A New Zero-Dimensional (CsK2)BiCl6 Metal Halide: Boosting Emission via B-Site Mn-Doping
Abstract
1. Introduction
2. Result and Discussion
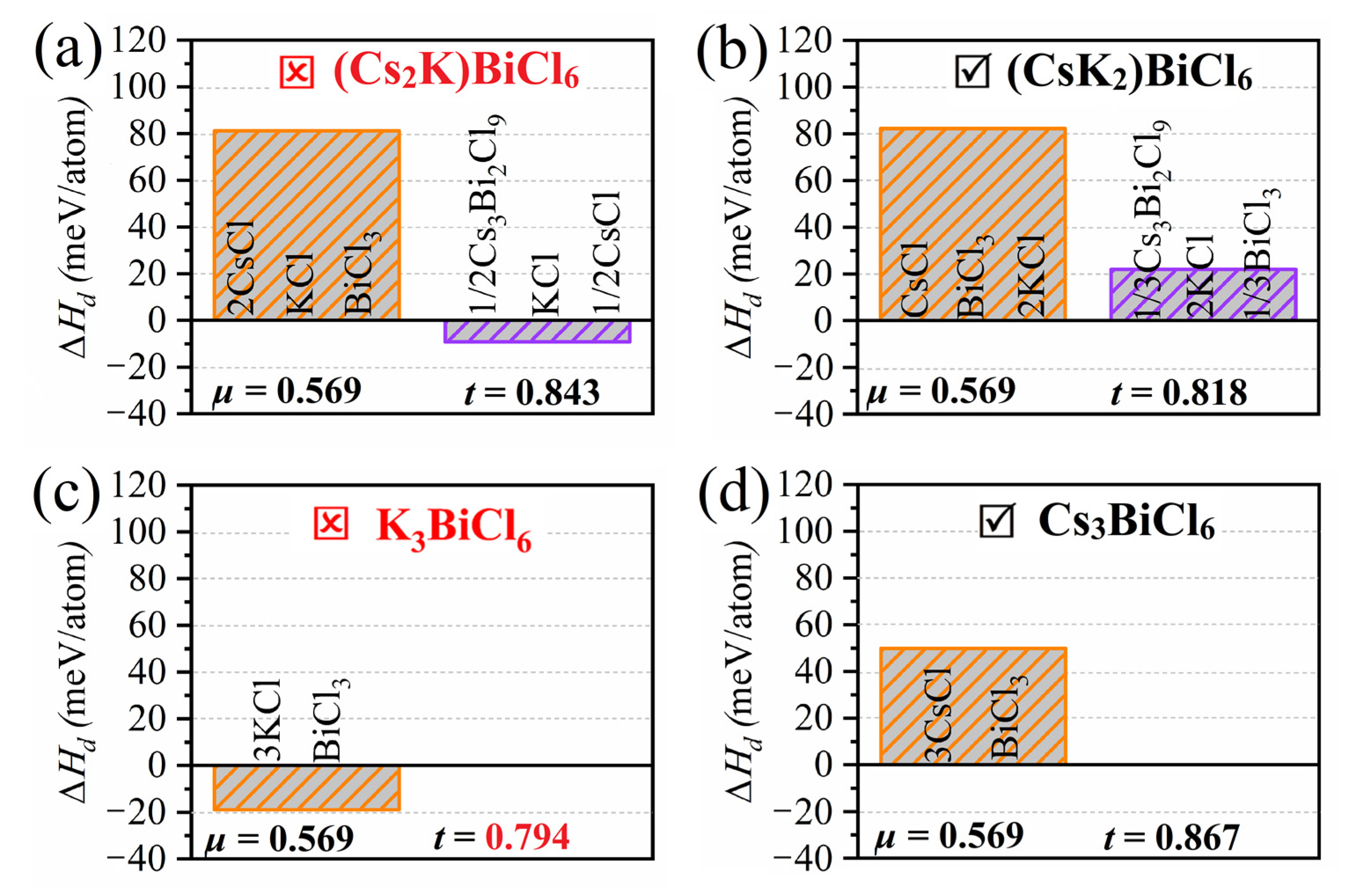
2.1. Theoretical Prediction of Crystallographic and Thermodynamic Stabilities of 0D A3BiCl6
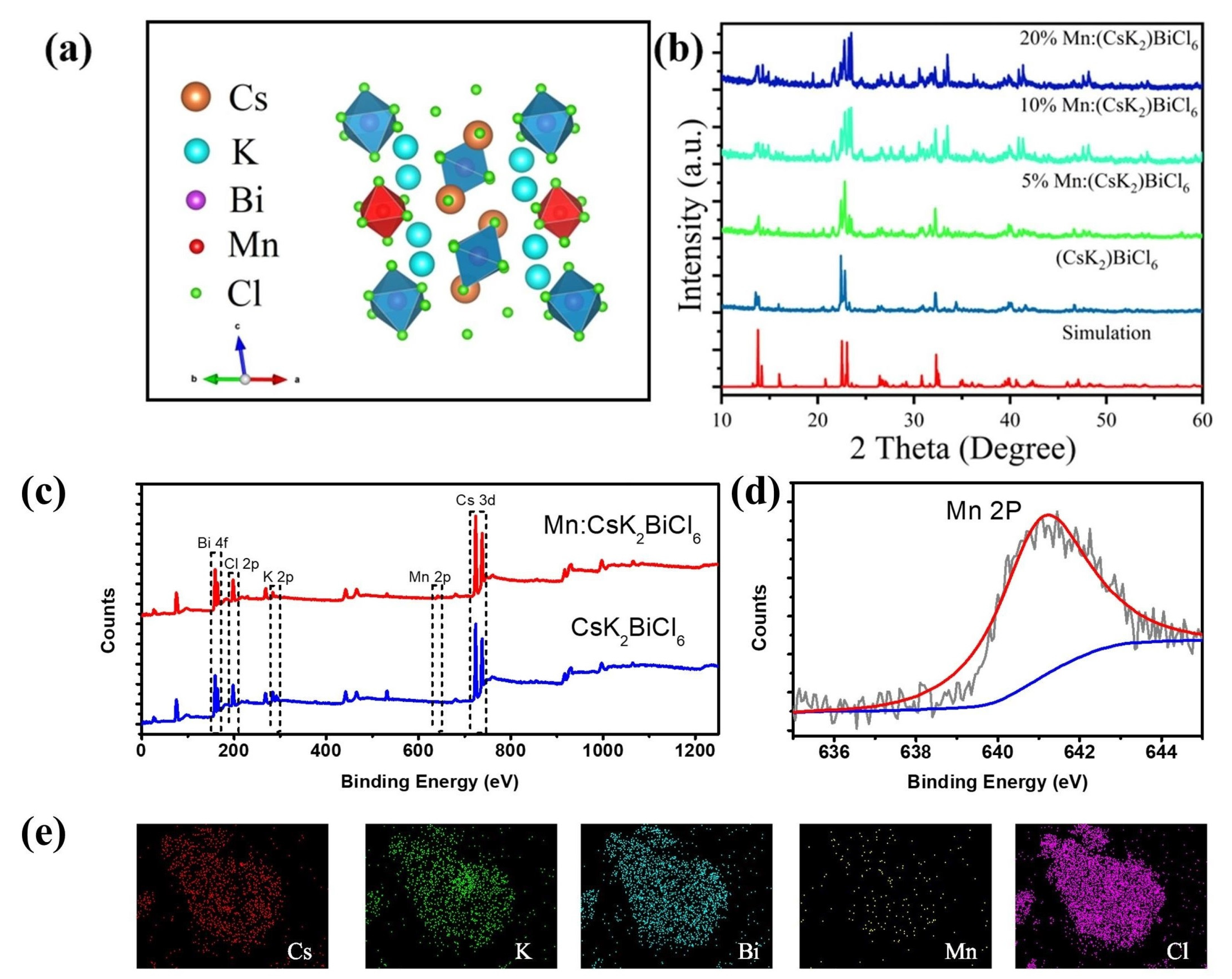
2.2. Electronic Structure of (CsK2)BiCl6
2.3. Experimental Crystal Structure of (CsK2)BiCl6
2.4. Optical Properties of (CsK2)BiCl6
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Cai, T.; Liu, E.; Hills-Kimball, K.; Gao, J.; Chen, O. Synthesis and transformation of zero-dimensional Cs3BiX6 (X = Cl, Br) perovskite-analogue nanocrystals. Nano Res. 2019, 13, 282–291. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Raino, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yan, Q. Recent Advances in Lead Halide Perovskites for Radiation Detectors. Sol. RRL 2019, 4, 210. [Google Scholar] [CrossRef]
- He, T.; Li, S.; Jiang, Y.; Qin, C.; Cui, M.; Qiao, L.; Xu, H.; Yang, J.; Long, R.; Wang, H.; et al. Reduced-dimensional perovskite photovoltaics with homogeneous energy landscape. Nat. Commun. 2020, 11, 1672. [Google Scholar] [CrossRef]
- Li, F.; Huang, S.; Liu, X.; Bai, Z.; Wang, Z.; Xie, H.; Bai, X.; Zhong, H. Highly Stable and Spectrally Tunable Gamma Phase RbxCs1–xPbI3 Gradient-Alloyed Quantum Dots in PMMA Matrix through A Sites Engineering. Adv. Funct. Mater. 2021, 31, 2008211. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, Y.; Wang, S. Controllable printing of large-scale compact perovskite films for flexible photodetectors. Nano Res. 2022, 15, 1547–1553. [Google Scholar] [CrossRef]
- Shynkarenko, Y.; Bodnarchuk, M.I.; Bernasconi, C.; Berezovska, Y.; Verteletskyi, V.; Ochsenbein, S.T.; Kovalenko, M.V. Direct Synthesis of Quaternary Alkylammonium-Capped Perovskite Nanocrystals for Efficient Blue and Green Light-Emitting Diodes. ACS Energy Lett. 2019, 4, 2703–2711. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, M.; Huang, S. Recent advances and perspective on the synthesis and photocatalytic application of metal halide perovskite nanocrystals. Nano Res. 2021, 14, 3773–3794. [Google Scholar] [CrossRef]
- Yuan, M.; Voznyy, O.; Zhitomirsky, D.; Kanjanaboos, P.; Sargent, E.H. Synergistic Doping of Fullerene Electron Transport Layer and Colloidal Quantum Dot Solids Enhances Solar Cell Performance. Adv. Mater. 2015, 27, 917–921. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, K.; Chen, H.; Yi, Y.; Zhang, T.; Long, X.; Li, J.; Zhang, L.; Wang, J.; Yang, S. Cost-efficient clamping solar cells using candle soot for hole extraction from ambipolar perovskites. Energy Environ. Sci. 2014, 7, 3326–3333. [Google Scholar] [CrossRef]
- Zhuang, Q.; Wang, H.; Zhang, C. Ion diffusion-induced double layer doping toward stable and efficient perovskite solar cells. Nano Res. 2022, 15, 5114–5122. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Wang, C.; Sun, M.; Luo, X.; Yang, Y.; Chang, S.; Zhang, D.; Duan, L. Chlorine distribution management for spectrally stable and efficient perovskite blue light-emitting diodes. Nano Energy 2021, 79, 105486. [Google Scholar] [CrossRef]
- Chang, T.; Wang, H.; Gao, Y.; Cao, S.; Zhao, J.; Zou, B.; Zeng, R. Component Engineering to Tailor the Structure and Optical Properties of Sb-Doped Indium-Based Halides. Inorg. Chem. 2022, 61, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Gao, F. Structural and Functional Diversity in Lead-Free Halide Perovskite Materials. Adv. Mater. 2019, 31, e1900326. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Cao, S.; Zhao, J.; Zou, B.; Zeng, R. Advances and Challenges in Two-Dimensional Organic-Inorganic Hybrid Perovskites toward High-Performance Light-Emitting Diodes. Nano-Micro Lett. 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Searching for promising new perovskite-based photovoltaic absorbers: The importance of electronic dimensionality. Mater. Horiz. 2017, 4, 206–216. [Google Scholar] [CrossRef]
- Yu, W.; Sun, X.; Xiao, M.; Hou, T.; Liu, X.; Zheng, B.; Yu, H.; Zhang, M.; Huang, Y.; Hao, X. Recent advances on interface engineering of perovskite solar cells. Nano Res. 2021, 15, 85–103. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Lee, S.J.; Shin, S.S.; Kim, Y.C.; Kim, D.; Ahn, T.K.; Noh, J.H.; Seo, J.; Seok, S.I. Fabrication of Efficient Formamidinium Tin Iodide Perovskite Solar Cells through SnF2-Pyrazine Complex. J. Am. Chem. Soc. 2016, 138, 3974–3977. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, D.; Yu, Y.; Grice, C.R.; Wang, C.; Cimaroli, A.J.; Schulz, P.; Meng, W.; Zhu, K.; Xiong, R.G.; et al. Lead-Free Inverted Planar Formamidinium Tin Triiodide Perovskite Solar Cells Achieving Power Conversion Efficiencies up to 6.22. Adv. Mater. 2016, 28, 9333–9340. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Wang, C.; Bai, Y.; Guo, Q.; Zhao, C.; Zhang, J.; Hu, S.; Hayat, T.; Alsaedi, A.; Tan, Z. Enhancing charge transport in an organic photoactive layer via vertical component engineering for efficient perovskite/organic integrated solar cells. Nanoscale 2019, 11, 4035–4043. [Google Scholar] [CrossRef]
- Fu, S.; Li, X.; Wan, L.; Zhang, W.; Song, W.; Fang, J. Effective Surface Treatment for High-Performance Inverted CsPbI2Br Perovskite Solar Cells with Efficiency of 15.92. Nano-Micro Lett. 2020, 12, 170. [Google Scholar] [CrossRef]
- Huang, J.; Chang, T.; Zeng, R.; Yan, J.; Wei, Q.; Zhou, W.; Cao, S.; Zou, B. Controlled Structural Transformation in Sb-Doped Indium Halides A3InCl6 and A2InCl5∙H2O Yields Reversible Green-to-Yellow Emission Switch. Adv. Opt. Mater. 2021, 9, 2002267. [Google Scholar] [CrossRef]
- Park, M.-H. 3D and 2D Metal Halide Perovskites for Blue Light-Emitting Diodes. Materials 2022, 15, 4571. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Zeng, R.; Zhao, Z.; Wei, Q.; Xue, X.; Bai, K.; Cai, C.; Zhou, W.; Xia, Z.; Zou, B. Homo- and Heterovalent Doping-Mediated Self-Trapped Exciton Emission and Energy Transfer in Mn-Doped Cs2Na1-xAgxBiCl6 Double Perovskites. J. Phys. Chem. Lett. 2020, 11, 340–348. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, L.; Xue, Y.; Ke, B.; Zhao, Z.; Huang, D.; Wei, Q.; Zhou, W.; Zou, B. Highly Efficient Blue Emission from Self-Trapped Excitons in Stable Sb(3+)-Doped Cs2NaInCl6 Double Perovskites. J. Phys. Chem. Lett. 2020, 11, 2053–2061. [Google Scholar] [CrossRef]
- Jin, M.; Zheng, W.; Gong, Z. Unraveling the triplet excited-state dynamics of Bi3+ in vacancy-ordered double perovskite Cs2SnCl6 nanocrystals. Nano Res. 2022, 15, 6422–6429. [Google Scholar] [CrossRef]
- Liao, K.; Li, C.; Xie, L.; Yuan, Y.; Wang, S.; Cao, Z.; Ding, L.; Hao, F. Hot-Casting Large-Grain Perovskite Film for Efficient Solar Cells: Film Formation and Device Performance. Nano-Micro Lett. 2020, 12, 156. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, Y.; Cui, M.; Qiao, L.; Wei, J.; Huang, Y.; Zhang, L.; He, T.; Li, S.; Hsu, H.Y.; et al. High-performance large-area quasi-2D perovskite light-emitting diodes. Nat. Commun. 2021, 12, 2207. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shan, T.; Leng, S.; Zhong, H.; Bao, Q.; Lu, Z.H.; Deng, L.L.; Chen, C.C. Low-Temperature Aging Provides 22% Efficient Bromine-Free and Passivation Layer-Free Planar Perovskite Solar Cells. Nano-Micro Lett. 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, Z.; Molokeev, M.S.; Xiao, Z.; Xia, Z.; Zhang, X.-M. Manipulation of Cl/Br transmutation in zero-dimensional Mn2+-based metal halides toward tunable photoluminescence and thermal quenching behaviors. J. Mater. Chem. C 2021, 9, 2047–2053. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, A.; Zhang, X.; Ou, Q.; Zhang, S. Metal cation substitution of halide perovskite nanocrystals. Nano Res. 2022, 15, 6522–6550. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Wu, J.; Wang, F.; Zhang, T.; Wang, Y.; Liu, D.; Li, S.; Penty, R.V.; White, I.H. Flexible optoelectronic devices based on metal halide perovskites. Nano Res. 2020, 13, 1997–2018. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly Monodisperse Insulator Cs4PbX6 (X = Cl, Br, I) Nanocrystals, Their Mixed Halide Compositions, and Their Transformation into CsPbX3 Nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef]
- Giorgi, G.; Yamashita, K. Zero-Dimensional Hybrid Organic-Inorganic Halide Perovskite Modeling: Insights from First Principles. J. Phys. Chem. Lett. 2016, 7, 888–899. [Google Scholar] [CrossRef]
- Lehner, A.J.; Fabini, D.H.; Evans, H.A.; Hébert, C.-A.; Smock, S.R.; Hu, J.; Wang, H.; Zwanziger, J.W.; Chabinyc, M.L.; Seshadri, R. Crystal and Electronic Structures of Complex Bismuth Iodides A3Bi2I9 (A = K, Rb, Cs) Related to Perovskite: Aiding the Rational Design of Photovoltaics. Chem. Mat. 2015, 27, 7137–7148. [Google Scholar] [CrossRef]
- Öz, S.; Hebig, J.-C.; Jung, E.; Singh, T.; Lepcha, A.; Olthof, S.; Jan, F.; Gao, Y.; German, R.; van Loosdrecht, P.H.M.; et al. Zero-dimensional (CH3NH3)3Bi2I9 perovskite for optoelectronic applications. Sol. Energy Mater. Sol. Cells. 2016, 158, 195–201. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, S.; Wei, Q.; Cao, S.; Zhao, J.; Zou, B.; Zeng, R. Stoichiometry-Controlled Phase Engineering of Cesium Bismuth Halides and Reversible Structure Switch. Adv. Opt. Mater. 2022, 10, 2101406. [Google Scholar] [CrossRef]
- Cheng, X.; Li, R.; Zheng, W.; Tu, D.; Shang, X.; Gong, Z.; Xu, J.; Han, S.; Chen, X. Tailoring the Broadband Emission in All-Inorganic Lead-Free 0D In-Based Halides through Sb3+ Doping. Adv. Opt. Mater. 2021, 9, 2100434. [Google Scholar] [CrossRef]
- Shil, S.K.; Wang, F.; Lai, Z.; Meng, Y.; Wang, Y.; Zhao, D.; Hossain, M.K.; Egbo, K.O.; Wang, Y.; Yu, K.M.; et al. Crystalline all-inorganic lead-free Cs3Sb2I9 perovskite microplates with ultra-fast photoconductive response and robust thermal stability. Nano Res. 2021, 14, 4116–4124. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Deng, H.; Farooq, U.; Yang, X.; Khan, J.; Tang, J.; Song, H. High Quantum Yield Blue Emission from Lead-Free Inorganic Antimony Halide Perovskite Colloidal Quantum Dots. ACS Nano. 2017, 11, 9294–9302. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Li, W.G.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem.-Int. Edit. 2019, 58, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B-Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simpl. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Diao, X.; Diao, Y.; Tang, Y. High-throughput screening of stable and efficient double inorganic halide perovskite materials by DFT. Sci Rep. 2022, 12, 12633. [Google Scholar] [CrossRef]
- Garoufalis, C.S.; Galanakis, I.; Zeng, Z.; Hayrapetyan, D.B.; Baskoutas, S. Structural and Electronic Properties of Small Perovskite Nanoparticles of the Form ABX3 (A = MA, DEA, FA, GA, B = Pb, Sn, X = Cl, Br, I). Electron. Mater. 2021, 2, 382–393. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, J.; Wu, D. Impact of A-Site Cations on Fluorescence Quenching in Organic–Inorganic Hybrid Perovskite Materials. J. Phys. Chem. C. 2021, 125, 11524–11531. [Google Scholar] [CrossRef]
- Han, P.; Luo, C.; Yang, S.; Yang, Y.; Deng, W.; Han, K. All-Inorganic Lead-Free 0D Perovskites by a Doping Strategy to Achieve a PLQY Boost from <2% to 90. Angew. Chem. Int. Ed. Engl. 2020, 59, 12709–12713. [Google Scholar]
- Dai, J.; Ma, L.; Ju, M.; Huang, J.; Zeng, X.C. In- and Ga-based inorganic double perovskites with direct bandgaps for photovoltaic applications. Phys. Chem. Chem. Phys. 2017, 19, 21691–21695. [Google Scholar] [CrossRef] [PubMed]
- Fedorovskiy, A.E.; Drigo, N.A.; Nazeeruddin, M.K. The Role of Goldschmidt’s Tolerance Factor in the Formation of A2BX6 Double Halide Perovskites and its Optimal Range. Small Methods 2019, 4, 1900426. [Google Scholar] [CrossRef]
- Zeng, R.; Bai, K.; Wei, Q.; Chang, T.; Yan, J.; Ke, B.; Huang, J.; Wang, L.; Zhou, W.; Cao, S.; et al. Boosting triplet self-trapped exciton emission in Te(IV)-doped Cs2SnCl6 perovskite variants. Nano Res. 2020, 14, 1551–1558. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, J.; Zhou, W.; Li, A.; Ouyang, F. Direct/indirect band gap tunability in van der Waals heterojunctions based on ternary 2D materials Mo1-xW xY2. J. Phys.-Condes. Matter 2019, 31, 505302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, J.F.; Qin, Y.; Wang, X.D.; Wei, J.H.; Li, M.; Kuang, D.B.; He, R. Activation of Self-Trapped Emission in Stable Bismuth-Halide Perovskite by Suppressing Strong Exciton–Phonon Coupling. Adv. Funct. Mater. 2021, 31, 2102654. [Google Scholar] [CrossRef]
- Li, H.; Pi, C.; Chen, W.; Zhou, M.; Wei, J.; Yi, J.; Song, P.; Alexey, Y.; Zhong, Y.; Yu, X.; et al. A Highly Stable Photodetector Based on a Lead-Free Double Perovskite Operating at Different Temperatures. J. Phys. Chem. Lett. 2021, 12, 5682–5688. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, X.; Wang, Y. Water triggered interfacial synthesis of highly luminescent CsPbX3:Mn2+ quantum dots from nonluminescent quantum dots. Nano Res. 2020, 13, 3387–3395. [Google Scholar] [CrossRef]
- Su, B.; Molokeev, M.S.; Xia, Z. Unveiling Mn2+ Dopant States in Two-Dimensional Halide Perovskite toward Highly Efficient Photoluminescence. J. Phys. Chem. Lett. 2020, 11, 2510–2517. [Google Scholar] [CrossRef]
- Peng, L.; Huang, K.; Zhang, Z.; Zhang, Y.; Shi, Z.; Xie, R.; Yang, W. Bandgap- and Radial-Position-Dependent Mn-Doped Zn–Cu–In–S/ZnS Core/Shell Nanocrystals. ChemPhysChem 2016, 17, 752–758. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhang, S.; Yan, J.; Zou, B.; Zeng, R. A New Zero-Dimensional (CsK2)BiCl6 Metal Halide: Boosting Emission via B-Site Mn-Doping. Crystals 2022, 12, 1681. https://doi.org/10.3390/cryst12111681
Wu J, Zhang S, Yan J, Zou B, Zeng R. A New Zero-Dimensional (CsK2)BiCl6 Metal Halide: Boosting Emission via B-Site Mn-Doping. Crystals. 2022; 12(11):1681. https://doi.org/10.3390/cryst12111681
Chicago/Turabian StyleWu, Jie, Shuai Zhang, Jun Yan, Bingsuo Zou, and Ruosheng Zeng. 2022. "A New Zero-Dimensional (CsK2)BiCl6 Metal Halide: Boosting Emission via B-Site Mn-Doping" Crystals 12, no. 11: 1681. https://doi.org/10.3390/cryst12111681
APA StyleWu, J., Zhang, S., Yan, J., Zou, B., & Zeng, R. (2022). A New Zero-Dimensional (CsK2)BiCl6 Metal Halide: Boosting Emission via B-Site Mn-Doping. Crystals, 12(11), 1681. https://doi.org/10.3390/cryst12111681







