Variation in Gemological Characteristics in Tsavorites with Different Tones from East Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. X-ray Fluorescence
2.3. Ultraviolet–Visible Spectroscopy
2.4. Infrared Spectroscopy and Raman Spectroscopy
2.5. Colorimetric Analysis
2.6. CIE1976 L*a*b* Color System
3. Results and Discussion
3.1. Gemological and Mineralogical Characteristics
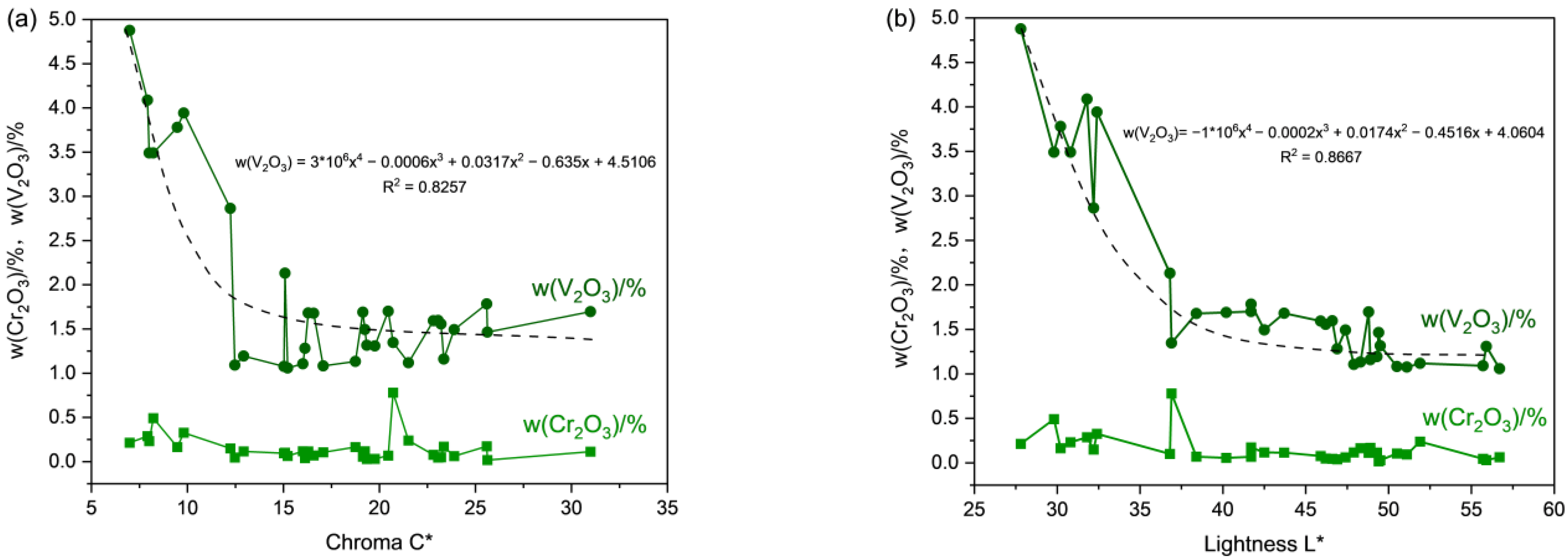
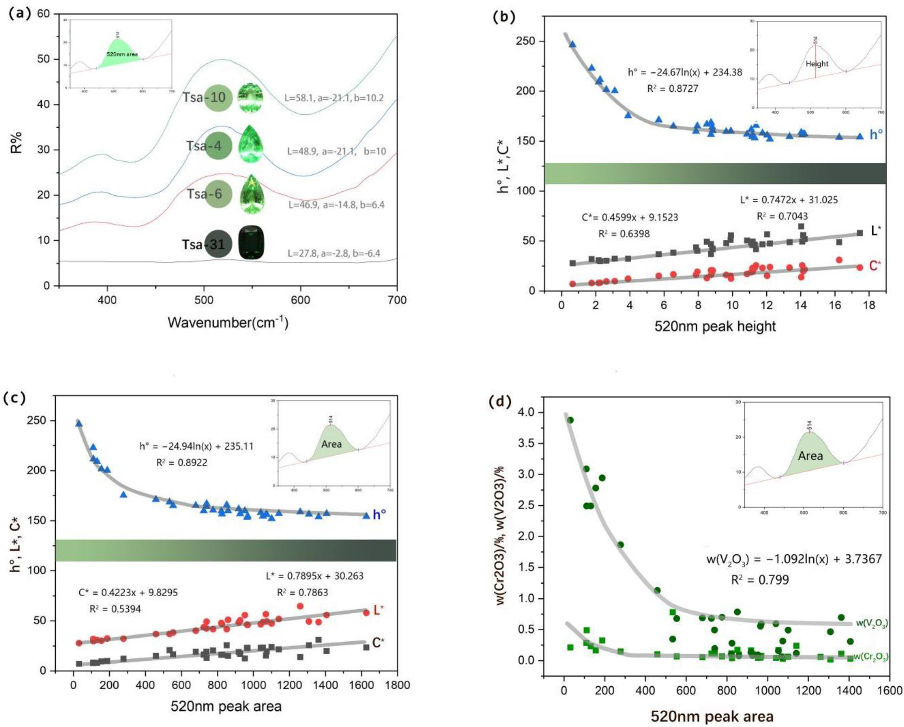
3.2. Color Quantification and Classification
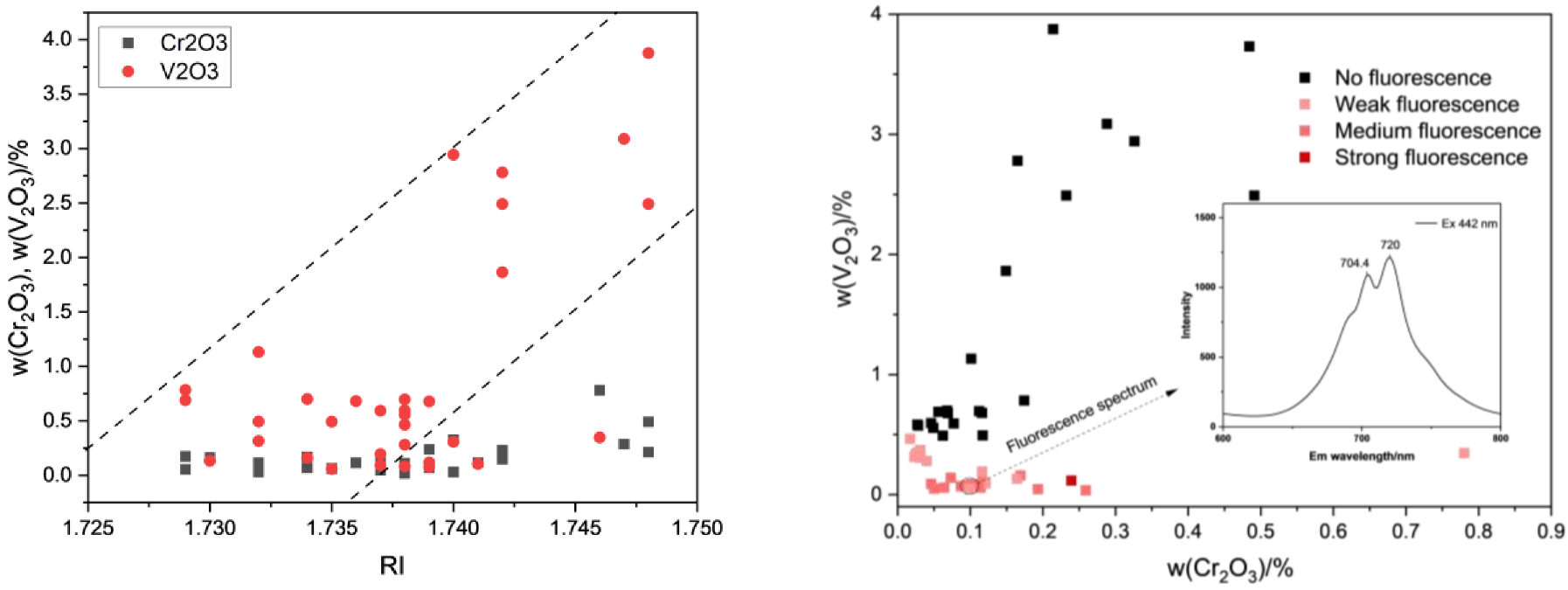
3.3. Chemical Composition Analysis
3.4. UV-VIS Spectrum Analysis
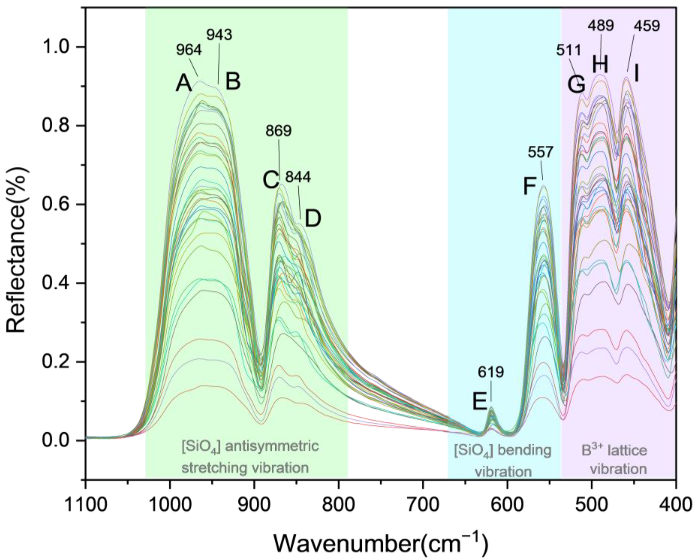
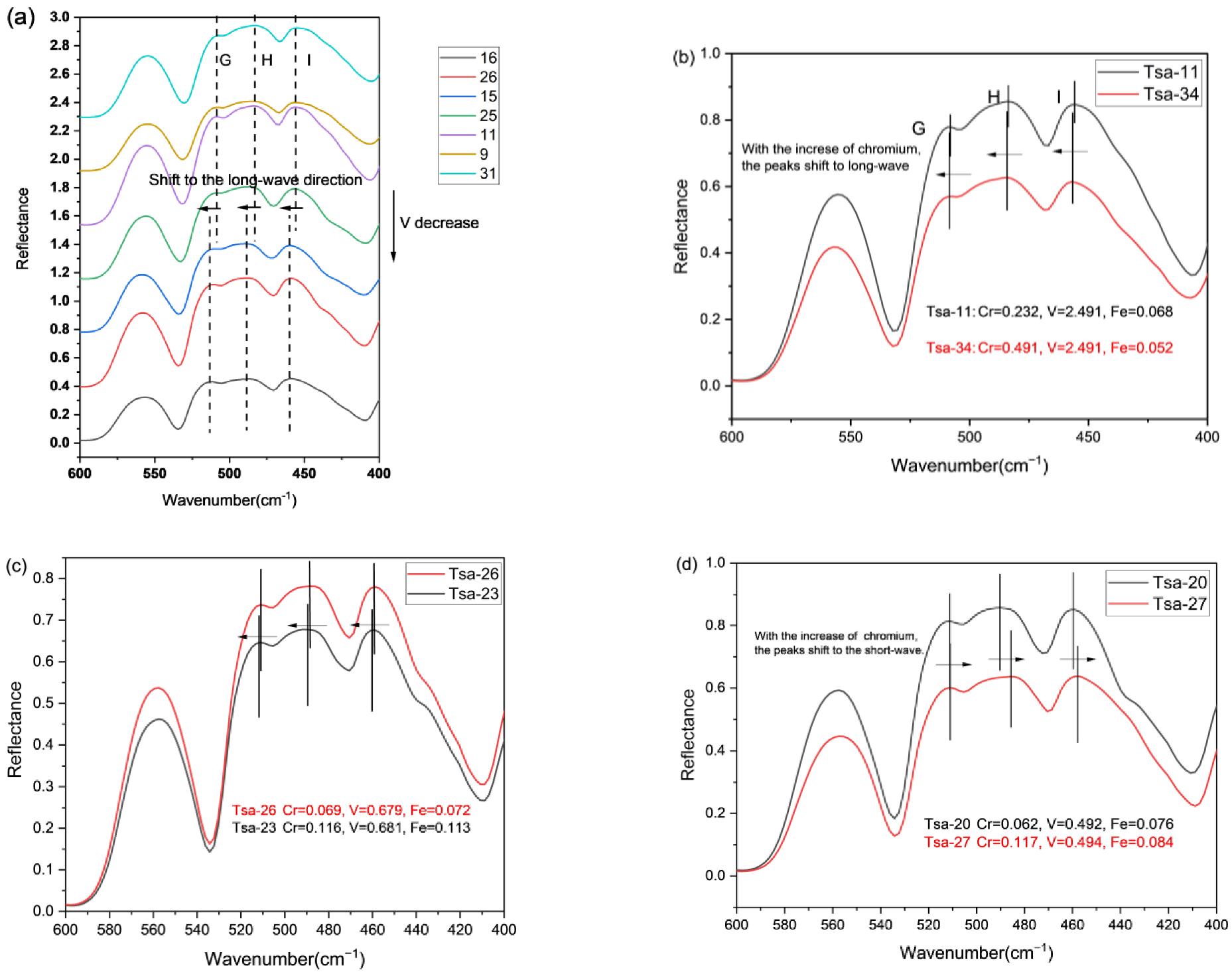
3.5. Infrared Spectroscopy
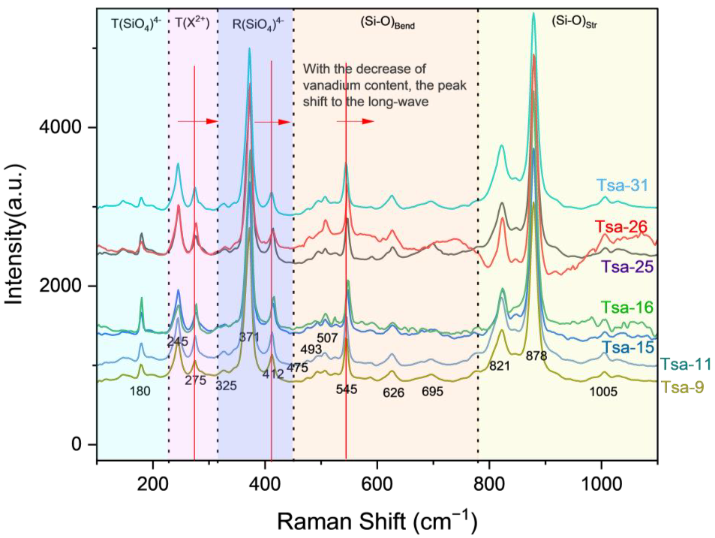
3.6. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Le Goff, E.; Malisa, E.P.J.; Saul, M.; Saul, E.; Saul, J.M.; Pardieu, V. Lithostratigraphic and structural controls of ‘tsavorite’ deposits at Lemshuku, Merelani area, Tanzania. C. R. Geosci. 2010, 342, 778–785. [Google Scholar] [CrossRef]
- Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Saul, M.; Saul, E.; Saul, J.M. Le district minier à ‘tsavorite’ de Lemshuku, Tanzanie. Rev. Gemmol. A. F. G. 2010, 172, 11–22. [Google Scholar]
- Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Galoisy, L.; Pardieu, V. Is the V/Cr ratio a fingerprint of the geographical origin of ‘tsavorite’ in the Mozambique Belt? In Proceedings of the General Meeting of the International Mineralogical Association: Acta Mineralogica Petrographica, Budapest, Hungary, 21–27 August 2010; p. 776. [Google Scholar]
- Switzer, G.S. Composition of green garnet from Tanzania and Keny. Gems Gemol. 1974, 14, 296–297. [Google Scholar]
- Pardieu, V. Tsavorite: Une pierre africaine. Rev. Gemmol. A.F.G. 2005, 154, 8–11. [Google Scholar]
- Pardieu, V.; Hughes, R.W. Tsavorite—The untamed beauty. Incolour 2008, 9, 12–20. [Google Scholar]
- Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Martelat, J.E.; Monié, P.; Dubessy, J.; Rollion-Bard, C.; Le Goff, E.; Malisa, E.; et al. New aspects and perspectives on tsavorite deposits. Ore Geol. Rev. 2013, 53, 1–25. [Google Scholar] [CrossRef]
- Pohl, W.; Niedermayr, G. Geology of the Mwatate Quadrangle and the Vanadium Grossularite Deposits of the area. Geol. Surv. Kenya 1979, 101, 55. [Google Scholar]
- Gubelin, E.J.; Weibel, M. Gruner vanadium grossular, Kenya. Lapis 1997, 2, 17–21. [Google Scholar]
- Shackleton, R.M. The final collision zone between East and West Gondwana: Where is it? J. Afr. Earth Sci. 1996, 23, 271–287. [Google Scholar] [CrossRef]
- Meert, J.G. A synopsis of events related to the assembly of eastern Gondwana. Tectonophysics 2003, 362, 1–40. [Google Scholar] [CrossRef]
- Stern, R.J. Arc assembly and continental collision in the Neoproterozoic East African Orogen: Implications for the consolidation of Gondwanaland. Annu. Rev. Earth Planet. Sci. 1994, 22, 319–351. [Google Scholar] [CrossRef]
- Jöns, N.; Schenk, V. Relics of the Mozambique Ocean in the central East African Orogen: Evidence from the Vohibory Block of Southern Madagascar. J. Metamorph. Geol. 2008, 26, 17–28. [Google Scholar] [CrossRef]
- Hauzenberger, C.A.; Bauernhofer, A.H.; Hoinkes, G.; Wallbrecher, E.; Mathu, E.M. PanAfrican high pressure granulites from SE-Kenya: Petrological and geothermobarometric evidence for a polycyclic evolution in the Mozambique belt. J. Afr. Earth Sci. 2004, 40, 245–268. [Google Scholar] [CrossRef]
- Hauzenberger, C.A.; Sommer, H.; Fritz, H.; Bauernhofer, A.; Kröner, A.; Hoinkes, G.; Wallbrecher, E.; Thöni, M. SHRIMP U–Pb zircon and Sm–Nd garnet ages from the granulite-facies basement of SE Kenya: Evidence for Neoproterozoic polycyclic assembly of the Mozambique Belt. J. Geol. Soc. 2007, 164, 189–201. [Google Scholar] [CrossRef]
- Malisa, E. Geology of the Tanzanite Gemstone Deposits in the Lelatema Area, NE Tanzania. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 1987; 146p. [Google Scholar]
- Suwa, K.; Suzuki, K.; Agata, T. Vanadium grossular from the Mozambique metamorphic rocks, south Kenya. J. Southeast Asian Earth Sci. 1996, 14, 299–308. [Google Scholar] [CrossRef]
- Olivier, B. The Geology and Petrology of the Merelani Tanzanite Deposit, NE Tanzania. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2006; 352p. [Google Scholar]
- Giuliani, G.; Ohnenstetter, D.; Palhol, F.; Feneyrol, J.; Boutroy, E.; De Boissezon, H.; Lhomme, T. Karelianite and vanadian phlogopite from the Merelani Hills gem zoisite deposits, Tanzania. Can. Mineral. 2008, 46, 1183–1194. [Google Scholar] [CrossRef]
- Department of Inorganic Chemistry, Beijing Normal University. Inorganic Chemistry; High Education Press: Beijing, China, 2010. [Google Scholar]
- Tang, J.; Guo, Y.; Xu, C. Metameric efects on peridot by changing background colour. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2019, 36, 2030–2039. [Google Scholar] [CrossRef]
- Guo, Y. Quality evaluation of tourmaline red based on uniform colour space. Clust. Comput. 2017, 20, 3393–3408. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Du, H. The foundation of a colour-chip evaluation system of jadeite-jade green with colour diference control of medical device. Multimed. Tools Appl. 2016, 75, 14491–14502. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Li, X.; Dong, S. Metamerism appreciation of jadeite-jade green under the standard light sources D65, A and CWF. Acta Geol. Sin.-Engl. Ed. 2016, 90, 2097–2103. [Google Scholar] [CrossRef]
- Guo, Y. Quality grading system of Jadeite-Jade green based on three colourimetric parameters under CIE standard light sources D65, CWF and A. Bulg. Chem. Commun. 2017, 49, 961–968. [Google Scholar]
- Guo, Y.; Zong, X.; Qi, M. Feasibility study on quality evaluation of Jadeite-jade colour green based on GemDialogue colour chip. Multimed. Tools Appl. 2019, 78, 841–856. [Google Scholar] [CrossRef]
- Guo, Y.; Zong, X.; Qi, M.; Zhang, Y.; Wang, H. Feasibility study on colour evaluation of jadeite based on GemDialogue colour chip images. EURASIP J. Image Video Process. 2018, 2018, 95. [Google Scholar] [CrossRef]
- Cheng, R.; Guo, Y. Study on the effect of heat treatment on amethyst color and the cause of coloration. Sci. Rep. 2020, 10, 14927. [Google Scholar] [CrossRef]
- King, J.; Moses, T.; Shigley, J.; Liu, Y. Colour grading of coloured diamonds in the GIA Gem Trade Laboratory. Gems Gemol. 1994, 30, 220–242. [Google Scholar] [CrossRef]
- Schiffer, V.V. Spectroscopy, Luminescence and Radiation Center in Minerals; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979. [Google Scholar]
- Li, L.P.; Ye, D. Role of Cr and V in colour change effect of gemstones. Gems Gemol. 2003, 5, 17–21, (In Chinese with English abstract). [Google Scholar]
- Zhao, S. The Gemology Characteristics of Green Garnet. Master’s Thesis, China University of Geosciences, Beijing, China, 2019. [Google Scholar]
- Hofmeister, A.M.; Fagan, T.J.; Campbell, K.M.; Schaal, R.B. Single-crystal IR spectroscopy of pyrope-almandine garnets with minor amounts of Mn and Ca. Am. Mineral. 1996, 81, 418–428. [Google Scholar] [CrossRef]
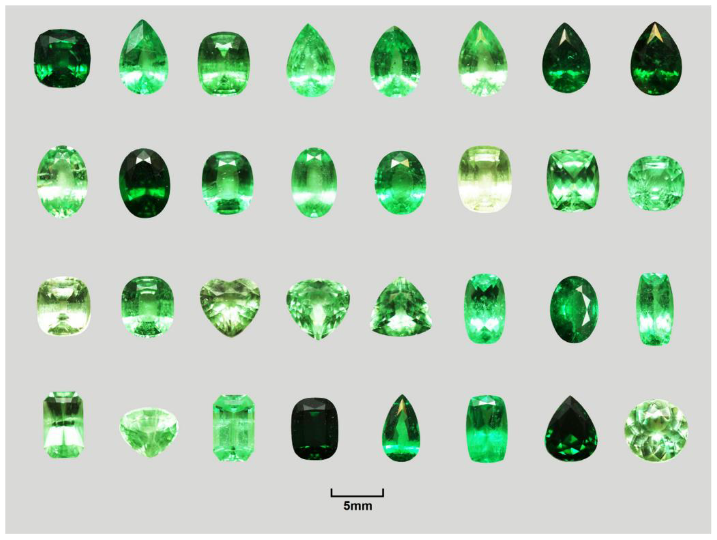




| Properties | Observation Data |
|---|---|
| Color | Light green to dark green |
| Diaphaneity | Transparent to opaque |
| RI | 1.729–1.748 |
| SG | 3.55–3.68 |
| Fluorescence reaction | Long-wave UV: Inert to red fluorescence Short-wave UV: Inert to light orange fluorescence |
| Clustering Number | 3 | 4 | 5 | 6 | 7 |
| Error number | 1 | 2 | 1 | 0 | 2 |
| Accuracy | 97.14 | 94.29 | 97.14 | 100 | 94.29 |
| [SiO4]4- Internal Vibration | Lattice Vibration | |||||||
|---|---|---|---|---|---|---|---|---|
| ν3(antisymmetric stretching vibration) | ν4, ν2(bending vibration) | Related to B3+(trivalent cation) vibration | ||||||
| A | B | C | D | E | F | G | H | I |
| 964 ± 5 cm−1 | 943 ± 3 cm−1 | 869 ± 3 cm−1 | 844 ± 3 cm−1 | 619 ± 1 cm−1 | 557 ± 2 cm−1 | 511 ± 1 cm−1 | 489 ± 3 cm−1 | 459 ± 3 cm−1 |
| Si-OStr | Si-OBend | R(SiO4)4− | T(X2+) | T(SiO4)4− | ||||
|---|---|---|---|---|---|---|---|---|
| A1g | Eg + F2g | A1g | Eg + F2g | A1g | Eg + F2g | Eg + F2g | Eg + F2g | |
| 878 | 1005, 821 | 545 | 493, 507, 626 | 371 | 412 | 245 | 275 | 180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Guo, Y. Variation in Gemological Characteristics in Tsavorites with Different Tones from East Africa. Crystals 2022, 12, 1677. https://doi.org/10.3390/cryst12111677
Ma Y, Guo Y. Variation in Gemological Characteristics in Tsavorites with Different Tones from East Africa. Crystals. 2022; 12(11):1677. https://doi.org/10.3390/cryst12111677
Chicago/Turabian StyleMa, Yuanmeng, and Ying Guo. 2022. "Variation in Gemological Characteristics in Tsavorites with Different Tones from East Africa" Crystals 12, no. 11: 1677. https://doi.org/10.3390/cryst12111677
APA StyleMa, Y., & Guo, Y. (2022). Variation in Gemological Characteristics in Tsavorites with Different Tones from East Africa. Crystals, 12(11), 1677. https://doi.org/10.3390/cryst12111677







