7-ADCA or phenylglycine) was dissolved in 100 mL of deionized water in a 250 mL flask with stirring at 300 rpm. The solution was turbid due to the undissolved solute. A thermostat was used to maintain a constant temperature. By adding 20% ammonia (or 20% sulfuric acid) into the solution, the pH changed, and the solute dissolved, becoming salt form in the solution. When the laser light intensity increased to the maximum value (about 92%), the system was considered to reach solid–liquid equilibrium and the pH of the system was recorded. Measurements were repeated three times.
The molar fractional solubility of 7-ADCA and phenylglycine can be calculated from the following equation:
where
mA,
mB, and
mC represent the mass of 7-ADCA (or phenylglycine), water, ammonia (or sulfuric acid),
MA,
MB, and
MC represent the molar mass of 7-ADCA (or phenylglycine), water, ammonia (or sulfuric acid), respectively [
22,
23].
Kinetic Determination of Cephalexin
Experiment 1: Determination and comparison of Km and Vmax of the cephalexin, cefradine, and cefadroxil.
A total of 100 mL of cephalosporin solution was added into a 250 mL four-necked flask with mixing at 30 °C and pH 8.0. Immobilized penicillin acylase was added with stirring for 5 min, and then immobilized penicillin acylase was separated, 0.2 mL was sampled to 50 mL volumetric flask, mobile phase was added to dilute to 50 mL, the residual concentration of cephalosporin was determined by HPLC, and the reaction rate was estimated [
16,
18].
Experiment 2: The effect of different additives, different methanol dosage, different particle size enzymes, and different alcohol solvents on the reaction rate of the cephalexin.
A total of 100 mL of cephalosporin solution was added into a 250 mL four-necked flask, and 0.5 g phenylacetic acid, 0.5 g phenylglycine, and 0.5 g 7-ADCA were added respectively, with mixing at 30 °C and pH 8.0. Immobilized penicillin acylase was added with stirring for 5 min, immobilized penicillin acylase was separated, and 0.2 mL was sampled to 50 mL volumetric flask, then mobile phase was added to dilute to 50 mL. The residual concentration of cephalosporin was determined by HPLC, and the effect of additives on the reaction speed was investigated [
16,
18].
A total of 50 mL of pH 8.0 phosphate buffer was added into a 150 mL beaker, and different amounts of cephalexin was added to adjust pH to 8.0 [
24]. The solution was poured into a 100 mL volumetric flask, and the volume of pH 8.0 phosphate buffer was fixed at 100 mL. Then, it was transferred to a four port reaction flask with a volume of 250 mL at 30 °C and pH 8.0. Immobilized penicillin acylase was added with stirring for 5 min to investigate the reaction rate [
16,
18].
About 50 mL of purified water was added into a 150 mL beaker. 10%, 20%, 30%, and 40% of methanol concentration (
v/
v, total volume of cephalexin solution after adjust pH 8.0) was added, respectively. About 1 g cephalexin was added to adjust the pH to 8.0, and it was poured into a 100 mL volumetric flask, the volume was diluted to 100 mL, and then it was transferred to a four port reaction flask with a volume of 250 mL at 30 °C and pH 8.0. Immobilized penicillin acylase was added with stirring for 5 min to investigate the reaction rate [
14].
A total of 100 mL cephalosporin solution was added into a 250 mL four-necked flask, and immobilized penicillin acylase was analyzed and the reaction rate was investigated with different particle sizes (100 μm, 300 μm) [
16]. About 50 mL purified water was added into a 150 mL beaker, and 20 mL of methanol, ethanol, isopropanol, N-butanol, ethylene glycol (EG), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF), and 1,4-dioxane were added respectively. About 1 g cephalexin was added to adjust the pH to 8.0, and it was poured into a 100 mL volumetric flask to dilute the volume to 100 mL, then it was moved to a four port reaction flask with a volume of 250 mL at 30 °C and pH 8.0. Immobilized penicillin acylase was added with stirring for 5 min to investigate the reaction rate [
14].
Preparation of 7-ADCA by Cephalexin Enzyme Hydrolysis
About 150 mL cephalosporin solution was added into a 250 mL four-necked flask with mixing at 30 °C and pH 8.0. Immobilized penicillin acylase was added with stirring to the end. The immobilized penicillin acylase was added to adjust the pH of the lysate to 6.0, and ethanol was added to adjust the pH to 5.0. For obtaining the crystal, phenylglycine was filtered and removed, and then the pH was adjusted to 4.0 to obtain the crystal, and 7-ADCA product was filtered [
16,
18].
3. Results and Discussion
3.1. Thermodynamics Research
3.1.1. Determination of Solubility of 7-ADCA and Phenylglycine
Cephalexin is completely converted to 7-ADCA and phenylglycine after hydrolysis, so the thermodynamic behavior of 7-ADCA and phenylglycine is the basis of separation and purification of feed liquid after hydrolysis. The solubility of 7-ADCA and phenylglycine measured in this section under different pH and temperature is shown in
Tables S1 and S2 and
Figure 4. The solubility of phenylglycine and 7-ADCA varies greatly when pH > 6.5, and the solubility of phenylglycine also decreases with the decrease of temperature. In addition, Felix, IMB.et al. reported the solubility of phenylglycine in ethanol–water mixed solvent, and found that the solubility of phenylglycine decreased gradually with the increase of ethanol content [
25]. Therefore, temperature, pH, and solvent composition have a great influence on the solubility of 7-ADCA and phenylglycine.
In order to obtain the relationship between solubility and temperature and pH more intuitively, three-dimensional graphs of the solubility of 7-ADCA and phenylglycine as a function of temperature and pH were drawn, respectively. The results are shown in the
Figure 4.
3.1.2. Solubility–Temperature Correlation Model
The Apelblat equation, proposed by Apelblat, is a semi-empirical model widely used to correlate the solubility data of the same solvent composition with temperature [
26,
27,
28,
29], and is calculated with Equation (1):
In the Equation (2),
xA is the molar solubility of the solute;
A,
B, and
C are the model parameters. The Apelblat equation was used to fit the experimental data, and the values of parameters
A,
B, and
C were obtained by regression, and the root mean square deviation (RMSD) was used to describe the fitting effect.
In the Equation (3), xie and xic are the experimental and fitting values, respectively; N is the number of experimental points.
It can be seen from
Table 2 and
Table 3 that the maximum root means square deviation obtained by the Apelblat equation fitting is 8.6498 × 10
−5, and the consistency between the obtained solubility value and the experimental value is greater than 0.9, indicating that the fitting effect is good.
3.1.3. Solubility-pH Correlation Model
- (1)
Tsuji equation
In the Equation (4),
xA is the molar fraction solubility of the solute;
x0 is the inherent molar fraction solubility of the solute;
pK1 and
pK2 are the negative logarithms of the dissociation constant, that is,
pKi =
−lgKi [
30];
- (2)
Nezhad equation
The Equation (5) is an ordinary differential equation representing the influence of pH variations on the ampholyte solubilities in water at a given pressure and temperature [
31].
Table 4,
Table 5,
Table 6 and
Table 7 show that the Tsuji equation and the Nezhad equation have good fitting effects on the solubility data of 7-ADCA and phenylglycine in pure water at different temperatures and pH conditions. Among them, the Tsuji equation has a better fitting effect than the Nezhad equation.
3.1.4. Akaike Information Criterion (AIC) Analysis
Akaike Information Criterion (AIC) was proposed by Japanese scholar Hiroshi (Akaike) in the study of information theory. It is an effective method to measure the quality of the model by taking into account the accuracy and practicability of the model. Thus, the AIC is used to compare the applicability of thermodynamic models to the solubility data in the solvent to find the best applicable model. In general, the smaller the
AIC value, the better the thermodynamic model fits the experimental data [
32]. The Akaike Information Criterion (AIC) can be calculated as:
where
L(
θ)—maximum likelihood function values for thermodynamic models;
k—the number of independent parameters of the thermodynamic model.
The above equation can be simplified to:
where
N—number of experimental values; RMSD—root mean square deviation of experimental values.
In order to further evaluate the experimental results, Akaike weight (
ωi) was introduced to evaluate the fitting effect of the thermodynamic equation. In general, the larger the value of
ωi, the better the fitting effect of the thermodynamic model.
where
n—the number of thermodynamic models to be compared; AIC
min—the minimum AIC value in the thermodynamic model; AIC
i—AIC value of the this model.
The sum of the AIC results for the correlated solubility and temperature model is shown in
Table 8 and
Table 9.
Since there is only the Apelblat model for the correlation solubility and temperature model, the optimal applicable model cannot be screened.
From the analysis results of the Akaike Information Criterion (AIC), it can be seen that the fitting results of the Tsuji equation are better than the Nezhad equation, indicating that the Tsuji equation is more suitable for the analysis of the solubility data of 7-ADCA and phenylglycine. In the analysis process of Akaike weight (ωi), from the calculation results of the ωi value of the 7-ADCA correlation solubility and pH model, the Nezhad equation generally has a large ωi value. From the calculation results of the ωi value, the calculation results of the two equations are similar. Taken together, the Tsuji equation is more applicable.
3.2. Kinetic Determination
3.2.1. Determination and Comparison of Km and Vmax of Cephalexin, Cefradine, and Cefadroxil
Substrate solutions of different concentrations was prepared, immobilized penicillin acylase was added, and the initial reaction rate was determined.
Table 14 shows the determination results.
Take
1/[S] and
1/V as abscissa and ordinate respectively, and use Lineweaver Burk double reciprocal equation to draw the graph, as shown in
Figure 5. Its longitudinal intercept is
Vmax−1, and the slope is
Km/
Vmax. According to
Figure 5,
Km and
Vmax of cephalexin are obtained. In addition, the kinetics of enzyme lysis reaction of cefradine and cefadroxil was also determined, and the double reciprocal equation is also shown in
Table S3 and
Figure 5, and the results of
Km/
Vmax are shown in
Figure 6.
Michaelis constant
Km represents the affinity of enzyme to substrate. The smaller the value is, the greater the affinity of enzyme to substrate becomes.
Figure 6 of the experimental results shows that the order of the affinity of the enzyme for cephalexin, cefradine, and cefadroxil is: cefadroxil > cephalexin > cefradin. It is also predicted that the amount of enzyme used for the hydrolysis of cefadroxil will be larger than cefadroxil and smaller than cephalosporin.
3.2.2. Effect of Different Additives on the Reaction Rate of Cephalexin
The effect of additives on the reaction rate was investigated by adding 0.5 g phenylacetic acid, 0.5 g phenylglycine, 7-ADCA 0.5 g, different doses of methanol or phosphate buffer respectively. In addition, immobilized penicillin acylase with different particle sizes (100 μm, 300 μm) were added to investigate the reaction rate. The results are shown in
Table S5 and
Figure 7.
It can be seen that phenylglycine 0.5 g, 7-ADCA 0.5 g, and phosphate buffer are beneficial to the reaction rate due to the positive and negative ion buffering effect of amphoteric compounds and buffer, while phenylacetic acid inhibits the enzyme hydrolysis reaction rate due to its competitive effect with the reaction substrate. In addition, the particle size of the enzyme, due to the diffusion limitation of reaction substrate and output, the immobilized enzyme with large particles is not conducive to accelerating the reaction speed, and the immobilized enzyme with small particles should be selected as far as possible under the condition of ensuring the removal of enzyme by filtration.
3.2.3. Effect of Different Doses of Methanol on the Reaction Rate of Cephalexin
Generally, organic solvents can inactivate the enzyme, and methanol is more prominent. The investigation results of reaction rate of different doses of methanol (volume percentage concentration) are shown in
Table S6 and
Figure 8A.
As shown in
Table S6 and
Figure 8A, the reaction rate of cephalexin without organic solvent was 0.36 mg/(mL·min
−1). When 10% of methanol was added, the reaction rate was increased by 22.22%. But when 20%, 30%, or 40% of methanol was added, the reaction rate was decreased by 2.78%, 50.00%, and 83.33%, respectively. With the increase of concentration of methanol, the enzyme cracking reaction rate became slower, indicating that the low concentration of methanol was conducive to the enzyme cracking reaction, while the high concentration of methanol was not conducive to the enzyme cracking reaction.
3.2.4. Effect of Different Organic Solvents on the Reaction Rate of Cephalexin
Different organic solvents resulted in different reaction rates for cephalexin hydrolysis. The investigation results are shown in
Table S7 and
Figure 8B.
Table S7 and
Figure 8B of the experimental results show that different organic solvents have different effects on the cleavage reaction rate of cephalexin, and the effect on the cleavage reaction rate of cephalexin is ethanol > isopropanol > THF > DMSO > EG > 1,4-dioxane > methanol. The reaction rate is 0.36 mg/mL min
−1 when no organic solvent is added during the cleavage of cephalexin. After adding ethanol, isopropanol, THF, DMSO, EG, 1,4-dioxane, methanol, the reaction rate decreased by 63.89%, 58.33%, 47.22%, 25%, 16.67%, and 2.78% respectively.
3.3. Research on 7-ADCA Separation and Purification Process
Cephalexin enzymatic catalytic hydrolysis technology consists of two steps: adding purified water to enzymatic hydrolysis cephalexin and adjusting the pH value to the isoelectric point of 7-ADCA and then the 7-ADCA can crystallize and precipitate. However, due to the high concentration in the system, phenylglycine also can crystallize and precipitate together. The presence of a large amount of phenylglycine will affect the hydrolysis process and the purity of 7-ADCA. The recovery method of cephalexin crystallization mother liquor developed in this study adopts the antisolvent crystallization/cooling crystallization method, giving priority to crystallization and effective separation of phenylglycine from the cracked feed liquor, and then adjusting the pH of the purified feed liquor to isoelectric point crystallization to obtain high-quality 7-ADCA.
Based on the thermodynamic basic data of 7-ADCA and phenylglycine, it was found that the thermodynamic behavior and solubility characteristics of 7-ADCA and phenylglycine are different in the hydrolysis liquid system with different pH and different alcohol solvent composition. The solubility of 7-ADCA increased rapidly around pH 6, while phenylglycine only increased after pH 9–10. After pH > 6.5, the solubility of 7-ADCA and phenylglycine was significantly different. 7-ADCA and PG were obtained by enzymatic hydrolysis of cephalexin; adjust the pH of the feed liquid to 6.0–7.0 and precipitate a small amount of phenylglycine. At the same time, add alcohol solvent to reduce the system temperature, which further promotes the crystallization and precipitation of phenylglycine in the hydrolysis liquid, while 7-ADCA is not precipitated. Therefore, phenylglycine in the hydrolysis liquid system is highly separated. Finally, adjust the pH to the isoelectric point of 7-ADCA around pH 4.0, and then crystallize 7-ADCA with high purity in high yield. Thus, two high-quality products are obtained respectively, and cephalexin is prepared by returning to the previous synthesis reaction step to improve the overall yield of the process.
A series of process parameters were screened for the separation of phenylglycine and the purification of 7-ADCA (
Table 15). The results showed that the products obtained by each process route had higher purity and yield than those of the original enzymatic pyrolysis process without phenylglycine separation. Therefore, the proposed process route of preferential removal of phenylglycine by antisolvent and/or cooling crystallization is reasonable and effective [
21].
Figure 9 shows the typical examples of PXRD, thermal analysis, infrared, electron microscope, and high-performance liquid chromatography (HPLC) of 7-ADCA products obtained in this study. The results showed that the product obtained by this process was confirmed to be 7-ADCA by PXRD, thermal analysis, and infrared characterization, and phenylglycine was not detected.
As shown in PXRD, thermal analysis, and infrared spectroscopy, the PXRD and infrared spectra of the recovered 7-ADCA are basically consistent with those of the genuine 7-ADCA, and the pyrolysis of 7-ADCA begins at 475–500 K. As shown in SEM, the recovered 7-ADCA and 7-ADCA genuine products are rectangular sheets, and the recovered products are slightly smaller than the genuine products. The purity of 7-ADCA product is >99%, the content of impurity phenylglycine is <0.5%, there is no 7-ADCA isomer, and the yield is about 90%, which meets the technical requirements.
4. Conclusions
The ecological environment is critical to human health, and all technologies for environmental improvement are important such as the enzyme catalysis technology, which is a green technology in the preparation of antibiotic.
In this work, the thermodynamic and kinetic parameters involved in the preparation of 7-ADCA by enzymatic hydrolysis of cephalexin were investigated. The solubility of 7-ADCA and phenylglycine tank in pure water at different temperatures and pH was determined. The correlation model of solubility and temperature was fitted by the Apelblat equation. The consistency between the fitted solubility value and the experimental value was above 0.9.
The correlation model of solubility and pH was studied, showing that the Tsuji equation had a better fitting effect than the Nezhad equation. The results of Akaike Information Criterion (AIC) analysis also show that the Tsuji equation had a better fitting effect than the Nezhad equation.
The Michaelis constant Km and the maximum reaction rate of enzymatic hydrolysis of cephalexin, cefradine, and cefadroxil were determined. The experimental results showed that the affinity of the enzyme followed the order: cefadroxil > cephalexin > cefradine, predicting that the amount of enzyme used for pyrolysis would increase. There were the positive and negative ion buffering effect of amphoteric compounds and buffers, accelerating the reaction speed. Low concentration of methanol was beneficial to the reaction, while high concentration of methanol inhibited the reaction. In addition, ethanol, isopropanol, THF, DMSO, EG, 1,4 -dioxane all had different degrees of inhibition on the reaction rate.
Finally, based on thermodynamic and kinetic studies, a process technology for the preparation of 7-ADCA by hydrolysis catalyzed of cephalexin was developed. It was confirmed that the proposed process route of preferential removal of phenylglycine by antisolvent and/or cooling crystallization was effective. The 7-ADCA crystal products were confirmed by PXRD, thermal analysis, infrared, electron microscope, and high-performance liquid chromatography (HPLC).