Combustion Synthesis of Metal-Intermetallic-Ceramic Laminate AlMg6-NiAl-TiC Composite
Abstract
1. Introduction
2. Materials and Methods
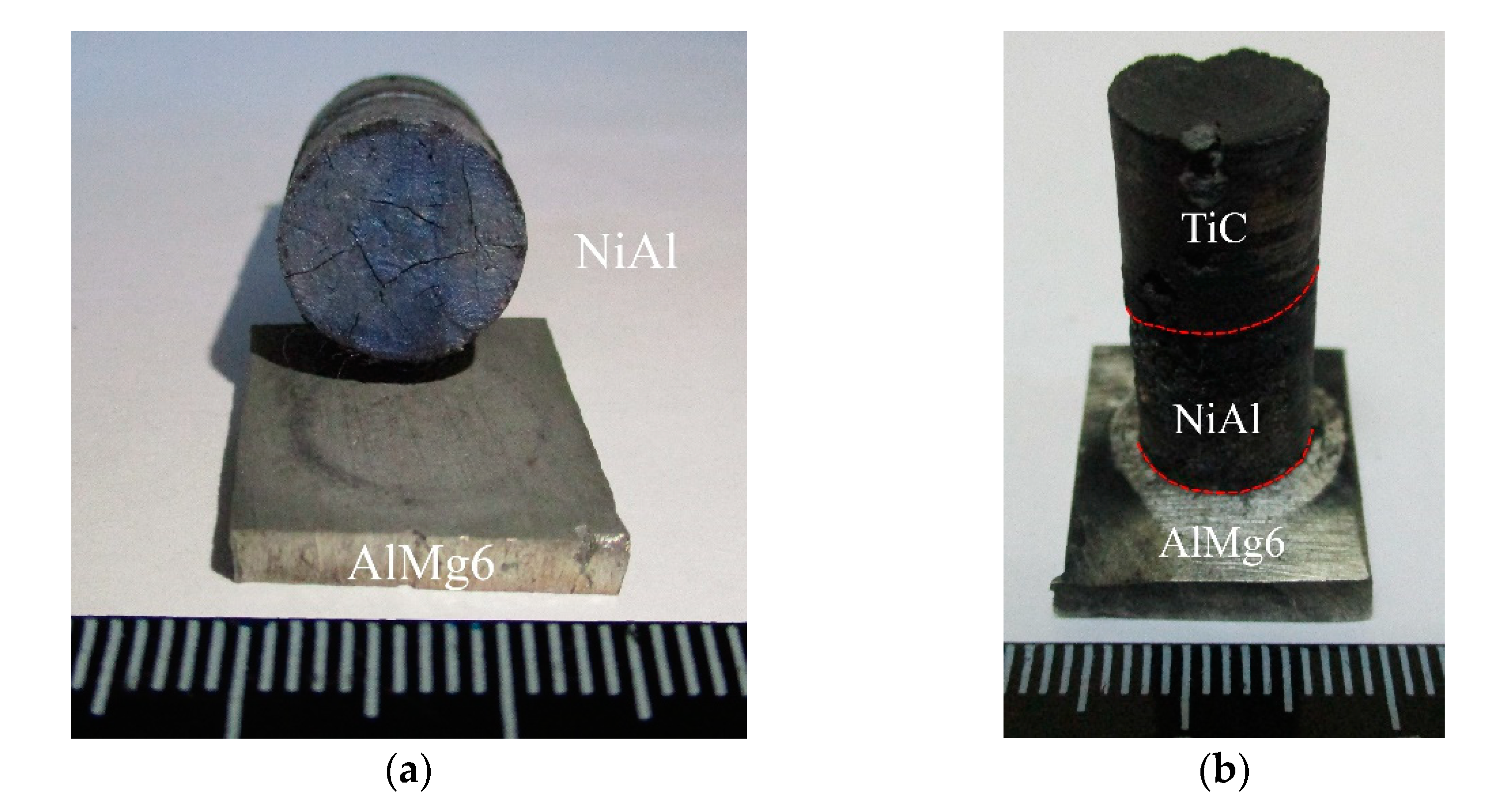
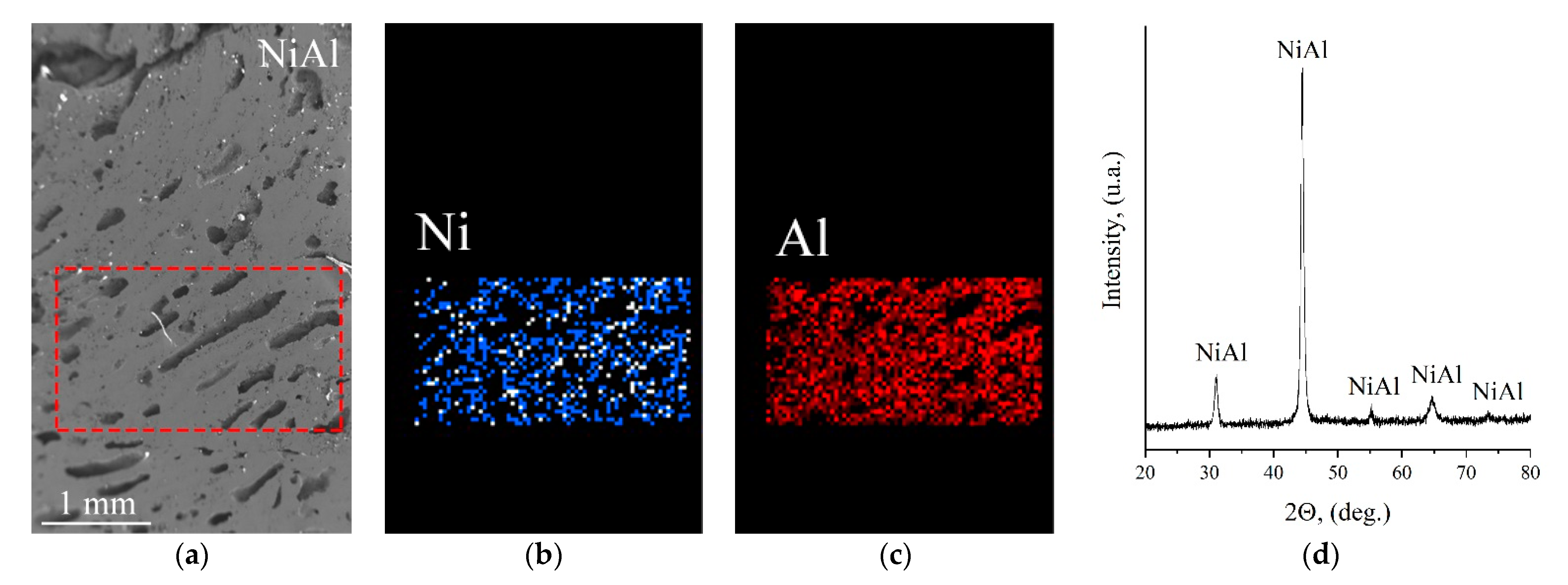
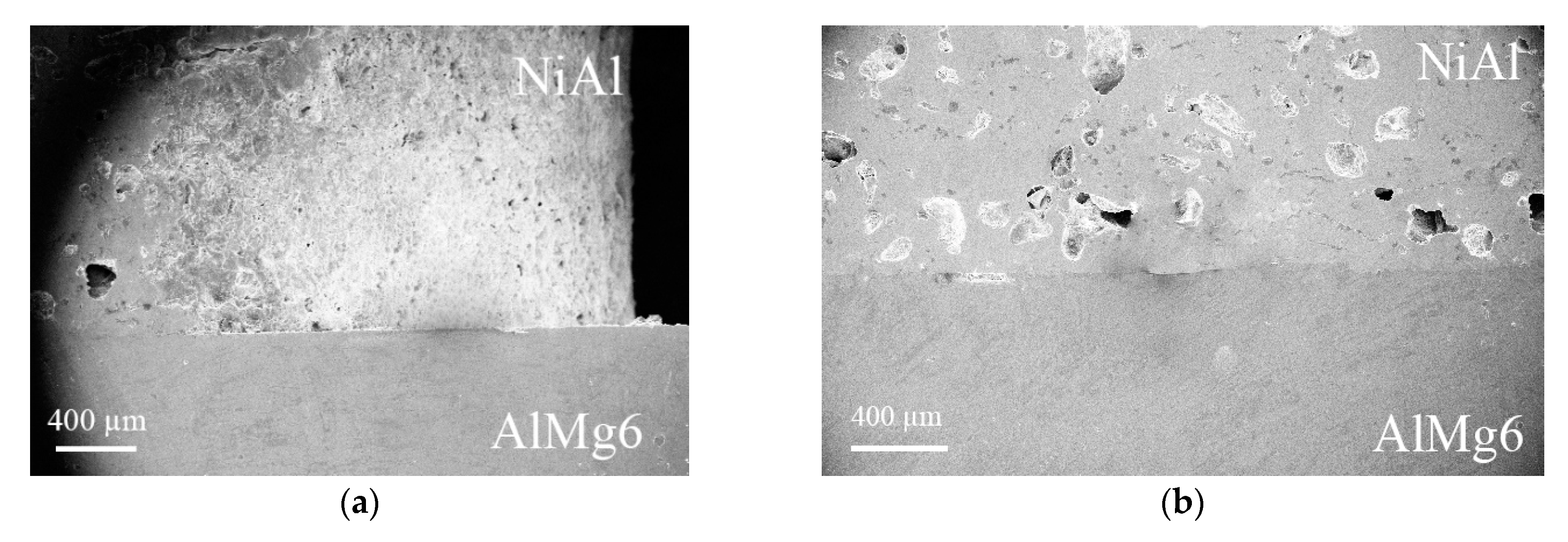
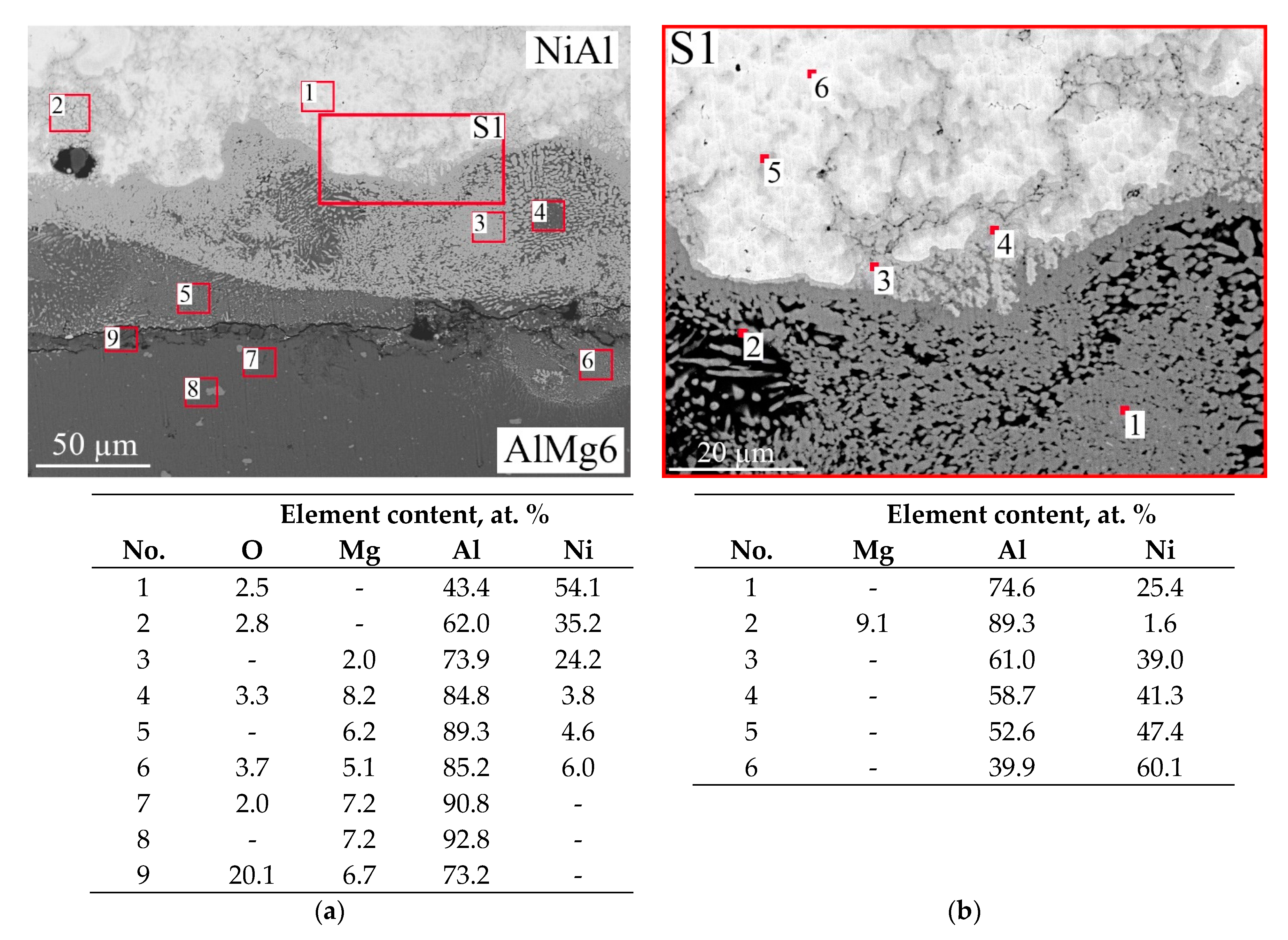
3. Results
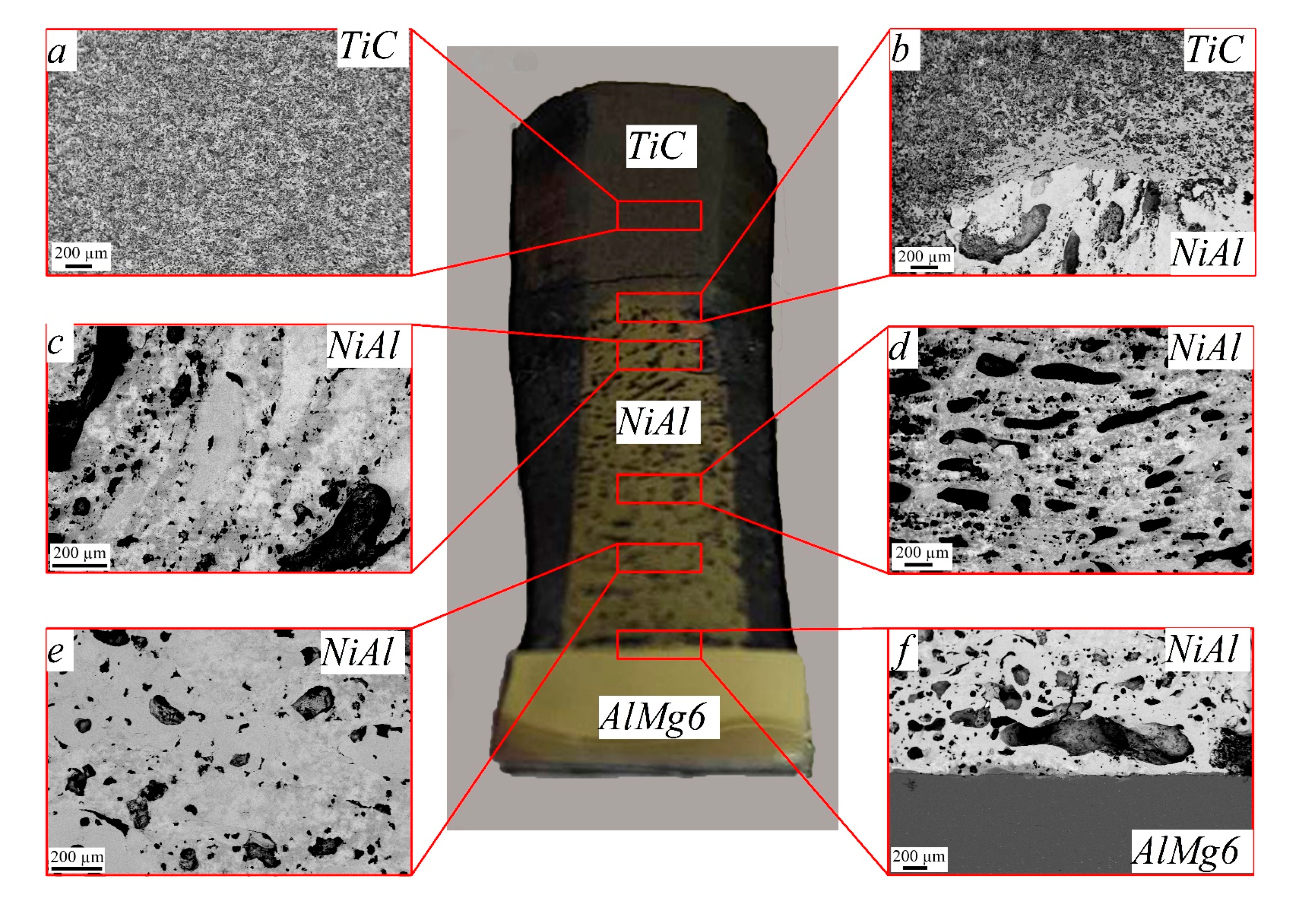
- Ceramic layer of 7-mm-thick TiC (Figure 4a);
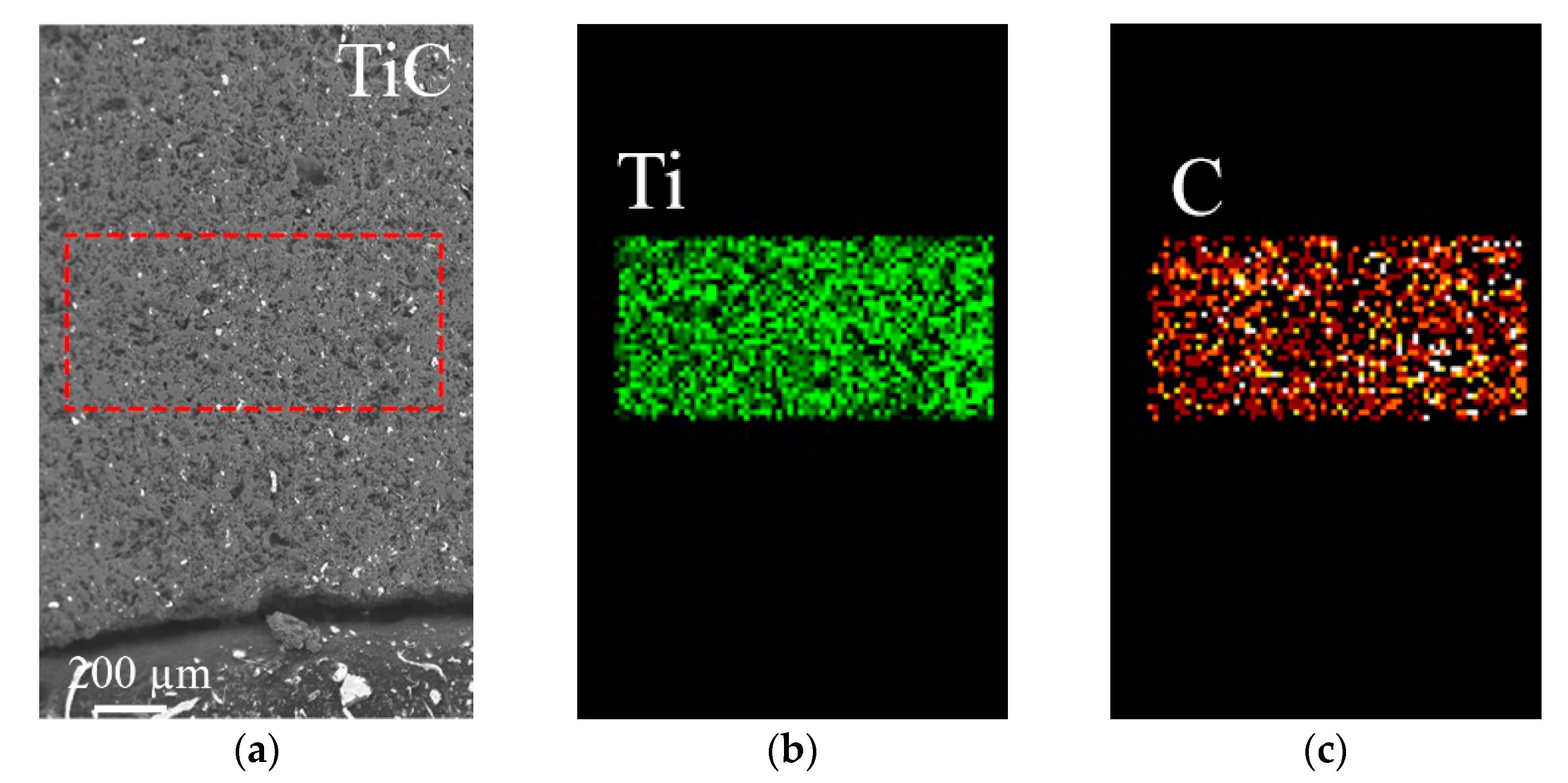
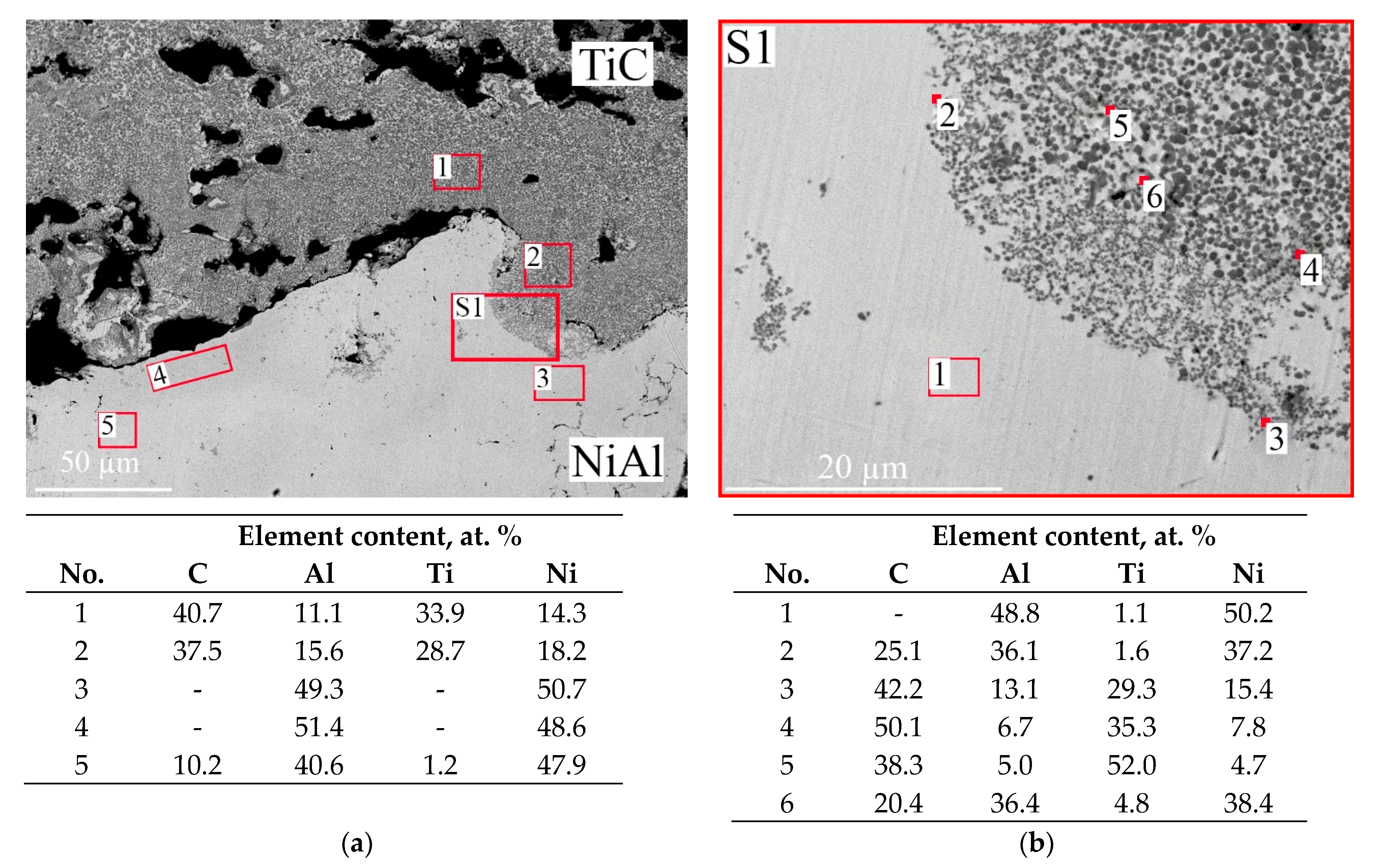
- An upper diffusion layer, which formed at the interface between NiAl and TiC (Figure 4b) and consisted of TiC + NiAl;
- An intermetallic layer, which consisted of 13-mm-thick NiAl (Figure 4c–e);
- A lower diffusion layer, which formed at the interface between NiAl and AlMg6 (Figure 4f);
- A layer of 4-mm-thick aluminum alloy AlMg6 (Figure 4f).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Fan, X.; Huang, W.; Zhou, X.; Zou, B. Preparation and characterization of NiAl–TiC–TiB2 intermetallic matrix composite coatings by atmospheric plasma spraying of SHS powders. Ceram. Int. 2020, 46, 10512–10520. [Google Scholar] [CrossRef]
- Hyjek, P.; Sulima, I.; Malczewski, P.; Bryła, K.; Jaworska, L. Effect of Reactive SPS on the Microstructure and Properties of a Dual-Phase Ni-Al Intermetallic Compound and Ni-Al-TiB2 Composite. Materials 2020, 13, 5668. [Google Scholar] [CrossRef]
- Nosewicz, S.; Jurczak, G.; Wejrzanowski, T.; HajIbrahim, S.; Grabias, A.; Węglewski, W.; Kaszyca, K.; Rojek, J.; Chmielewski, M. Thermal conductivity analysis of porous NiAl materials manufactured by spark plasma sintering: Experimental studies and modelling. Int. J. Heat Mass Transfer 2022, 194, 123070. [Google Scholar] [CrossRef]
- Eakins, D.E.; Thadhani, N.N. Shock compression of reactive powder mixtures. Int. Mater. Rev. 2009, 54, 181–213. [Google Scholar] [CrossRef]
- Ning, H.; Wang, D.; Zhao, J.; Wang, B.; Liu, G. Fabrication and joining of NiAl and TiAl intermetallics by additive sintering. Mater. Sci. Eng. A 2022, 849, 143439. [Google Scholar] [CrossRef]
- Sytschev, A.E.; Vrel, D.; Boyarchenko, O.D.; Roshchupkin, D.V.; Sachkova, N.V. Combustion synthesis in bi-layered (Ti−Al)/(Ni−Al) system. J. Mater. Process. Technol. 2017, 240, 60–67. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyan, A.S. Combustion Synthesis and Nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Leonelli, C. 4—Use of combustion synthesis/self-propagating high- temperature synthesis (SHS) for the joining of similar/dissimilar materials. In Joining Processes for Dissimilar and Advanced Materials; Rakesh, P., Davim, J.P., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 63–79. [Google Scholar] [CrossRef]
- Bazhin, P.M.; Konstantinov, A.S.; Chizhikov, A.P.; Pazniak, A.I.; Kostitsyna, E.V.; Prokopets, A.D.; Stolin, A.M. Laminated cermet composite materials: The main production methods, structural features and properties (review). Ceram. Int. 2021, 47, 1513–1525. [Google Scholar] [CrossRef]
- Lee, H.; Ikenaga, A.; Kim, S.H.; Kim, K.B. The effects of induction heating rate on properties of Ni-Al based intermetallic compound layer coated on ductile cast iron by combustion synthesis. Intermetallics 2007, 15, 1050–1056. [Google Scholar] [CrossRef]
- Cai, X.; Li, K.; Sang, C.; Ren, X.; Leihu, X.; Feng, P. Interfacial microstructure and mechanical properties of Ti/Cu joint manufactured by Ni-Al thermal explosion reaction. J. Manuf. Mater. Process. 2020, 57, 919–929. [Google Scholar] [CrossRef]
- Cai, X.; Ren, X.; Sang, C.; Zhu, L.; Li, P.Z.; Feng, Z. Dissimilar joining mechanism, microstructure and properties of Ni to 316 stainless steel via Ni-Al thermal explosion reaction. Mater. Sci. Eng. 2021, 807, 140868. [Google Scholar] [CrossRef]
- Thiers, L.; Mukasyan, A.S.; Varma, A. Thermal explosion in Ni-Al system: Influence of reaction medium microstructure. Combust. Flame 2002, 131, 198–209. [Google Scholar] [CrossRef]
- Herbold, E.B.; Jordan, J.L.; Thadhani, N.N. Effects of processing and powder size on microstructure and reactivity in arrested reactive milled Al + Ni. Acta Mater. 2011, 59, 6717–6728. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Zhao, J.; Ning, H.; Liu, G. Deformation behavior and fracture mechanism of a novel laminated Ni/Al sheets during in-situ tensile process. Mater. Sci. Eng. A 2021, 827, 142050. [Google Scholar] [CrossRef]
- Ozdemir, O.; Zeytin, S.; Bindal, C. Tribological properties of NiAl produced by pressure-assisted combustion synthesis. Wear 2008, 265, 979–985. [Google Scholar] [CrossRef]
- Church, P.; Claridge, R.; Ottley, P.; Lewtas, I.; Harrison, N.; Gould, P.; Braithwaite, C.; Williamson, D. Investigation of a Nickel-Aluminum Reactive Shaped Charge Liner. J. Appl. Mech. 2013, 80, 031701324234. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, R.; Lin, C.; Wang, E.; Wang, Y.; Song, L.; Han, W. Fabrication and characterization of NiTif-SiCf synergistically reinforced (Al3Ti+Al3Ni)-based metallic-intermetallic laminated composite. J. Alloys Compd. 2021, 881, 160520. [Google Scholar] [CrossRef]
- Liang, G.; Yuan, M.; Wang, H.; Pei, X.; Zhou, X.; Li, D. Microstructure evolution and mechanical properties of Ti-Ni-Al ternary Metallic-Intermetallic Laminate (MIL) composites. J. Alloys Compd. 2023, 935, 167853. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, S.; Ma, Z.; Lu, B.; Wang, Z. Effects of SiC Fibers and Laminated Structure on Mechanical Properties of Ti–Al Laminated Composites. Materials 2021, 14, 1323. [Google Scholar] [CrossRef]
- Zelepugin, S.A.; Shkoda, O.A.; Lepakova, O.K.; Zelepugin, A.S.; Kasatskii, N.G.; Shavnev, A.A.; Krasnov, E.I. Synthesis of the Ti–TiAl3 metallic-intermetallic laminate composite by various methods. Trudy VIAM 2016, 47, 23–31. [Google Scholar] [CrossRef]
- Konieczny, M. Mechanical properties and deformation behavior of laminated Ni-(Ni2Al3+NiAl3) and Ni-(Ni3Al+NiAl) composites. Mater. Sci. Eng. 2013, 586, 11–18. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, F.; Lin, C.; Yuan, D.; Huang, H.; Wang, E.; Wang, Z.; Guo, C. Microstructure and mechanical properties of continuous ceramic SiC and shape memory alloy NiTi hybrid fibers reinforced Ti-Al metal-intermetallic laminated composite. J. Alloys Compd. 2017, 729, 1145–1155. [Google Scholar] [CrossRef]
- Emurlaeva, Y.Y.; Ivanov, I.V.; Lazurenko, D.V.; Ogneva, T.S.; Chen, P.W.; Zhou, Q.; Bataev, A.A.; Ruktuev, A.A.; Tanaka, S.; Bataev, I.A. On the texture and superstructure formation in Ti-TiAl3-Al MIL composites. Intermetallics 2021, 135, 107231. [Google Scholar] [CrossRef]
- Ogneva, T.S.; Bataev, I.A.; Mali, V.I.; Anisimov, A.G.; Lazurenko, D.V.; Popelyukh, A.I.; Emurlaeva, Y.Y.; Bataev, A.A.; Tanaka, S.; Yegoshin, K.D. Effect of sintering pressure and temperature on structure and properties of Ni single bond Al metal-intermetallic composites produced by SPS. Mater. Charact. 2021, 180, 11415. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Singh, T.; Chowdhury, S.G.; Jindal, V. Microstructural Characteristics of Accumulative Roll-Bonded Ni-Al-Based Metal-Intermetallic Laminate Composite. J. Mater. Eng. Perform. 2012, 21, 1912–1918. [Google Scholar] [CrossRef]
- Riyadi, T.W.B.; Mujiyono, M.; Nurhadiyanto, D.; Mukhammad, A.F.H.; Hassan, S.B.A.; Wulandari, A.P.; Murni, M. Fabrication of NiAl and TiC intermetallic matrix composite coatings. Compos. Interfaces 2021, 29, 328–343. [Google Scholar] [CrossRef]
- Malakhov, A.; Shakhray, D.; Denisov, I.; Galiev, F.; Seropyan, S. Synthesis of NiAl Intermetallic Compound under Shock-Wave Extrusion. Materials 2022, 15, 6062. [Google Scholar] [CrossRef]
- Kim, Y.K.; Pouraliakbar, H.; Hong, S.I. Effect of interfacial intermetallic compounds evolution on the mechanical response and fracture of layered Ti/Cu/Ti clad materials. Mater. Sci. Eng. A 2020, 772, 138802. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Khalaj, G.; Jandaghi, M.R.; Fadaei, A.; Ghareh-Shiran, M.K.; Shim, S.H.; Hong, S.I. Three-layered SS321/AA1050/AA5083 explosive welds: Effect of PWHT on the interface evolution and its mechanical strength. Int. J. Press. Vessel. Pip. 2020, 188, 104216. [Google Scholar] [CrossRef]




| Pellet | Powder | Particle Size, µm | |
|---|---|---|---|
| to 50% wt. | to 90% wt. | ||
| Ni-Al | Ni | <9.1 | <21.0 |
| Al | <18.4 | <28.2 | |
| Ti-C | Ti | <52.5 | <85.4 |
| C | <2.5 | <4.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denisov, I.; Shakhray, D.; Malakhov, A.; Seropyan, S. Combustion Synthesis of Metal-Intermetallic-Ceramic Laminate AlMg6-NiAl-TiC Composite. Crystals 2022, 12, 1851. https://doi.org/10.3390/cryst12121851
Denisov I, Shakhray D, Malakhov A, Seropyan S. Combustion Synthesis of Metal-Intermetallic-Ceramic Laminate AlMg6-NiAl-TiC Composite. Crystals. 2022; 12(12):1851. https://doi.org/10.3390/cryst12121851
Chicago/Turabian StyleDenisov, Igor, Denis Shakhray, Andrey Malakhov, and Stepan Seropyan. 2022. "Combustion Synthesis of Metal-Intermetallic-Ceramic Laminate AlMg6-NiAl-TiC Composite" Crystals 12, no. 12: 1851. https://doi.org/10.3390/cryst12121851
APA StyleDenisov, I., Shakhray, D., Malakhov, A., & Seropyan, S. (2022). Combustion Synthesis of Metal-Intermetallic-Ceramic Laminate AlMg6-NiAl-TiC Composite. Crystals, 12(12), 1851. https://doi.org/10.3390/cryst12121851






